Hyperketonemia Predictions Provide an On-Farm Management Tool with Epidemiological Insights
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Day Records for Milk Components and Hyperketonemia Predictions
2.2. Cow- and Herd-Level Data Aggregation
2.3. Statistical Analysis
3. Results
3.1. General Descriptive Statistics of the Dataset
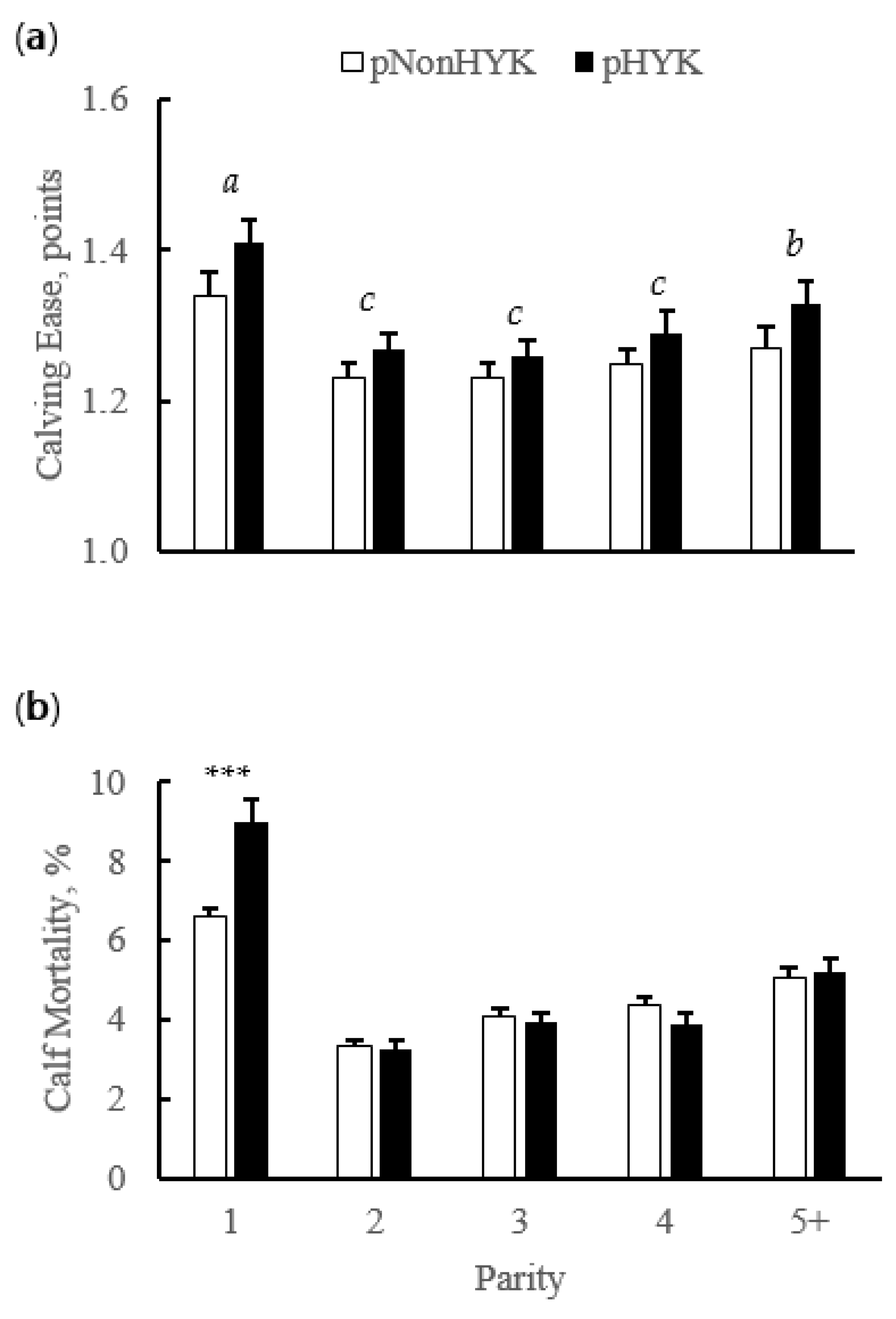
3.2. Relationships between Prediction of Hyperketonemia and Prior Cow and Parturition Factors
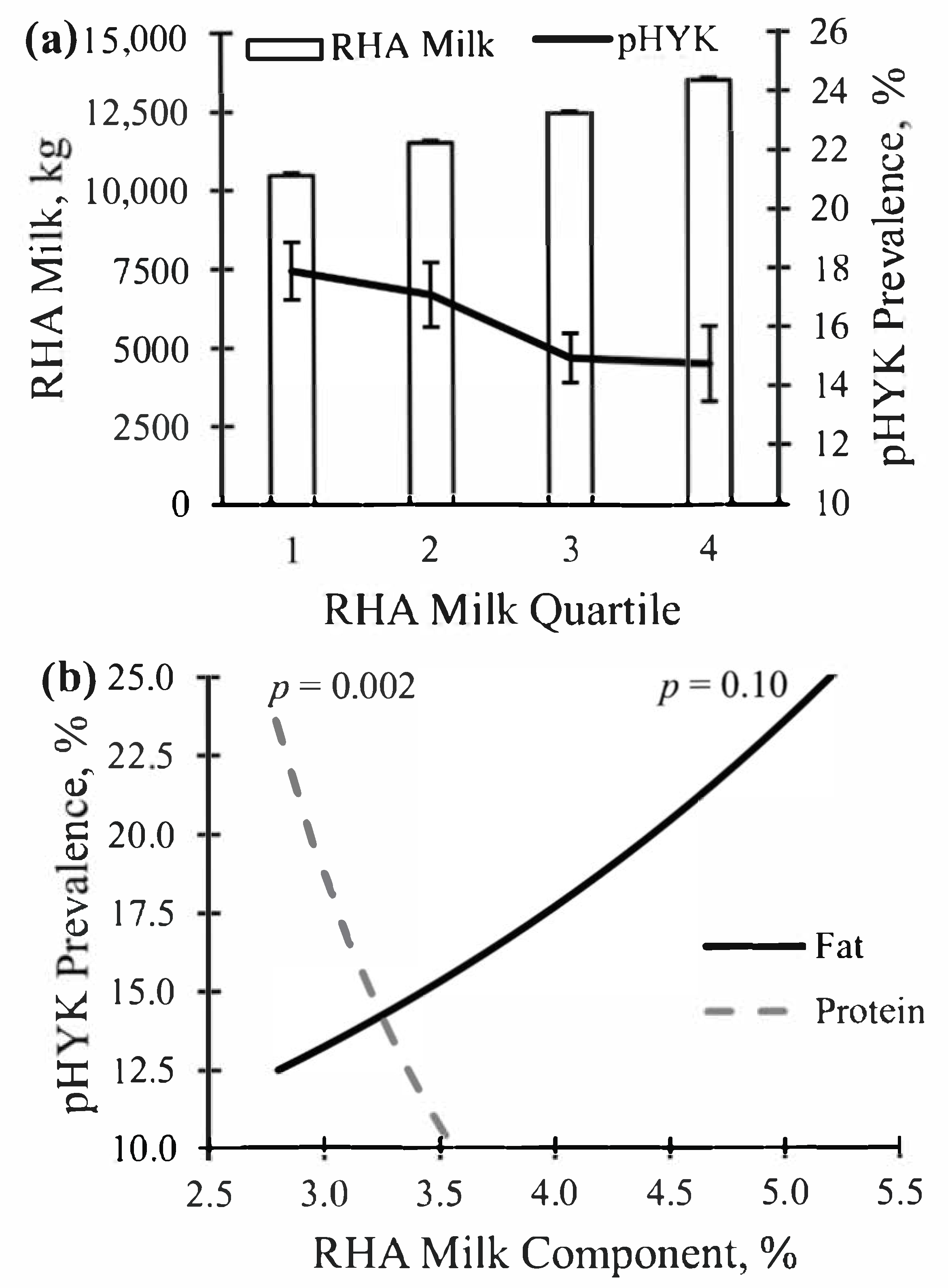
3.3. Relationships between Prediction of Hyperketonemia and Early Lactation Performance
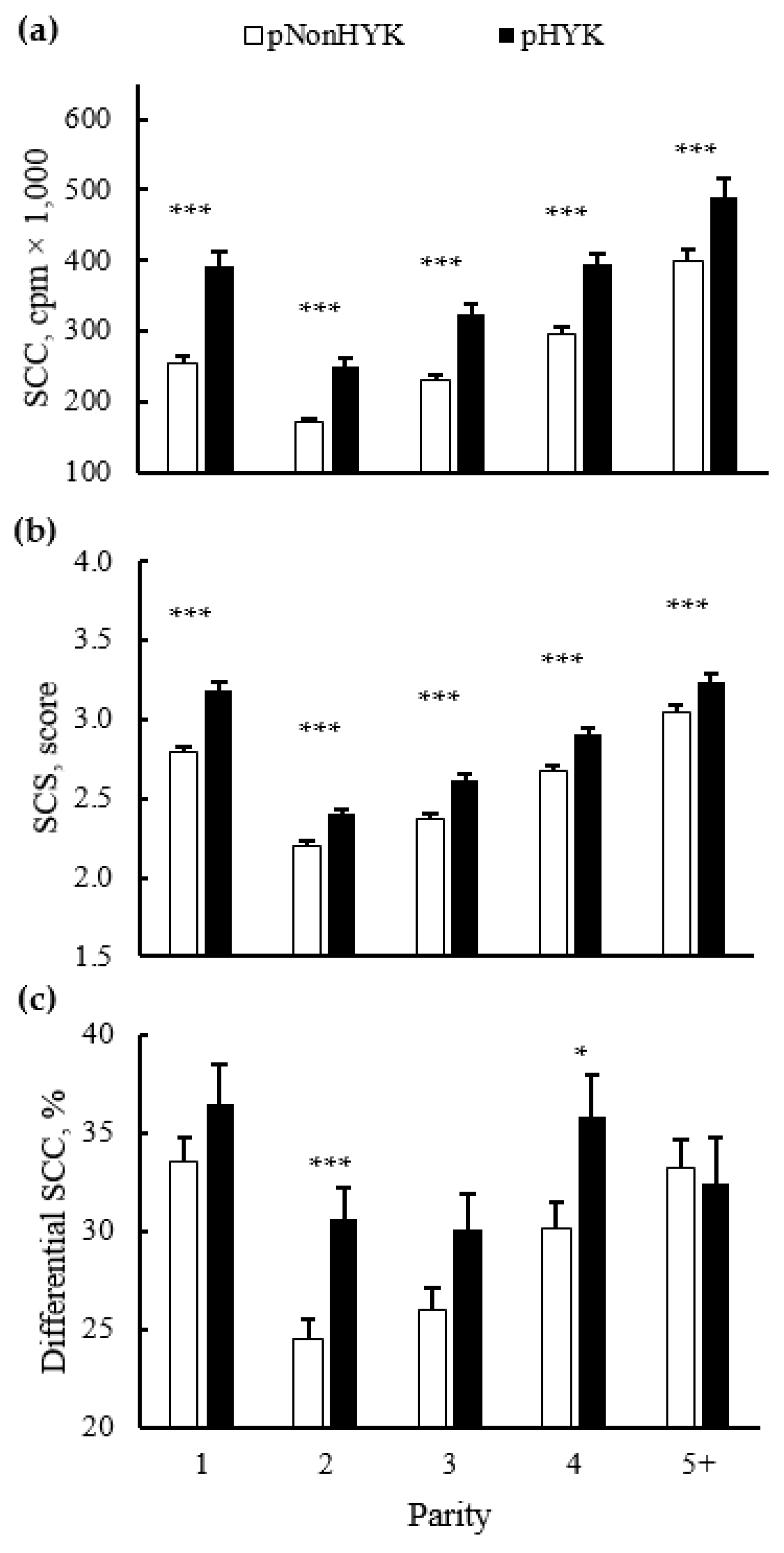
3.4. Relationships between Prediction of Hyperketonemia and Cow Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabrera, V.E.; Barrientos-Blanco, J.A.; Delgado, H.; Fadul-Pacheco, L. Symposium Review: Real-Time Continuous Decision Making Using Big Data on Dairy Farms. J. Dairy Sci. 2020, 103, 3856–3866. [Google Scholar] [CrossRef] [PubMed]
- Pralle, R.S.; White, H.M. Symposium Review: Big Data, Big Predictions: Utilizing Milk Fourier-Transform Infrared and Genomics to Improve Hyperketonemia Management. J. Dairy Sci. 2020, 103, 3867–3873. [Google Scholar] [CrossRef] [PubMed]
- Halachmi, I.; Guarino, M.; Bewley, J.; Pastell, M. Smart Animal Agriculture: Application of Real-Time Sensors to Improve Animal Well-Being and Production. Annu. Rev. Anim. Biosci. 2018, 7, 1–23. [Google Scholar] [CrossRef]
- Chandler, T.L.; Pralle, R.S.; Dórea, J.R.R.; Poock, S.E.; Oetzel, G.R.; Fourdraine, R.H.; White, H.M. Predicting Hyperketonemia by Logistic and Linear Regression Using Test-Day Milk and Performance Variables in Early-Lactation Holstein and Jersey Cows. J. Dairy Sci. 2018, 101, 2476–2491. [Google Scholar] [CrossRef]
- Suthar, V.S.; Canelas-Raposo, J.; Deniz, A.; Heuwieser, W. Prevalence of Subclinical Ketosis and Relationships with Postpartum Diseases in European Dairy Cows. J. Dairy Sci. 2013, 96, 2925–2938. [Google Scholar] [CrossRef] [Green Version]
- Santschi, D.E.; Lacroix, R.; Durocher, J.; Duplessis, M.; Moore, R.K.; Lefebvre, D.M. Prevalence of Elevated Milk β-Hydroxybutyrate Concentrations in Holstein Cows Measured by Fourier-Transform Infrared Analysis in Dairy Herd Improvement Milk Samples and Association with Milk Yield and Components. J. Dairy Sci. 2016, 99, 9263–9270. [Google Scholar] [CrossRef] [PubMed]
- McArt, J.A.A.; Nydam, D.V.; Overton, M.W. Hyperketonemia in Early Lactation Dairy Cattle: A Deterministic Estimate of Component and Total Cost per Case. J. Dairy Sci. 2015, 98, 2043–2054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pralle, R.S.; Weigel, K.W.; White, H.M. Predicting Blood β-Hydroxybutyrate Using Milk Fourier Transform Infrared Spectrum, Milk Composition, and Producer-Reported Variables with Multiple Linear Regression, Partial Least Squares Regression, and Artificial Neural Network. J. Dairy Sci. 2018, 101, 4378–4387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutten, M.J.M.; Bovenhuis, H.; Hettinga, K.A.; van Valenberg, H.J.F.; van Arendonk, J.A.M. Predicting Bovine Milk Fat Composition Using Infrared Spectroscopy Based on Milk Samples Collected in Winter and Summer. J. Dairy Sci. 2009, 92, 6202–6209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutten, M.J.M.; Bovenhuis, H.; Heck, J.M.L.; van Arendonk, J.A.M. Predicting Bovine Milk Protein Composition Based on Fourier Transform Infrared Spectra. J. Dairy Sci. 2011, 94, 5683–5690. [Google Scholar] [CrossRef] [PubMed]
- Van der Drift, S.G.A.; Jorritsma, R.; Schonewille, J.T.; Knijn, H.M.; Stegeman, J.A. Routine Detection of Hyperketonemia in Dairy Cows Using Fourier Transform Infrared Spectroscopy Analysis of β-Hydroxybutyrate and Acetone in Milk in Combination with Test-Day Information. J. Dairy Sci. 2012, 95, 4886–4898. [Google Scholar] [CrossRef] [Green Version]
- Denis-Robichaud, J.; Dubuc, J.; Lefebvre, D.; DesCôteaux, L. Accuracy of Milk Ketone Bodies from Flow-Injection Analysis for the Diagnosis of Hyperketonemia in Dairy Cows. J. Dairy Sci. 2014, 97, 3364–3370. [Google Scholar] [CrossRef]
- De Roos, A.P.W.; van den Bijgaart, H.J.C.M.; Hørlyk, J.; de Jong, G. Screening for Subclinical Ketosis in Dairy Cattle by Fourier Transform Infrared Spectrometry. J. Dairy Sci. 2007, 90, 1761–1766. [Google Scholar] [CrossRef] [Green Version]
- Chandler, T.L.; Pralle, R.S.; Oetzel, G.R.; Fourdraine, R.H.; White, H.M. Development of a Ketosis Prevalence Detection Tool in Holstein Dairy Cows Based on Milk Component Data and Cow Test-Day Information. J. Dairy Sci. 2015, 98 (Suppl. 2), 507. [Google Scholar]
- Borchers, M.R.; Bewley, J.M. An Assessment of Producer Precision Dairy Farming Technology Use, Prepurchase Considerations, and Usefulness. J. Dairy Sci. 2015, 98, 4198–4205. [Google Scholar] [CrossRef] [Green Version]
- Vanholder, T.; Papen, J.; Bemers, R.; Vertenten, G.; Berge, A.C.B. Risk Factors for Subclinical and Clinical Ketosis and Association with Production Parameters in Dairy Cows in the Netherlands. J. Dairy Sci. 2015, 98, 880–888. [Google Scholar] [CrossRef] [Green Version]
- Duffield, T. Subclinical Ketosis in Lactating Dairy Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 231–253. [Google Scholar] [CrossRef]
- Duffield, T.F.; Lissemore, K.D.; McBride, B.W.; Leslie, K.E. Impact of Hyperketonemia in Early Lactation Dairy Cows on Health and Production. J. Dairy Sci. 2009, 92, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Curtis, C.R.; Erb, H.N.; Sniffen, C.J.; Smith, R.D.; Kronfeld, D.S. Path Analysis of Dry Period Nutrition, Postpartum Metabolic and Reproductive Disorders, and Mastitis in Holstein Cows1. J. Dairy Sci. 1985, 68, 2347–2360. [Google Scholar] [CrossRef]
- Gröhn, Y.T.; Erb, H.N.; McCulloch, C.E.; Saloniemi, H.S. Epidemiology of Metabolic Disorders in Dairy Cattle: Association Among Host Characteristics, Disease, and Production. J. Dairy Sci. 1989, 72, 1876–1885. [Google Scholar] [CrossRef]
- Vergara, C.F.; Döpfer, D.; Cook, N.B.; Nordlund, K.V.; McArt, J.A.A.; Nydam, D.V.; Oetzel, G.R. Risk Factors for Postpartum Problems in Dairy Cows: Explanatory and Predictive Modeling. J. Dairy Sci. 2014, 97, 4127–4140. [Google Scholar] [CrossRef] [Green Version]
- McArt, J.A.A.; Nydam, D.V.; Oetzel, G.R. Epidemiology of Subclinical Ketosis in Early Lactation Dairy Cattle. J. Dairy Sci. 2012, 95, 5056–5066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathbun, F.M.; Pralle, R.S.; Bertics, S.J.; Armentano, L.E.; Cho, K.; Do, C.; Weigel, K.A.; White, H.M. Relationships between Body Condition Score Change, Prior Mid-Lactation Phenotypic Residual Feed Intake, and Hyperketonemia Onset in Transition Dairy Cows. J. Dairy Sci. 2017, 100, 3685–3696. [Google Scholar] [CrossRef] [Green Version]
- McArt, J.A.A.; Nydam, D.V.; Oetzel, G.R. A Field Trial on the Effect of Propylene Glycol on Displaced Abomasum, Removal from Herd, and Reproduction in Fresh Cows Diagnosed with Subclinical Ketosis. J. Dairy Sci. 2012, 95, 2505–2512. [Google Scholar] [CrossRef] [Green Version]
- Bauman, D.E.; Currie, W.B. Partitioning of Nutrients During Pregnancy and Lactation: A Review of Mechanisms Involving Homeostasis and Homeorhesis. J. Dairy Sci. 1980, 63, 1514–1529. [Google Scholar] [CrossRef]
- Samková, E.; Špička, J.; Hanuš, O.; Roubal, P.; Pecová, L.; Hasoňová, L.; Smetana, P.; Klimešová, M.; Čítek, J. Comparison of Fatty Acid Proportions Determined by Mid-Infrared Spectroscopy and Gas Chromatography in Bulk and Individual Milk Samples. Animals 2020, 10, 1095. [Google Scholar] [CrossRef] [PubMed]
- Woolpert, M.E.; Dann, H.M.; Cotanch, K.W.; Melilli, C.; Chase, L.E.; Grant, R.J.; Barbano, D.M. Management Practices, Physically Effective Fiber, and Ether Extract Are Related to Bulk Tank Milk de Novo Fatty Acid Concentration on Holstein Dairy Farms. J. Dairy Sci. 2017, 100, 5097–5106. [Google Scholar] [CrossRef] [Green Version]
- Miettinen, P.V.A. Relationship between Milk Acetone and Milk Yield in Individual Cows. J. Vet. Med. Ser. 1994, 41, 102–109. [Google Scholar] [CrossRef]
- White, H.M. ADSA Foundation Scholar Award: Influencing Hepatic Metabolism: Can Nutrient Partitioning Be Modulated to Optimize Metabolic Health in the Transition Dairy Cow? J. Dairy Sci. 2020, 103, 6741–6750. [Google Scholar] [CrossRef]
- Drackley, J.K. ADSA Foundation Scholar Award. Biology of Dairy Cows during the Transition Period: The Final Frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- Rukkwamsuk, T.; Geelen, M.J.; Kruip, T.A.; Wensing, T. Interrelation of Fatty Acid Composition in Adipose Tissue, Serum, and Liver of Dairy Cows during the Development of Fatty Liver Postpartum. J. Dairy Sci. 2000, 83, 52–59. [Google Scholar] [CrossRef]
- Weld, K.A.; Oliveira, R.C.; Bertics, S.J.; Erb, S.J.; White, H.M. Hepatic Pyruvate Carboxylase Expression Differed Prior to Hyperketonemia Onset in Transition Dairy Cows. PLoS ONE 2020, 15, e0241929. [Google Scholar] [CrossRef] [PubMed]
- Tessari, R.; Berlanda, M.; Morgante, M.; Badon, T.; Gianesella, M.; Mazzotta, E.; Contiero, B.; Fiore, E. Changes of Plasma Fatty Acids in Four Lipid Classes to Understand Energy Metabolism at Different Levels of Non-Esterified Fatty Acid (NEFA) in Dairy Cows. Animals 2020, 10, 1410. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Nydam, D.V.; Lock, A.L.; Overton, T.R.; McArt, J.A.A. Short Communication: Association of Milk Fatty Acids with Early Lactation Hyperketonemia and Elevated Concentration of Nonesterified Fatty Acids. J. Dairy Sci. 2016, 99, 5851–5857. [Google Scholar] [CrossRef]
- Jorjong, S.; van Knegsel, A.T.M.; Verwaeren, J.; Bruckmaier, R.M.; Baets, B.D.; Kemp, B.; Fievez, V. Milk Fatty Acids as Possible Biomarkers to Diagnose Hyperketonemia in Early Lactation. J. Dairy Sci. 2015, 98, 5211–5221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbano, D.M.; Melilli, C.; Overton, T.R. Advanced Use of FTIR Spectra of Milk for Feeding and Health Management. In Proceedings of the Cornell Nutrition Conference, Syracuse, NY, USA, 21 October 2014. [Google Scholar]
- Weld, A.K.; Oliveira, R.C.; Sailer, K.J.; Holdorf, H.T.; Bertics, S.J.; White, H.M. Hyperketonemia Does Not Affect Proportional Uptake of Fatty Acids by the Mammary Gland. J. Dairy Sci. 2018, 10 (Suppl. 2), 270. [Google Scholar]
- Schukken, Y.H.; Wilson, D.J.; Welcome, F.; Garrison-Tikofsky, L.; Gonzalez, R.N. Monitoring Udder Health and Milk Quality Using Somatic Cell Counts. Vet. Res. 2003, 34, 579–596. [Google Scholar] [CrossRef] [Green Version]
- Harmon, R.J. Physiology of Mastitis and Factors Affecting Somatic Cell Counts. J. Dairy Sci. 1994, 77, 2103–2112. [Google Scholar] [CrossRef]
- Schwarz, D.; Kleinhans, S.; Reimann, G.; Stückler, P.; Reith, F.; Ilves, K.; Pedastsaar, K.; Yan, L.; Zhang, Z.; Valdivieso, M.; et al. Investigation of Dairy Cow Performance in Different Udder Health Groups Defined Based on a Combination of Somatic Cell Count and Differential Somatic Cell Count. Prev. Vet. Med. 2020, 183, 105123. [Google Scholar] [CrossRef]
- Van Straten, M.; Friger, M.; Shpigel, N.Y. Events of Elevated Somatic Cell Counts in High-Producing Dairy Cows Are Associated with Daily Body Weight Loss in Early Lactation. J. Dairy Sci. 2009, 92, 4386–4394. [Google Scholar] [CrossRef] [Green Version]
- Shen, T.; Li, X.; Loor, J.J.; Zhu, Y.; Du, X.; Wang, X.; Xing, D.; Shi, Z.; Fang, Z.; Li, X.; et al. Hepatic Nuclear Factor Kappa B Signaling Pathway and NLR Family Pyrin Domain Containing 3 Inflammasome Is Over-Activated in Ketotic Dairy Cows. J. Dairy Sci. 2019, 102, 10554–10563. [Google Scholar] [CrossRef] [PubMed]
- Pralle, R.S.; Li, W.; White, H.M. Hepatic Differential Gene Expression of Cows Clustered by Postpartum Metabolites: A Model for Susceptibility to Lipid-Related Metabolic Disorders. J. Dairy Sci. 2020, 103 (Suppl. 1), 27. [Google Scholar]
- Damm, M.; Holm, C.; Blaabjerg, M.; Bro, M.N.; Schwarz, D. Differential Somatic Cell Count—A Novel Method for Routine Mastitis Screening in the Frame of Dairy Herd Improvement Testing Programs. J. Dairy Sci. 2017, 100, 4926–4940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkeby, C.; Toft, N.; Schwarz, D.; Farre, M.; Nielsen, S.S.; Zervens, L.; Hechinger, S.; Halasa, T. Differential Somatic Cell Count as an Additional Indicator for Intramammary Infections in Dairy Cows. J. Dairy Sci. 2020, 103, 1759–1775. [Google Scholar] [CrossRef] [Green Version]
- Economic Research Service. Milk Cost of Production Estimates. Available online: http://www.ers.usda.gov/data-products/milk-cost-of-production-estimates/ (accessed on 29 April 2021).
- LeBlanc, S.J.; Leslie, K.E.; Duffield, T.F. Metabolic Predictors of Displaced Abomasum in Dairy Cattle. J. Dairy Sci. 2005, 88, 159–170. [Google Scholar] [CrossRef] [Green Version]
- McConnel, C.S.; McNeil, A.A.; Hadrich, J.C.; Lombard, J.E.; Heller, J.; Garry, F.B. A Comparison of a Novel Time-Based Summary Measure of Dairy Cow Health against Cumulative Disease Frequency. Irish Vet. J. 2018, 71, 7. [Google Scholar] [CrossRef] [Green Version]
- Gaddis, K.L.P.; Cole, J.B.; Clay, J.S.; Maltecca, C. Genomic Selection for Producer-Recorded Health Event Data in US Dairy Cattle. J. Dairy Sci. 2014, 97, 3190–3199. [Google Scholar] [CrossRef]
- Mörk, M.; Lindberg, A.; Alenius, S.; Vågsholm, I.; Egenvall, A. Comparison between Dairy Cow Disease Incidence in Data Registered by Farmers and in Data from a Disease-Recording System Based on Veterinary Reporting. Prev. Vet. Med. 2009, 88, 298–307. [Google Scholar] [CrossRef] [PubMed]
- McArt, J.A.A.; Nydam, D.V.; Oetzel, G.R.; Overton, T.R.; Ospina, P.A. Elevated Non-Esterified Fatty Acids and β-Hydroxybutyrate and Their Association with Transition Dairy Cow Performance. Vet. J. 2013, 198, 560–570. [Google Scholar] [CrossRef]
- Pralle, R.S.; Erb, S.J.; Holdorf, H.T.; White, H.M. Greater Liver PNPLA3 Protein Abundance in Vivo and in Vitro Supports Lower Triglyceride Accumulation in Dairy Cows. Sci. Rep. 2021, 11, 2839. [Google Scholar] [CrossRef] [PubMed]
- Grummer, R.R. Nutritional and Management Strategies for the Prevention of Fatty Liver in Dairy Cattle. Vet. J. 2008, 176, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Weigel, K.A.; Pralle, R.S.; Adams, H.; Cho, K.; Do, C.; White, H.M. Prediction of Whole-genome Risk for Selection and Management of Hyperketonemia in Holstein Dairy Cattle. J. Anim. Breed. Genet. 2017, 134, 275–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pralle, R.S.; Schultz, N.E.; White, H.M.; Weigel, K.A. Hyperketonemia GWAS and Parity-Dependent SNP Associations in Holstein Dairy Cows Intensively Sampled for Blood β-Hydroxybutyrate Concentration. Physiol. Genom. 2020, 52, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, S.; Schenkel, F.; Fleming, A.; Kroezen, V.; Sargolzaei, M.; Baes, C.; Cánovas, A.; Squires, J.; Miglior, F. Genome-Wide Association Analysis for β-Hydroxybutyrate Concentration in Milk in Holstein Dairy Cattle. BMC Genet. 2019, 20, 58. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Huang, H.; Freebern, E.; Santos, D.J.A.; Dai, D.; Si, J.; Ma, C.; Cao, J.; Guo, G.; Liu, G.E.; et al. Integrating RNA-Seq with GWAS Reveals Novel Insights into the Molecular Mechanism Underpinning Ketosis in Cattle. BMC Genom. 2020, 21, 489. [Google Scholar] [CrossRef]



| Variable | N, Denominator 2 | Mean 3 | SD 4 | Min 5 | Q1 6 | Median | Q3 7 | Max 8 |
|---|---|---|---|---|---|---|---|---|
| All Records | 240,714 | — | — | — | — | — | — | — |
| Records/Cow 9 | 174,690 | 1.4 | 0.7 | 1.0 | 1.0 | 1.0 | 2.0 | 6.0 |
| Records/Herd 10 | 335 | 718.6 | 1328.0 | 11.0 | 116.0 | 255.0 | 641.0 | 13,204.0 |
| Cows/Herd 11 | 335 | 521.5 | 949.1 | 11.0 | 91.5 | 179.0 | 468.5 | 9550.0 |
| Primiparous Records | 88,782 | — | — | — | — | — | — | — |
| Records/Cow | 88,782 | 1.0 | 0.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Records/Herd | 333 | 266.6 | 499.5 | 1.0 | 42.0 | 97.0 | 232.0 | 5091.0 |
| Cows/Herd | 333 | 266.6 | 499.5 | 1.0 | 42.0 | 97.0 | 232.0 | 5091.0 |
| Records %, Herd 12 | — | 36.9 | 13.8 | 0.0 | 31.8 | 36.5 | 40.6 | 99.5 |
| Multiparous Records | 151,932 | — | — | — | — | — | — | — |
| Records/Cow | 115,745 | 1.3 | 0.6 | 1.0 | 1.0 | 1.0 | 2.0 | 6.0 |
| Records/Herd | 335 | 453.5 | 835.4 | 5.0 | 76.5 | 163.0 | 403.0 | 8113.0 |
| Cows/Herd | 335 | 345.5 | 632.6 | 5.0 | 59.5 | 122.0 | 311.5 | 6318.0 |
| Records %, Herd | — | 63.1 | 13.8 | 0.5 | 59.4 | 63.5 | 68.3 | 100.0 |
| pNonHYK Records | 202,659 | — | — | — | — | — | — | — |
| Records/Cow | 155,646 | 1.3 | 0.6 | 1.0 | 1.0 | 1.0 | 1.0 | 6.0 |
| Records/Herd | 335 | 605.0 | 1127.5 | 9.0 | 95.0 | 211.0 | 545.0 | 10,935.0 |
| Cows/Herd | 335 | 464.6 | 853.6 | 9.0 | 80.5 | 160.0 | 426.0 | 8516.0 |
| Records %, Herd | — | 83.8 | 7.6 | 52.1 | 79.5 | 85.3 | 89.2 | 97.7 |
| pHYK Records | 38,055 | — | — | — | — | — | — | — |
| Records/Cow | 34,427 | 1.1 | 0.3 | 1.0 | 1.0 | 1.0 | 1.0 | 4.0 |
| Records/Herd | 335 | 113.6 | 216.7 | 1.0 | 16.5 | 40.0 | 105.5 | 2269.0 |
| Cows/Herd | 335 | 102.8 | 194.5 | 1.0 | 16.0 | 36.0 | 94.0 | 2063.0 |
| Records %, Herd | — | 16.2 | 7.6 | 2.3 | 10.8 | 14.7 | 20.5 | 47.9 |
| Variable | Mean 1 | SD 2 | Min 3 | Q1 4 | Median | Q3 5 | Max 6 |
|---|---|---|---|---|---|---|---|
| All Records (11,539 Records; 321 Herds) | — | — | — | — | — | — | — |
| Records per Herd | 36.0 | 24.1 | 1.0 | 15.0 | 30.0 | 62.0 | 72.0 |
| Cows Tested, RHA | 467.7 | 681.5 | 32.8 | 125.3 | 228.0 | 562.8 | 7511.7 |
| Postpartum Cows Tested 7 | 220.0 | 327.9 | 11.0 | 57.0 | 105.0 | 250.0 | 3878.0 |
| Predicted HYK, % 8 | 15.9 | 8.4 | 0.0 | 9.8 | 14.9 | 20.7 | 58.8 |
| RHA Milk, kg | 12,125.8 | 1729.3 | 5539.3 | 11,136.6 | 12,265.6 | 13,264.6 | 18,196.3 |
| RHA Milk Fat, kg | 465.1 | 68.3 | 225.0 | 421.4 | 466.3 | 513.0 | 684.9 |
| RHA Milk Fat, % | 3.8 | 0.2 | 2.8 | 3.7 | 3.8 | 4.0 | 5.3 |
| RHA Milk Protein, kg | 376.2 | 51.9 | 176.0 | 345.6 | 380.6 | 409.6 | 536.2 |
| RHA Milk Protein, % | 3.1 | 0.1 | 2.8 | 3.0 | 3.1 | 3.2 | 3.6 |
| RHA Quartile 1 (2885 Records; 158 Herds) | — | — | — | — | — | — | — |
| Records per Herd | 18.3 | 17.2 | 1.0 | 5.0 | 13.0 | 24.8 | 69.0 |
| Cows Tested, RHA | 197.0 | 507.6 | 34.0 | 82.5 | 118.2 | 174.4 | 5596.1 |
| Postpartum Cows Tested | 84.3 | 195.5 | 11.0 | 39.0 | 56.0 | 80.0 | 2914.0 |
| Predicted HYK, % | 16.6 | 9.4 | 0.0 | 9.8 | 15.4 | 22.2 | 58.8 |
| RHA Milk, kg | 9870.7 | 1119.1 | 5539.3 | 9467.8 | 10,151.8 | 10,665.3 | 11,136.1 |
| RHA Milk Fat, kg | 382.6 | 45.5 | 225.0 | 362.4 | 388.7 | 412.8 | 585.1 |
| RHA Milk Fat, % | 3.9 | 0.2 | 3.1 | 3.7 | 3.9 | 4.0 | 5.3 |
| RHA Milk Protein, kg | 309.5 | 35.0 | 176.0 | 297.1 | 318.0 | 332.9 | 372.0 |
| RHA Milk Protein, % | 3.1 | 0.1 | 2.9 | 3.1 | 3.1 | 3.2 | 3.6 |
| RHA Quartile 2 (2884 Records; 147 Herds) | — | — | — | — | — | — | — |
| Records per Herd | 19.6 | 16.5 | 1.0 | 6.0 | 15.0 | 28.0 | 66.0 |
| Cows Tested, RHA | 378.8 | 730.9 | 32.8 | 113.2 | 180.7 | 296.0 | 7511.7 |
| Postpartum Cows Tested | 178.4 | 350.7 | 11.0 | 54.8 | 83.0 | 140.0 | 3878.0 |
| Predicted HYK, % | 15.8 | 8.4 | 0.0 | 9.7 | 14.9 | 20.6 | 58.3 |
| RHA Milk, kg | 11,746.0 | 323.8 | 11,137.0 | 11,482.6 | 11,745.3 | 12,039.4 | 12,264.7 |
| RHA Milk Fat, kg | 447.5 | 28.1 | 339.7 | 429.1 | 445.9 | 462.7 | 590.1 |
| RHA Milk Fat, % | 3.8 | 0.2 | 2.8 | 3.7 | 3.8 | 3.9 | 5.3 |
| RHA Milk Protein, kg | 365.4 | 14.0 | 327.0 | 355.6 | 364.7 | 375.1 | 414.1 |
| RHA Milk Protein, % | 3.1 | 0.1 | 2.8 | 3.1 | 3.1 | 3.2 | 3.4 |
| RHA Quartile 3 (2885 Records; 142 Herds) | — | — | — | — | — | — | — |
| Records per Herd | 20.0 | 16.1 | 1.0 | 6.8 | 17.0 | 29.3 | 70.0 |
| Cows Tested, RHA | 630.8 | 771.1 | 35.7 | 178.7 | 387.0 | 727.8 | 5227.7 |
| Postpartum Cows Tested | 297.1 | 381.2 | 11.0 | 80.0 | 159.0 | 323.0 | 2524.0 |
| Predicted HYK, % | 16.4 | 7.6 | 0.0 | 11.1 | 15.6 | 20.7 | 46.5 |
| RHA Milk, kg | 12,745.0 | 290.3 | 12,265.6 | 12,495.6 | 12,726.4 | 12,994.5 | 13,264.4 |
| RHA Milk Fat, kg | 489.3 | 31.0 | 361.1 | 468.1 | 488.1 | 508.0 | 622.0 |
| RHA Milk Fat, % | 3.8 | 0.2 | 2.9 | 3.7 | 3.8 | 4.0 | 4.8 |
| RHA Milk Protein, kg | 394.6 | 14.6 | 347.5 | 385.1 | 394.2 | 403.7 | 439.1 |
| RHA Milk Protein, % | 3.1 | 0.1 | 2.8 | 3.0 | 3.1 | 3.2 | 3.4 |
| RHA Quartile 4 (2885 Records; 100 Herds) | — | — | — | — | — | — | — |
| Records per Herd | 28.9 | 21.3 | 1.0 | 10.8 | 23.0 | 42.3 | 72.0 |
| Cows Tested, RHA | 664.3 | 570.5 | 29.4 | 324.4 | 527.6 | 756.3 | 3418.8 |
| Postpartum Cows Tested | 320.1 | 296.1 | 11.0 | 122.0 | 251.0 | 384.0 | 2156.0 |
| Predicted HYK, % | 14.9 | 7.8 | 0.0 | 8.7 | 13.5 | 19.3 | 48.3 |
| RHA Milk, kg | 14,141.4 | 848.1 | 13,264.8 | 13,550.6 | 13,908.5 | 14,453.3 | 18,196.3 |
| RHA Milk Fat, kg | 540.9 | 37.5 | 430.9 | 515.3 | 537.1 | 563.4 | 684.9 |
| RHA Milk Fat, % | 3.8 | 0.3 | 3.1 | 3.7 | 3.8 | 4.0 | 4.9 |
| RHA Milk Protein, kg | 435.3 | 27.1 | 382.4 | 415.0 | 429.6 | 450.0 | 536.2 |
| RHA Milk Protein, % | 3.1 | 0.1 | 2.9 | 3.0 | 3.1 | 3.2 | 3.4 |
| Lactation 2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | 1 | 2 | 3 | 4 | 5+ | p-Value | ||||||
| pNonHYK | pHYK | pNonHYK | pHYK | pNonHYK | pHYK | pNonHYK | pHYK | pNonHYK | pHYK | SEM | Health × Parity 3 | |
| Cull within 60d, % | 5.7 | 13.7 *** | 3.4 | 7.6 *** | 4.1 | 10.5 *** | 5.6 | 11.8 *** | 7.4 | 13.5 *** | 0.95 | 0.003 |
| Days open, d | 120.9 | 133.0 *** | 126.9 | 132.6 *** | 128.7 | 132.0 *** | 128.6 | 132.8 *** | 132.4 | 141.2 *** | 2.33 | <0.001 |
| AI 4 to Conception | 2.0 | 2.1 *** | 2.1 | 2.2 *** | 2.2 | 2.2 ** | 2.1 | 2.3 *** | 2.2 | 2.4 *** | 0.04 | <0.001 |
| PTA 5 milk, kg | −14.1 | −12.0 | −58.5 | −66.4 | −93.1 | −106.6 * | −132.2 | −143.9 * | −186.3 | −207.7 *** | 10.0 | 0.02 |
| PTA DPR 6 | −0.27 | −0.47 *** | −0.19 | −0.33 *** | −0.06 | −0.17 *** | 0.12 | 0.05 * | 0.33 | 0.36 | 0.05 | <0.001 |
| PTA productive life | 0.12 | −0.34 | −0.12 | −0.39 | −0.20 | −0.47 * | −0.23 | −0.43 * | −0.22 | −0.28 *** | 0.07 | <0.001 |
| PTA SCS, score | 2.99 | 3.01 *** | 3.00 | 3.01 *** | 3.01 | 3.02 *** | 3.02 | 3.03 *** | 3.02 | 3.03 *** | 0.004 | <0.001 |
| PTA net merit | 5.48 | −22.95 *** | −45.39 | −63.04 *** | −82.78 | −103.37 *** | −119.86 | −136.83 *** | −165.74 | −176.40 * | 7.18 | 0.01 |
| PTA ketosis | 0.05 | −0.11 *** | −0.09 | −0.18 *** | −0.19 | −0.29 *** | −0.30 | −0.38 *** | −0.41 | −0.46 *** | 0.02 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pralle, R.S.; Amdall, J.D.; Fourdraine, R.H.; Oetzel, G.R.; White, H.M. Hyperketonemia Predictions Provide an On-Farm Management Tool with Epidemiological Insights. Animals 2021, 11, 1291. https://doi.org/10.3390/ani11051291
Pralle RS, Amdall JD, Fourdraine RH, Oetzel GR, White HM. Hyperketonemia Predictions Provide an On-Farm Management Tool with Epidemiological Insights. Animals. 2021; 11(5):1291. https://doi.org/10.3390/ani11051291
Chicago/Turabian StylePralle, Ryan S., Joel D. Amdall, Robert H. Fourdraine, Garrett R. Oetzel, and Heather M. White. 2021. "Hyperketonemia Predictions Provide an On-Farm Management Tool with Epidemiological Insights" Animals 11, no. 5: 1291. https://doi.org/10.3390/ani11051291
APA StylePralle, R. S., Amdall, J. D., Fourdraine, R. H., Oetzel, G. R., & White, H. M. (2021). Hyperketonemia Predictions Provide an On-Farm Management Tool with Epidemiological Insights. Animals, 11(5), 1291. https://doi.org/10.3390/ani11051291






