Effect of Finishing Diet and Lairage Time on Steers Welfare in Uruguay
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Determinations
2.1.1. Productivity
2.1.2. Temperament
2.1.3. Health Status
2.2. Transport and Slaughter Plant
2.2.1. Physiological Indicators
- Time A: before leaving the farm (basal values)
- Time B: immediately after arriving at the slaughterhouse (transport effect)
- Time C: after lairage (lairage effect)
- Time D: during bleeding immediately post slaughter (effect of the last handling procedures)
2.2.2. Behavior in Lairage Pen
2.2.3. Carcass Traits Indicators at the Abattoir as a Means of Assessing Overall Animal Welfare
Bruising
pH
2.3. Statistical Analysis
3. Results and Discussion
3.1. Field Determinations
3.1.1. Productivity
3.1.2. Temperament and ADG
3.1.3. Health Status
3.2. Transport and Slaughter Plant
3.2.1. Physiological Indicators
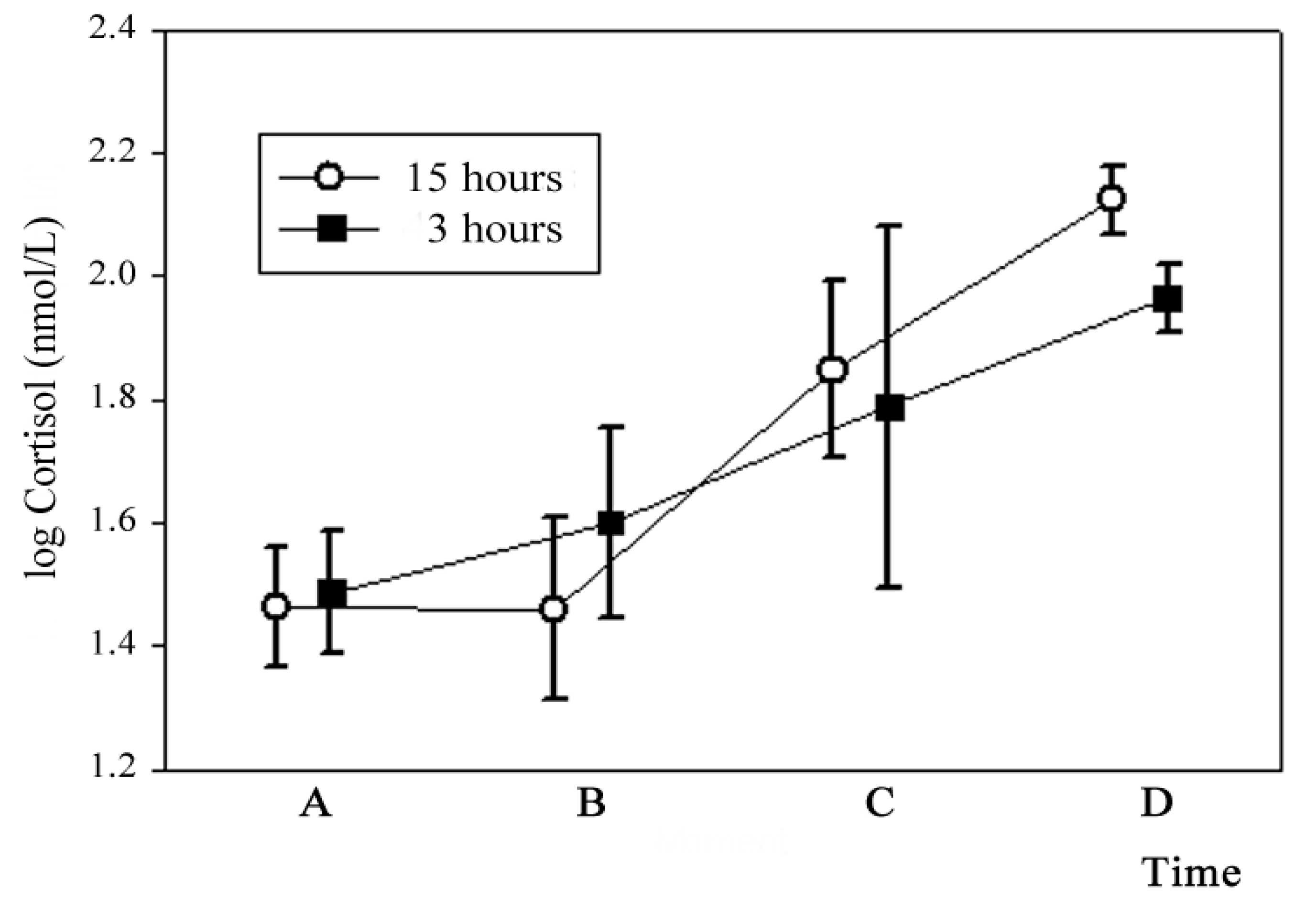
CORTISOL
Temperament and Cortisol
CPK
Temperament and CPK
NEFA
Temperament and NEFA
β-HIDROXIBUTIRATE (βHB)
3.2.2. Behavior in Lairage Pen
Temperament and Behavior
3.2.3. Carcass Traits Indicators at the Abattoir as a Means of Assessing Overall Animal Welfare
Bruising
pH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Gebresenbet, G.; Eriksson, B. Effect of Transport and Handling on Animal Welfare, Meat Quality and Environment with Special Emphasis on Tied Cows; Report 233; Department of Agricultural Engineering, Swedish University of Agricultural Sciences: Uppsala, Sweden, 1998. [Google Scholar]
- Gross, W.B.; Siegel, P.B. General Principle of stress and welfare. In Livestock Handling and Transport; Grandin, T., Ed.; CABI Publishing: Wallingford, UK, 1993. [Google Scholar]
- Honkavaara, M.; Kortesniemi, P. Effect of long distance transport on cattle stress and meat quality. Meat Focus Int. 1994, 3, 405–409. [Google Scholar]
- Warris, P.D.; Brown, S.N.; Knowles, T.G.; Kestin, S.C.; Edwards, J.E.; Dolan, S.K.; Phillip, A.J. Effect on cattle of transport by road for up to 15 h. Vet. Rec. 1995, 136, 319–323. [Google Scholar] [CrossRef]
- Terlouw, E.M.C.; Picard, B.; Deiss, V.; Berri, C.; Hocquette, J.-F.; Lebret, B.; Lefèvre, F.; Hamill, R.; Gagaoua, M. Understanding the Determination of Meat Quality Using Biochemical Characteristics of the Muscle: Stress at Slaughter and Other Missing Keys. Foods 2021, 10, 84. [Google Scholar] [CrossRef]
- Bourguet, C.; Deiss, V.; Boissy, A.; Terlouw, E.M.C. Young Blond d’Aquitaine, Angus and Limousin bulls differ in emotional reactivity: Relationships with animal traits, stress reactions at slaughter and post-mortem muscle metabolism. Appl. Anim. Behav. Sci. 2015, 164, 41–55. [Google Scholar] [CrossRef]
- Deters, E.L.; Hansen, S.L. Invited Review: Linking road transportation with oxidative stress in cattle and other species. Appl. Anim. Sci. 2020, 36, 183–200. [Google Scholar] [CrossRef]
- Ferguson, D.M.; Warner, R.D. Have we underestimated the impact of pre-slaughter stress on meat quality. Meat Sci. 2008, 80, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Gallo, C.B.; Huertas, S.M. Main animal welfare problems in ruminant livestock during preslaughter operations: A South American view. Animal 2015, 10, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Huertas, S.M.; Kempener, R.E.A.M.; van Eerdenburg, F.J.C.M. Relationship between Methods of Loading and Unloading, Carcass Bruising, and Animal Welfare in the Transportation of Extensively Reared Beef Cattle. Animals 2018, 8, 119. [Google Scholar] [CrossRef] [Green Version]
- Losada-Espinosa, N.; Villarroel, M.; María, G.A.; Miranda-de la Lama, G.C. Pre-slaughter cattle welfare indicators for use in commercial abattoirs with voluntary monitoring systems: A systematic review. Meat Sci. 2018, 34–48. [Google Scholar] [CrossRef] [Green Version]
- Schwartzkopf-Genswein, K.; Ahola, J.; Edwards-Callaway, L.; Hale, D.; Paterson, J. Transportation issues affecting cattle wellbeing and considerations for the future. Symposium Paper Prof. Anim. Sci. 2016, 32, 707–716. [Google Scholar] [CrossRef] [Green Version]
- Wigham, E.E.; Butterworth, A.; Wotton, S. Review: Assessing cattle welfare at slaughter—Why is it important and what challenges are faced? Meat Sci. 2018, 145, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Costa, F.O.; Brito, G.; Soares de Lima, J.M.; Sant’Anna, A.C.; Paranhos da Costa, M.J.R.; del Campo, M. Lairage time effect on meat quality in Hereford steers in rangeland conditions. Rev. Bras. Zootec. 2019, 48, e20180020. [Google Scholar] [CrossRef] [Green Version]
- Chulayo, A.Y.; Muchenje, V. Activities of some stress enzymes as indicators of slaughter cattle welfare and their relationship with physico-chemical characteristics of beef. Animal 2017, 11, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Frimpong, S.; Bebresenbet, G.; Bobobee, E.; Aklaku, E.D.; Hamdu, I. Effect of transportation and pre-slaughter handling on welfare and meat quality of cattle: Case study of Kumasi abattoir, Ghana. Vet. Sci. 2014, 1, 174–191. [Google Scholar] [CrossRef] [Green Version]
- Mounier, L.; Dubroeucq, H.; Andanson, S.; Veissier, I. Variations in meat pH of beef bulls in relation to conditions of transfer to slaughter and previous history of the animals. J. Anim. Sci. 2006, 84, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Teke, B.; Akdag, F.; Ekiz, B.; Ugurlu, M. Effects of different lairage times after long distance transportation on carcass and meat quality characteristics of Hungarian Simmental bulls. Meat Sci. 2014, 96, 24–229. [Google Scholar] [CrossRef]
- LI, X.; Xia, A.Q.; Chen, L.J.; Du, M.T.; Chen, L.; Kang, N.; Zhang, D.Q. Effects of lairage after transport on post mortem muscle glycolysis, protein phosphorylation and lamb meat quality. J. Integr. Agric. 2018, 17, 2336–2344. [Google Scholar] [CrossRef] [Green Version]
- Amtmann, V.A.; Gallo, C.; Van Schaik, G.; Tadich, N. Relaciones entre el manejo antemortem, variables sanguíneas indicadoras de estrés y pH de la canal en novillos. Arch. Med. Vet. 2006, 38, 259–264. [Google Scholar] [CrossRef] [Green Version]
- Gallo, C.; Lizondo, G.; Knowles, T.G. Effects of journey and lairage time on steers transported to slaughter in Chile. Vet. Rec. 2003, 152, 361–364. [Google Scholar] [CrossRef]
- Jarvis, A.M.; Harrington, D.W.J.; Cockram, M.S. Effect of source and lairage on some behavioural and biochemical measurements of feed restriction and dehydration in cattle at a slaughterhouse. Appl. Anim. Behav. Sci. 1996, 50, 83–94. [Google Scholar] [CrossRef]
- Loredo-Osti, J.; Sánchez-López, E.; Barreras-Serrano, A.; Figueroa-Saavedra, F.; Linares, C.P.; Ruiz-Albarrán, M.; Domínguez-Muñoz, M.A. An evaluation of environmental, intrinsic and pre- and post-slaughter risk factors associated to dark-cutting beef in a Federal Inspected Type slaughter plant. Meat Sci. 2019, 150, 85–92. [Google Scholar] [CrossRef]
- European Union Council. Council Regulation (EC) N° 1099/2009 of 24 September 2009 on the protection of the animals at the time of killing. Off. J. Eur. Union 2009, 303, 1–30. [Google Scholar]
- OIE. Terrestrial Animal Health Code, 18th ed.; World Organisation for Animal Health: Paris, France, 2009; p. 349. [Google Scholar]
- Gallo, C. Animal welfare in the Americas. In Compendium of Technical Items Presented to the International Committee or to the Regional Commissions of the OIE; OIE, Ed.; World Animal Health Organization (OIE): Paris, France, 2007; pp. 151–166. [Google Scholar]
- Gallo, C.; Tadich, T.A. South America. In Long Distance Transport and Welfare of Farm Animals; Appleby, M.C., Cussen, V., Garcés, L., Lambert, L., Turner, J., Eds.; CABI: Wallingford, UK, 2008; pp. 261–287. [Google Scholar]
- Herrera, C.; Gallo, C. Análisis descriptivo de la presentación de canales con pH elevado en bovinos de distinta procedencia geográfica y tiempo de espera prefaena. In Proceedings of the XXXIV Congreso Anual de la Sociedad Chilena de Producción Animal, Pucón, Chile, 21–23 October 2009; pp. 270–271. [Google Scholar]
- del Campo, M.; Toyos, G.; Albin, A.; Borca, A.; Correa, D.; Robaina, R.; Brito, G. Third Uruguayan National Beef Quality Audit: Bruises characterization. In Proceedings of the ICoMST. 2017. Available online: https://digicomst.ie/2017/2017_06_12/ (accessed on 15 March 2021).
- Grandin, T.; Deesing, M.J.; Struthers, J.J.; Swinker, A.M. Cattle With Hair Whorl Patterns Above the Eyes Are More Behaviorally Agitated During Restraint. Appl. Anim. Behav. Sci. 1995, 46, 117–123. [Google Scholar] [CrossRef]
- Hearnshaw, H.; Morris, C.A. Genetic and environmental effects on temperament score in beef cattle. Aust. J. Agric. Res. 1984, 35, 723–733. [Google Scholar] [CrossRef]
- Saaty, T.L. Analytic Hierarchy Pocess; McGrawHill: New York, NY, USA, 1980. [Google Scholar]
- Martin, P.; Bateson, P. Measuring Behaviour; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- SAS. Statistical Analysis Sistems (SAS); SAS Institute Inc.: Cary, NS, USA, 2014. [Google Scholar]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Rovira, J. Manejo Nutritivo de los Rodeos de Cría en Pastoreo; Editorial Hemisferio Sur.: Montevideo, Uruguay, 1996; pp. 3–58. [Google Scholar]
- Carámbula, M. Pasturas Naturales Mejoradas; Editorial Hemisferio Sur.: Montevideo, Uruguay, 1996; p. 524. [Google Scholar]
- Andreo, N.; Castro, H.; Vottero, D. Las Bajas Ganancias de Peso de Los Novillos Durante el Otoño. 2001. Available online: http://www.produccionbovina.com/informacion_tecnica/invernada_o_engorde_pastoril_o_acampo/+4las_bajas_ganancias_de_peso_de_los_novillos_en_otono (accessed on 30 March 2021).
- Voisinet, B.D.; Grandin, T.; Tatum, J.D.; O’Connor, S.F.; Struthers, J.J. Feedlot Cattle with Calm Temperaments have higher average daily gain than cattle with Excitable Temperaments. J. Anim. Sci. 1997, 75, 892–896. [Google Scholar] [CrossRef] [Green Version]
- Barnett, J.L.; Hemsworth, P.H.; Mand, A.M. The effect of chronic stress on some blood parameters in the pig. Appl. Anim. Ethol. 1983, 9, 273–277. [Google Scholar] [CrossRef]
- Hemsworth, P.H.; Price, E.O.; Borgwardt, R. Behavioural responses of domestic pig and cattle to humans and novel stimuli. Appl. Anim. Behav. Sci. 1996, 50, 43–56. [Google Scholar] [CrossRef]
- Boivin, X.; Le Neindre, P.; Garel, J.P.; Chupin, J.M. Influence of breed and rearing management on cattle reactions during human handling. Appl. Anim. Behav. Sci. 1994, 39, 115–122. [Google Scholar] [CrossRef]
- Appleby, M.C.; Hughes, B. Animal Welfare; Appleby, M.C., Hughes, B., Eds.; CABI Publishing: Wallingford, UK, 2005; p. 76. [Google Scholar]
- Broom, D.M. Causes of poor welfare in large animals during transport. Vet. Res. Commun. 2003, 27, 515–518. [Google Scholar] [CrossRef]
- Hartung, G. Effects of transport on health of farm animals. Vet. Res. Commun. 2003, 27, 525–527. [Google Scholar] [CrossRef]
- Tarrant, V.; Grandin, T. Cattle transport. In Livestock Handling and Transport; Grandin, T., Ed.; CAB International: Wallingford, UK, 1993; pp. 109–126. [Google Scholar]
- Ishiwata, T.; Uetake, K.; Eguchi, Y.; Tanaka, T. Physical conditions in a cattle vehicle during spring and autumn conditions in Japan, and reactions of steers to long distance transport. Anim. Sci. J. 2008, 79, 620–627. [Google Scholar] [CrossRef]
- Fazio, E.; Medica, P.; Alberghina, D.; Cavaleri, S.; Ferlazzo, A. Effect of Long Distance Road Transport on Thyroid and Adrenal Function and Hematocrit Values in Limousin Cattle: Influence of Body Weight Decrease. Vet. Res. Commun. 2005, 29, 713–719. [Google Scholar] [CrossRef]
- Trunkfield, H.R.; Broom, D.M. Welfare of calves during handling and transport. Appl. Anim. Behav. Sci. 1990, 28, 135–152. [Google Scholar] [CrossRef]
- Villarroel, M.; Maria, G.; Sañudo, C.; Garcia-Belenguer, S.; Chacon, G.; Gebresenbet, G. Effect of commercial transport in Spain on cattle welfare and meat Quality. Dtsch. Tierarztl. Wochenschr. 2003, 110, 105–107. [Google Scholar] [PubMed]
- Marahrens, M.; von Richthofen, I.; Schmeiduch, S.; Hartung, I. Special problems of long-distance transport of cattle. Dtsch. Tierärztliche Wochenschr. 2003, 110, 120–125. [Google Scholar]
- Sartorelli, P.; Dominoni, S.; Agnes, F. Influence of duration of simulated transport on plasma stress markers in the calf. J. Vet. Med. Ser. A 1992, 39, 401. [Google Scholar] [CrossRef]
- Boissy, A.; Le Neindre, P. Behavioral, cardiac and cortisol responses to brief peer separation and reunion in cattle. Physiol. Behav. 1997, 61, 693–699. [Google Scholar] [CrossRef]
- Lay, D.C., Jr.; Friend, T.H.; Bowers, C.L.; Grissom, K.K.; Jenkins, O.C. A comparative physiological and behavioural study of freeze and hot iron branding using dairy cows. J. Anim. Sci. 1992, 70, 1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadich, N.; Gallo, C.; Bustamante, H.; Schwertera, M.; van Schaik, G. Effects of transport and lairage time on some blood constituents of Friesian-cross steers in Chile. Livest. Prod. Sci. 2005, 93, 223–233. [Google Scholar] [CrossRef]
- Curley, K.O., Jr.; Neuendorff, D.A.; Lewis, A.W.; Cleere, J.J.; Welsh, T.H., Jr.; Randel, R.D. Functional characteristics of the bovine hypothalamic–pituitary–adrenal axis vary with temperament. Horm. Behav. 2008, 53, 20–27. [Google Scholar] [CrossRef]
- Café, L.M.; Robinson, D.L.; Ferguson, D.M.; Geesink, G.H.; Greenwood, P.L. Temperament and hypothalamic-pituitary-adrenal axis function are related and combine to affect growth, efficiency, carcass, and meat quality traits in Brahman steers. Domest. Anim. Endocrinol. 2011, 40, 230–240. [Google Scholar] [CrossRef]
- Burdick, N.C.; Randel, R.D.; Carroll, J.A.; Welsh, T.H., Jr. Interactions between temperament, stress, and immune functioning cattle. Int. J. Zool. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Curley, K.O., Jr. Influence of Temperament on Bovine Hypothalamic-Pituitary–Adrenal Function. Master’s Thesis, Texas A&M University, College Station, TX, USA, December 2004. [Google Scholar]
- Curley, K.O., Jr.; Paschal, J.C.; Welsh, T.H.; Randel, R.D. Technical note: Exit velocity as a measure of cattle temperament is repeatable and associated with serum concentration of cortisol in Brahman bulls. J. Anim. Sci. 2006, 84, 3100–3103. [Google Scholar] [CrossRef]
- Fell, L.R.; Colditz, I.G.; Walker, K.H.; Watson, D.L. Associations between temperament, performance and immune function in cattle entering a commercial feedlot. Aust. J. Exp. Agric. 1999, 39, 795–802. [Google Scholar] [CrossRef]
- Ryjnberk, A.; Mol, J.A. Adrenocortical function. In Clinical Biochemistry of Domestic Animals, 4th ed.; Kaneko, J.J., Ed.; Academic Press: New York, NY, USA, 1989; pp. 610–629. [Google Scholar]
- Terlouw, E.M.C.; Schouten, W.G.P.; Ladewig, J. Phisiology. In Animal Welfare; Appleby, M.C., Hughes, B.O., Eds.; CABI Publishing: Wallingford, UK, 2005; p. 143. [Google Scholar]
- Grasso, F.; Gambacorta, E.; Montemurro, N.; Berardino, D.; di Zullo, A.; Matassino, D. Assessment of transport and slaughter in beef cattle on the basis of some biological tests. Prod. Anim. 1989, 2, 91–119. [Google Scholar]
- Groth, W.; Granzer, W. Changes due to transport in the blood values of fattening calves compared with early weaned calves. Dtsch. Tierarztl. Wochenschr. 1977, 84, 89–93. [Google Scholar] [PubMed]
- Van de Water, G.; Verjans, F.; Geers, R. The effect of short distance transport under commercial conditions on the physiology of slaughter calves; pH and colour profiles of veal. Livest. Prod. Sci. 2003, 82, 171–179. [Google Scholar] [CrossRef]
- Lefebvre, H.P.; Laroute, V.; Braun, J.P.; Lassourd, V.; Toutain, P.L. Non-invasive and quantitative evaluation of post-injection muscle damage by pharmacokinetic analysis of creatine-kinase release. Vet. Res. 1996, 27, 343–361. [Google Scholar]
- Mpakama, T.; Chulayo, A.; Muchenje, V. Bruising in slaughter cattle and its relationship with Creatine kinase levels and beef quality as affected by animal related factors. Asian Australas. J. Anim. Sci. 2014, 27, 717–725. [Google Scholar] [CrossRef] [Green Version]
- Simova, V.; Voslarova, E.; Vecerek, V.; Passantino, A.; Bedanova, I. Effects of travel distance and season of the year on transport-related mortality in cattle. Anim. Sci. J. 2016, 88, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.H.; Berret, S.; Patterson, D.S.P. The significance of elevated plasma creatine phosphokinase activity in muscle disease of cattle. J. Comp. Pathol. 1976, 86, 531–538. [Google Scholar] [CrossRef]
- Berg, A.; Haralambie, G. Changes in serum creatine kinase and hexose phosphate isomerase activity with exercise duration. Eur. J. Appl. Physiol. 1978, 39, 191. [Google Scholar] [CrossRef] [PubMed]
- Lensink, B.J.; Fernandez, X.; Boivin, X.; Pradel, P.; Le Neindre, P.; Veissier, I. The impact of gentle contact on ease of handling, welfare and growth of calves and on quality of veal meat. J. Anim. Sci. 2000, 78, 1219–1226. [Google Scholar] [CrossRef]
- Moberg, G.P.; Mench, J.A. The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; CABI Publishing: Wallingford, UK, 2000. [Google Scholar]
- Balm, P.H.M. Stress Physiology in Animals; Sheffield Academic Press Ltd.: Sheffield, UK, 1990. [Google Scholar]
- Shaw, F.D.; Tume, R.K. The assessment of pm-slaughter and slaughter treatments of livestock by measurement of plasma constituents—A review of recent work. Meat Sci. 1992, 32, 311–329. [Google Scholar] [CrossRef]
- Mellor, D.J.; Stafford, K.J. Interpretation of cortisol responses in calf dibudding studies. New Zealand Vet. J. 1997, 45, 126–127. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.J.; Cook, C.J.; Stafford, K.J. Quantifing some responses to pain as a stressor. In The Biology of Animal Stress Basic Principles and Implications for Animal Welfare; Moberg, G.P., Mench, J.A., Eds.; CABI Publishing: Wallingford, UK, 2000. [Google Scholar]
- von Borell, E.H. The biology of stress and its application to livestock housing and transportation assessment. J. Anim. Sci. 2001, 79, e260–e267. [Google Scholar] [CrossRef]
- Gupta, S.; Earley, B.; Ting, S.T.L.; Crowe, M.A. Effect of repeated regrouping and relocation on the physiological, immunological, and haematological variables and performance of steers. J. Anim. Sci. 2005, 83, 1948–1958. [Google Scholar] [CrossRef] [Green Version]
- Cockram, M.S.; Corley, K.T.T. Effect of pre-slaughter handling on the behaviour and blood composition of beef-cattle. Vet. J. 1991, 147, 444–454. [Google Scholar] [CrossRef]
- Moberg, G.P. Biological response to stress: Key to assessment of animal well being? In Animal Stress; Moberg, G.P., Ed.; American Physiological Society: Bethesda, MD, USA, 1985; pp. 23–49. [Google Scholar]
- Turner, S.P.; Navajas, E.A.; Hyslop, J.J.; Ross, D.W.; Richardson, R.I.; Prieto, N. Associations between response to handling and growth and meat quality in frequently handled beef cattle. J. Anim. Sci. 2011, 89, 4239–4248. [Google Scholar] [CrossRef]
- Llonch, P.; Miguel Somarriba, M.; Duthie, C.A.; Haskell, M.J.; Rooke, J.A.; Troy, S.; Roehe, R.; Turner, S.P. Association of Temperament and Acute Stress Responsiveness with Productivity, Feed Efficiency, And Methane Emissions in Beef Cattle: An Observational Study. Front. Vet. Sci. 2016, 3, 43. [Google Scholar] [CrossRef] [Green Version]
- Clariget, J.; Banchero, G.; Luzardo, S.; Fernández, E.; Pérez, E.; La Manna, A.; Saravia, A.; del Campo, M.; Ferrés, A.; Andrighetto, M.E. Effect of preslaughter fasting duration on physiology, carcass and meat quality in beef cattle finished on pastures or feedlot. Res. Vet. Sci. 2021. [Google Scholar] [CrossRef]
- Alende, M.; Lagreca, G.V.; Pordomingo, A.J.; Pighín, D.; Grigioni, G.; Carduza, F.; Pazos, A.; Babinec, F.; Sancho, A.M. Efectos del tiempo de transporte, espera pre-faena y maduración de novillos sobre indicadores de estrés, calidad instrumental y sensorial de la carne. Arch. Med. Vet. 2014, 46, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Díaz, M.T.; Vieira, C.; Pérez, C.; Lauzurca, S.; Gonzáles de Chávarri, E.; Sánchez, M.; de la Fuente, J. Effect of lairage time (0 h, 3 h, 6 h or 12 h) on glycogen content and meat quality parameters in suckling lambs. Meat Sci. 2014, 96, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Grigor, P.N.; Cockram, M.S.; Steele, W.B.; McIntyre, J.; Williams, C.L.; Leushuis, I.E.; van Reenen, C.G. A comparison of the welfare and meat quality of veal calves slaughtered on the farm with those subjected to transportation and lairage. Livest. Prod. Sci. 2004, 91, 9–228. [Google Scholar] [CrossRef]
- Hartung, J.; Marahrens, M.; Parvizi, N.; Schmeiduch, S.; Hiegemann, H.; Aussel, M.; Zerbe, F.; Ulbrich, H. Investigations on stress response of heifers during long distance road transport (Zur Belastung von Rindern beim Straßentransport auf Langstrecken). In Proceedings Congress Bundesverband der beamteten Tierarzte (BbT); Congress Bundesverband der beamteten Tierarzte: Staffelstein, Germany, 2000; pp. 33–41. [Google Scholar]
- Tribe, D.E. The behaviour of grazing animals. In Progress in the Physiology of Farm Animals; Hammond, J., Ed.; Butterworths: London, UK, 1955; Volume 2, p. 285. [Google Scholar]
- del Campo, M.; Manteca, X.; Soares de Lima, J.M.; Brito, G.; Hernández, P.; Sañudo, C.; Montossi, F. Effect of Different Finishing Strategies and Steer Temperament on Animal Welfare and Instrumental Meat Tenderness. Animals 2021, 11, 859. [Google Scholar] [CrossRef]
- Adams, D.C.; Reynolds, W.L. Winter grazing patterns of three- and six-year-old crossbred cows in the Northern Great Plains. J. Anim. Sci. 1983, 57 (Suppl. 1), 134. [Google Scholar]
- Dwyer, D.D. Activities and grazing preferences of cows with calves in the Northern Osage County, Oklahoma. Oklahoma. Agr. Exp. Sta. Bull 1961, No.B-588. 61. [Google Scholar]
- del Campo, M.; Brito, G.; de Oliveira Costa, F.; Vergara, E.; Anchaño, M.; Frugoni, J.; Bottero, D.; Levratto, J.; Rodríguez, H.; Hernández, S.; et al. Efecto del manejo previo a la faena sobre el bienestar animal y la calidad de producto. Año 2. In Alternativas Tecnológicas Para los Sistemas Ganaderos del Basalto; Berretta, E., Montossi, F., Brito, G., Eds.; Serie Técnica INIA: Montevideo, Uruguay, 2014; pp. 529–553. Available online: http://www.inia.uy/Publicaciones/Paginas/st-217_2014.aspx (accessed on 15 March 2021).
- Krizsan, S.J.; Ahvenjärvi, S.; Huhtanenl, P. A meta-analysis of passage rate estimated by rumen evacuation with cattle and evaluation of passage rate prediction models. J. Dairy Sci. 2010, 93, 5890–5901. [Google Scholar] [CrossRef]
- Dawkins, M.S. Animal Suffering. In The Science of Animal Welfare; Chapman and Hall: London, UK, 1980. [Google Scholar]
- Mc Nally, P.W.; Warris, P.D. Recent bruising in cattle at abattoirs. Vet. Rec. 1996, 138, 126–128. [Google Scholar] [CrossRef]
- Brito, G.; Correa, D.; San Julián, R. Tercera auditoría de calidad de carne vacuna del Uruguay; Serie Técnica; INIA: Montevideo, Uruguay, 2017; 50p, Available online: http://www.ainfo.inia.uy/digital/bitstream/item/6771/1/st-229-2017.pdf (accessed on 15 March 2021).
- Kline, C.; Weller, Z.D.; Temple, G.; Algino, R.J.; Edwards-Callaway, L.N. From unloading to trimming: Studying bruising in individual slaughter cattle. Transl. Anim. Sci. 2020, 4, 1–9. [Google Scholar] [CrossRef]
- Barnett, J.L.; Eldridge, G.A.; McCausland, I.P.; Caple, I.W.; Millar, H.W.C.; Truscott, T.G.; Hollier, T. Stress and bruising in cattle. Proc. Aust. Soc. Anim. Prod. 1984, 15, 653. [Google Scholar]
- Bray, A.; Graafhuis, A.; Chrystall, B. The cumulative effect of nutritional, shearing and pre-slaughter washing stresses on the quality of lamb meat. Meat Sci. 1989, 25, 59. [Google Scholar] [CrossRef]
- FAWC. Farm Animal Welfare in Great Britain: Past, Present and Future; FAWC: London, UK, 2009; 70p. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/319292/Farm_Animal_Welfare_in_Great_Britain_-_Past__Present_and_Future.pdf (accessed on 30 March 2021).
- van Staaveren, N.; Doyle, B.; Manzanilla, E.; Calderón Díaz, J.; Hanlon, A.; Boyle, L. Validation of carcass lesions as indicators for on-farm health and welfare of pigs. J. Anim. Sci. 2017, 95, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Warriss, P. The handling of cattle pre-slaughter and its effects on carcass and meat quality. Appl. Anim. Behav. Sci. 1990, 28, 171–186. [Google Scholar] [CrossRef]
- Gregory, N.G.; Grandin, T. Animal Welfare and Meat Science; CAB International: Wallingford, UK, 1998. [Google Scholar]



| Log CPK (U/L) | Time A Basal Value | Time B after Transportation | Time C after Lairage | Time D at Slaughter |
|---|---|---|---|---|
| 3 h | 1.99 c ± 0.50 | 2.48 b ± 0.51 | 2.39 b ± 0.52 b | 2.71 a ± 0.51 |
| 15 h | 2.08 c ± 0.50 | 2.44 b ± 0.51 | 2.40 b ± 0.51 b | 2.67 a ± 0.50 |
| NEFA (mmol/L) | Time A Basal Value | Time B after Transportation | Time C after Lairage | Time D at Slaughter |
|---|---|---|---|---|
| 3 h | 0.36 d ± 0.02 | 0.55 b ± 0.03 | 0.48 b,c,d ± 0.08 | 0.43 c,d ± 0.03 |
| 15 h | 0.37 d ± 0.02 | 0.49 b,c ± 0.03 | 0.68 a ± 0.04 | 0.52 b ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Campo Gigena, M.; Soares de Lima, J.M.; Brito, G.; Manteca, X.; Hernández, P.; Montossi, F. Effect of Finishing Diet and Lairage Time on Steers Welfare in Uruguay. Animals 2021, 11, 1329. https://doi.org/10.3390/ani11051329
del Campo Gigena M, Soares de Lima JM, Brito G, Manteca X, Hernández P, Montossi F. Effect of Finishing Diet and Lairage Time on Steers Welfare in Uruguay. Animals. 2021; 11(5):1329. https://doi.org/10.3390/ani11051329
Chicago/Turabian Styledel Campo Gigena, Marcia, Juan Manuel Soares de Lima, Gustavo Brito, Xavier Manteca, Pilar Hernández, and Fabio Montossi. 2021. "Effect of Finishing Diet and Lairage Time on Steers Welfare in Uruguay" Animals 11, no. 5: 1329. https://doi.org/10.3390/ani11051329
APA Styledel Campo Gigena, M., Soares de Lima, J. M., Brito, G., Manteca, X., Hernández, P., & Montossi, F. (2021). Effect of Finishing Diet and Lairage Time on Steers Welfare in Uruguay. Animals, 11(5), 1329. https://doi.org/10.3390/ani11051329






