Early Heat Exposure Effects on Proteomic Changes of the Broiler Liver under Acute Heat Stress
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
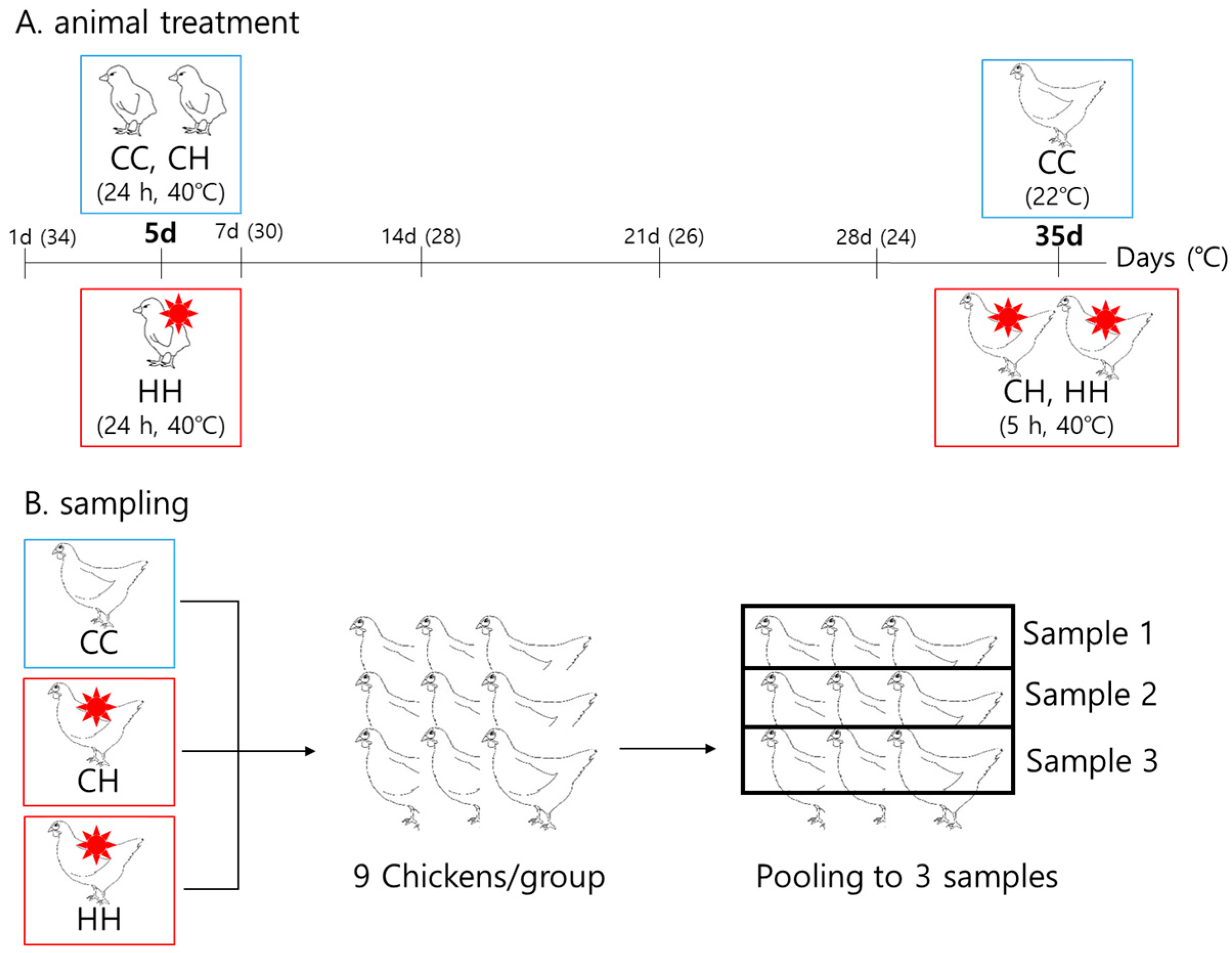
2.1. Animals and Heat Exposure Conditions
2.2. Protein Extraction and Digestion
2.3. LC-MS/MS Analysis
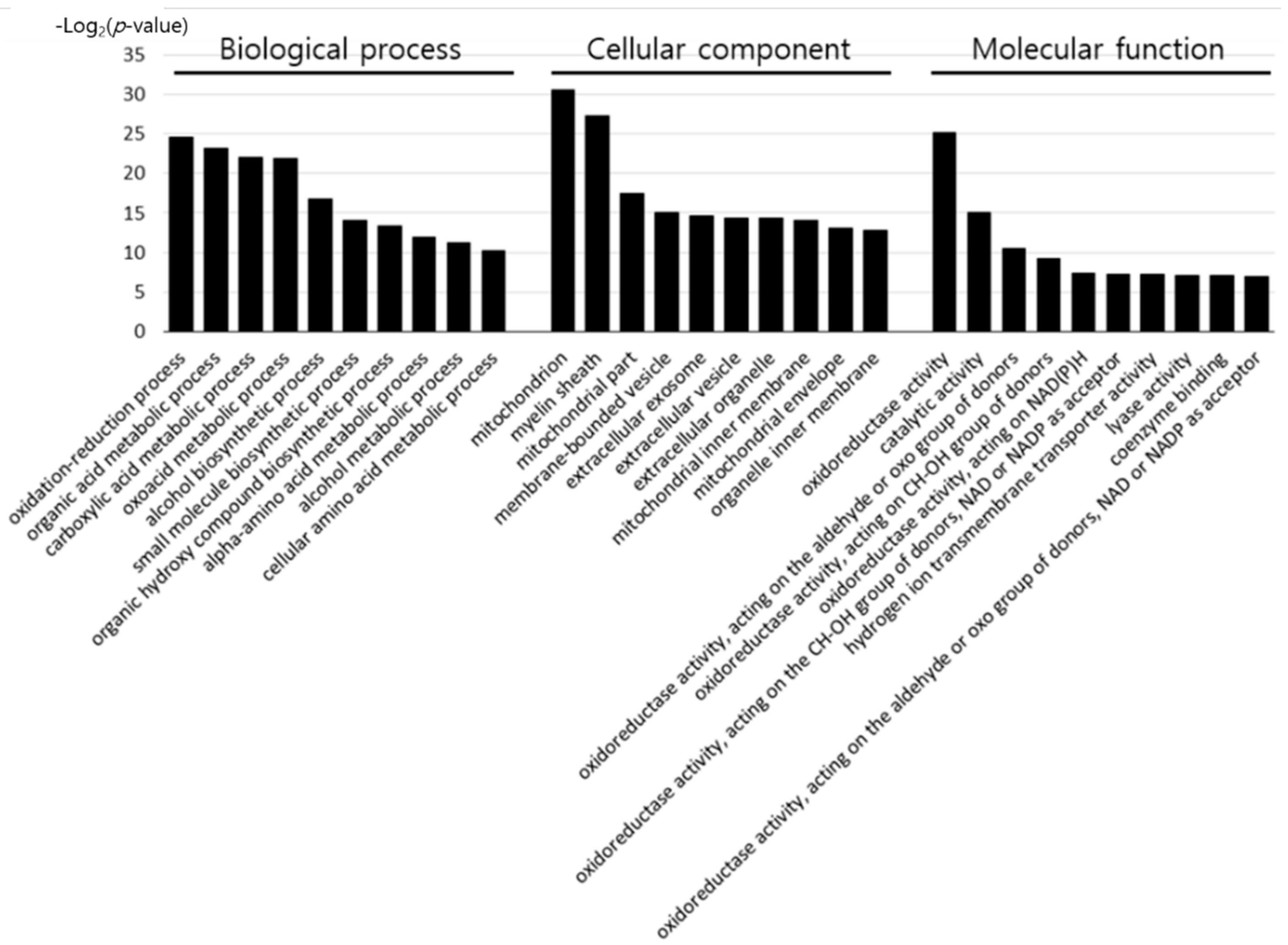
2.4. Gene Ontology Enrichment Analysis
2.5. Gene Expression by qPCR
2.6. Statistical Analysis
3. Results
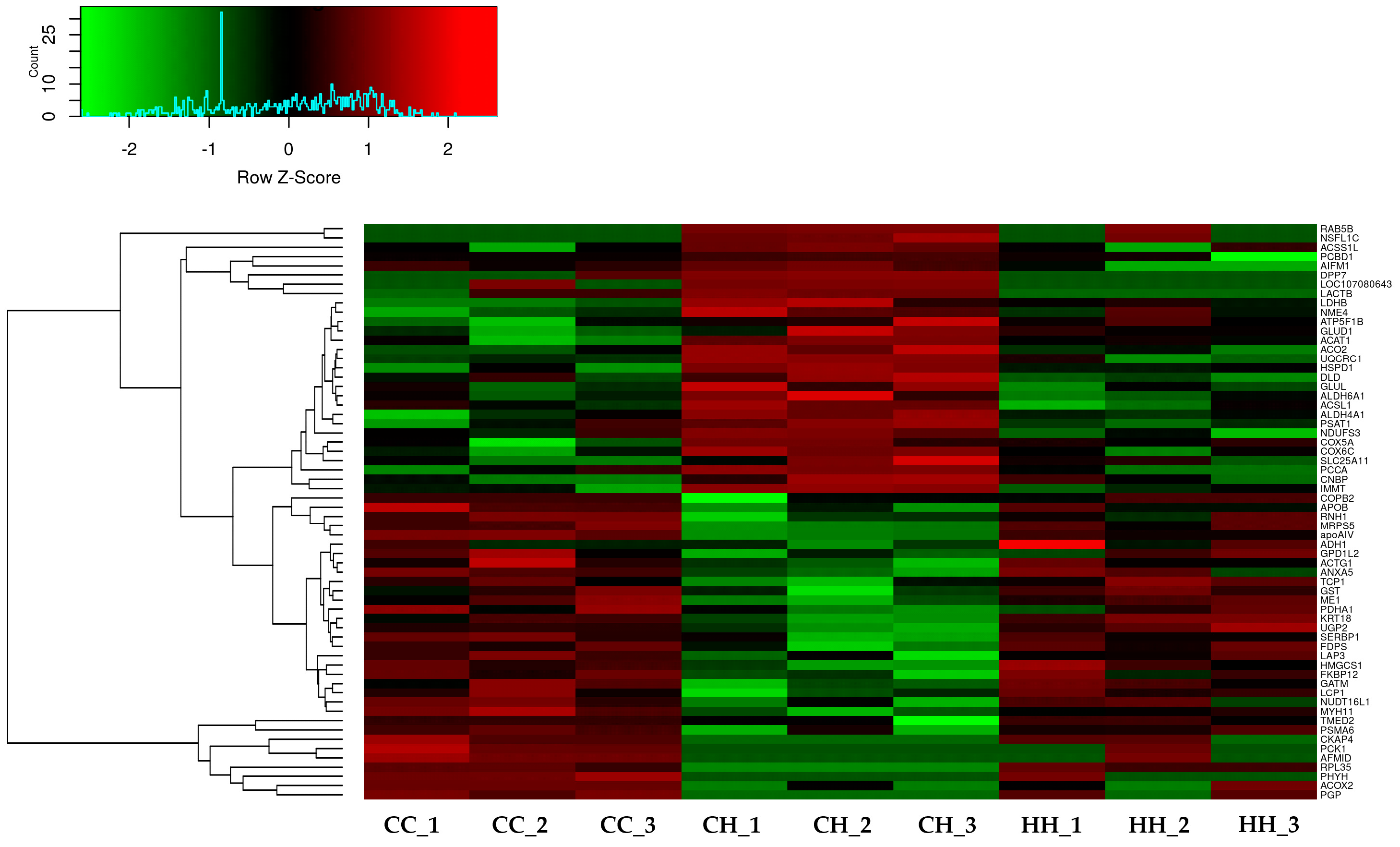
3.1. Bioinformatic Analysis of Differentially Expressed Proteins by Early Heat Exposure under Acute HS
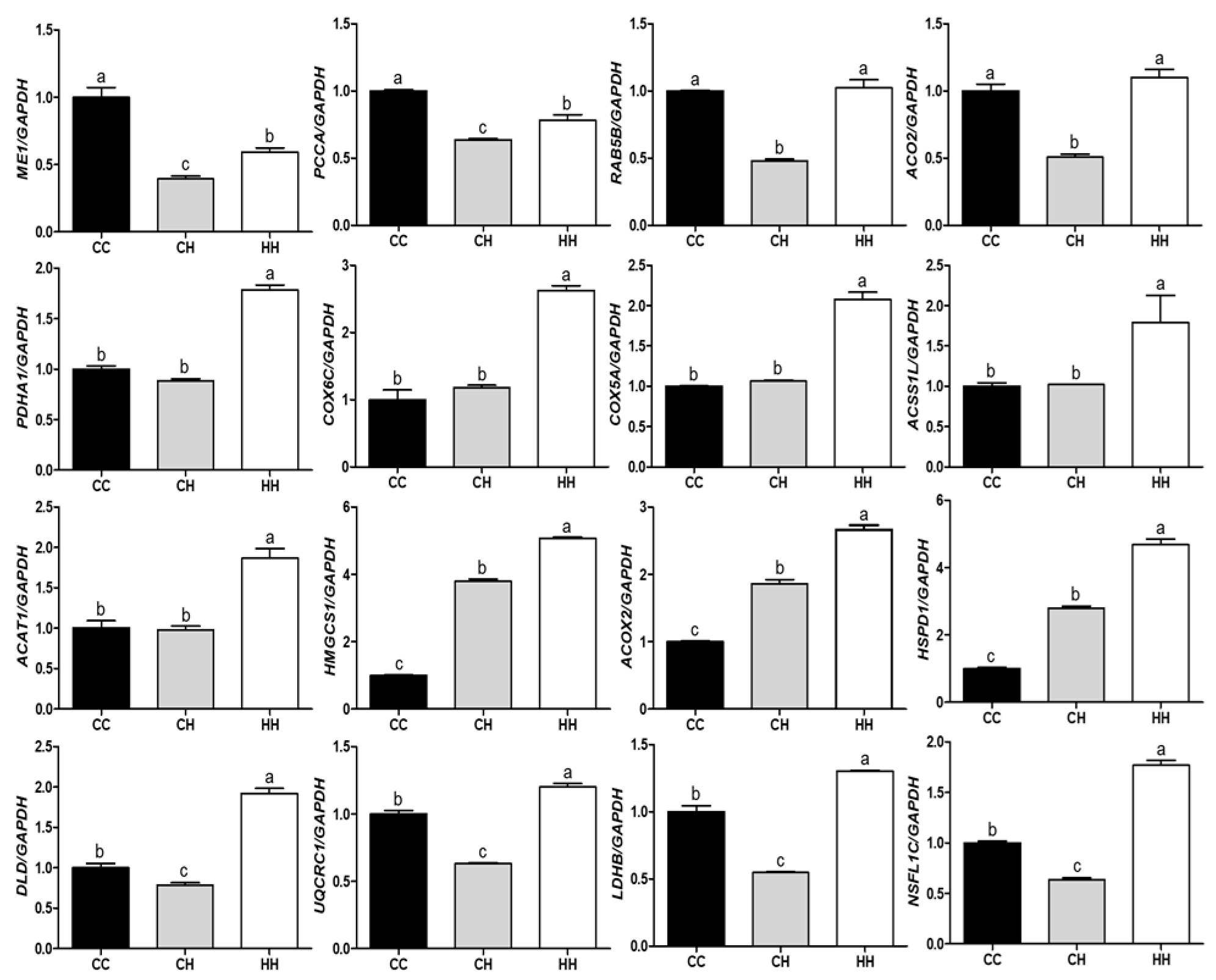
3.2. Validation of Differentially Expressed Proteins by Their Gene Expression
4. Discussion
4.1. Carbohydrate Metabolism
4.2. Energy Metabolism
4.3. Lipid Metabolism
4.4. RT- qPCR Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Dozier, W., III; Purswell, J.; Kidd, M.; Corzo, A.; Branton, S. Apparent metabolizable energy needs of broilers from two to four kilograms as influenced by ambient temperature. J. Appl. Poult. Res. 2007, 16, 206–218. [Google Scholar] [CrossRef]
- Jahejo, A.R.; Rajput, N.; Rajput, N.M.; Leghari, I.H.; Kaleri, R.R.; Mangi, R.A.; Sheikh, M.K.; Pirzado, M.Z. Effects of heat stress on the performance of hubbard broiler chicken. Cells 2016, 2, 1–5. [Google Scholar]
- Park, S.O.; Hwangbo, J.; Ryu, C.M.; Yoon, J.S.; Park, B.S.; Kang, H.K.; Seo, O.S.; Chae, H.S.; Choi, H.C.; Choi, Y.H. Effects of extreme heat stress and continuous lighting on growth performance and blood lipid in broiler chickens. J. Korean Appl. Sci. Technol. 2013, 30, 78–87. [Google Scholar] [CrossRef]
- Yahav, S.; Goldfeld, S.; Plavnik, I.; Hurwitz, S. Physiological responses of chickens and turkeys to relative humidity during exposure to high ambient temperature. J. Therm. Biol. 1995, 3, 245–253. [Google Scholar] [CrossRef]
- Lin, H.; Decuypere, E.; Buyse, J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 144, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, A.; Pumford, N.R.; Bottje, W.; Nakagawa, K.; Miyazawa, T.; Akiba, Y.; Toyomizu, M. Mitochondrial oxidative damage in chicken skeletal muscle induced by acute heat stress. J. Poult. Sci. 2007, 44, 439–445. [Google Scholar] [CrossRef]
- Lin, H.; Jiao, H.; Buyse, J.; Decuypere, E. Strategies for preventing heat stress in poultry. Worlds Poult. Sci. J. 2006, 62, 71–86. [Google Scholar] [CrossRef]
- Yahav, S.; Hurwitz, S. Induction of thermotolerance in male broiler chickens by temperature conditioning at an early age. Poult. Sci. 1996, 75, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Gu, X. Proteomic changes of the porcine small intestine in response to chronic heat stress. J. Mol. Endocrinol. 2015, 55, 277. [Google Scholar] [CrossRef]
- He, S.; Hou, X.; Xu, X.; Wan, C.; Yin, P.; Liu, X.; Chen, Y.; Shu, B.; Liu, F.; Xu, J. Quantitative proteomic analysis reveals heat stress-induced injury in rat small intestine via activation of the MAPK and NF-κB signaling pathways. Mol. Biosyst. 2015, 11, 826–834. [Google Scholar] [CrossRef]
- Min, L.; Zheng, N.; Zhao, S.; Cheng, J.; Yang, Y.; Zhang, Y.; Yang, H.; Wang, J. Long-term heat stress induces the inflammatory response in dairy cows revealed by plasma proteome analysis. Biochem. Biophys. Res. Commun. 2016, 471, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Skibiel, A.L.; Zachut, M.; do Amaral, B.C.; Levin, Y.; Dahl, G.E. Liver proteomic analysis of postpartum Holstein cows exposed to heat stress or cooling conditions during the dry period. J. Dairy Sci. 2018, 101, 705–716. [Google Scholar] [CrossRef]
- Zeng, T.; Jiang, X.; Li, J.; Wang, D.; Li, G.; Lu, L.; Wang, G. Comparative proteomic analysis of the hepatic response to heat stress in Muscovy and Pekin ducks: Insight into thermal tolerance related to energy metabolism. PLoS ONE 2013, 8, e76917. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Shim, K. Early heat exposure effect on the heat shock proteins in broilers under acute heat stress. Poult. Sci. 2021, 100, 100964. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Lara, L.J.; Rostagno, M.H.J.A. Impact of heat stress on poultry production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef]
- Alam, M.M.; Hashem, M.A.; Rahman, M.M.; Hossain, M.M.; Haque, M.R.; Sobhan, Z.; Islam, M.S. Effect of heat stress on behavior, physiological and blood parameters of goat. Progress. Agric. 2011, 22, 37–45. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P., Jr. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.L.; McCue, P.M.; Petrie, M.A.; Shields, R.K. Whole body heat exposure modulates acute glucose metabolism. Int. J. Hyperth. 2018, 35, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Miova, B.; Dinevska-Kjovkarovska, S.; Djimrevska, A.; Mitev, S. Prior heat stress induces moderation of diabetic alterations in glycogen metabolism of rats. Open Life Sci. 2014, 9, 249–259. [Google Scholar] [CrossRef]
- Cai, Z.; Li, C.F.; Han, F.; Liu, C.; Zhang, A.; Hsu, C.C.; Peng, D.; Zhang, X.; Jin, G.; Rezaeian, A.H.; et al. Phosphorylation of PDHA by AMPK drives TCA cycle to promote cancer metastasis. Mol. Cell 2020, 80, 263–278.e267. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, K.; Vemuri, G.N.; Mahadevan, R. Economics of membrane occupancy and respiro-fermentation. Mol. Syst. Biol. 2011, 7, 500. [Google Scholar] [CrossRef] [PubMed]
- Čunátová, K.; Reguera, D.P.; Houštěk, J.; Mráček, T.; Pecina, P. Role of cytochrome c oxidase nuclear-encoded subunits in health and disease. Physiol. Res. 2020, 69, 947–965. [Google Scholar] [CrossRef] [PubMed]
- Taurino, F.; Stanca, E.; Siculella, L.; Trentadue, R.; Papa, S.; Zanotti, F.; Gnoni, A. Mitochondrial proteome analysis reveals depression of the Ndufs3 subunit and activity of complex I in diabetic rat brain. J. Proteom. 2012, 75, 2331–2341. [Google Scholar] [CrossRef]
- Suhane, S.; Kanzaki, H.; Arumugaswami, V.; Murali, R.; Ramanujan, V.K. Mitochondrial NDUFS3 regulates the ROS-mediated onset of metabolic switch in transformed cells. Biol. Open 2013, 2, 295–305. [Google Scholar] [CrossRef]
- Lu, Z.; He, X.; Ma, B.B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Increased fat synthesis and limited apolipoprotein B cause lipid accumulation in the liver of broiler chickens exposed to chronic heat stress. Poult. Sci. 2019, 98, 3695–3704. [Google Scholar] [CrossRef]
- Qu, H.; Ajuwon, K.M. Metabolomics of heat stress response in pig adipose tissue reveals alteration of phospholipid and fatty acid composition during heat stress. J. Anim. Sci. 2018, 96, 3184–3195. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Monte, M.J.; Alonso-Peña, M.; Briz, O.; Herraez, E.; Berasain, C.; Argemi, J.; Prieto, J.; Marin, J.J. ACOX2 deficiency: An inborn error of bile acid synthesis identified in an adolescent with persistent hypertransaminasemia. J. Hepatol. 2017, 66, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cui, H.; Fu, R.; Zheng, M.; Liu, R.; Zhao, G.; Wen, J. The regulation of IMF deposition in pectoralis major of fast-and slow-growing chickens at hatching. J. Anim. Sci. Biotechno. 2017, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Ayyagari, V.N.; Wang, X.; Diaz-Sylvester, P.L.; Groesch, K.; Brard, L. Assessment of acyl-CoA cholesterol acyltransferase (ACAT-1) role in ovarian cancer progression—An in vitro study. PLoS ONE 2020, 15, e0228024. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zheng, A.; Meng, K.; Chang, W.; Bai, Y.; Li, K.; Cai, H.; Liu, G.; Yao, B. Proteome changes in the intestinal mucosa of broiler (Gallus gallus) activated by probiotic Enterococcus faecium. J. Proteom. 2013, 91, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Cherkasov, V.; Grousl, T.; Theer, P.; Vainshtein, Y.; Gläßer, C.; Mongis, C.; Kramer, G.; Stoecklin, G.; Knop, M.; Mogk, A.; et al. Systemic control of protein synthesis through sequestration of translation and ribosome biogenesis factors during severe heat stress. FEBS Lett. 2015, 589, 3654–3664. [Google Scholar] [CrossRef] [PubMed]
- Yost, H.J.; Lindquist, S. Translation of unspliced transcripts after heat shock. Science 1988, 242, 1544–1548. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, D.; Shim, K. Early Heat Exposure Effects on Proteomic Changes of the Broiler Liver under Acute Heat Stress. Animals 2021, 11, 1338. https://doi.org/10.3390/ani11051338
Kang D, Shim K. Early Heat Exposure Effects on Proteomic Changes of the Broiler Liver under Acute Heat Stress. Animals. 2021; 11(5):1338. https://doi.org/10.3390/ani11051338
Chicago/Turabian StyleKang, Darae, and Kwanseob Shim. 2021. "Early Heat Exposure Effects on Proteomic Changes of the Broiler Liver under Acute Heat Stress" Animals 11, no. 5: 1338. https://doi.org/10.3390/ani11051338
APA StyleKang, D., & Shim, K. (2021). Early Heat Exposure Effects on Proteomic Changes of the Broiler Liver under Acute Heat Stress. Animals, 11(5), 1338. https://doi.org/10.3390/ani11051338





