Longitudinal Metabolic Biomarker Profile of Hyperketonemic Cows from Dry-Off to Peak Lactation and Identification of Prognostic Classifiers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Study Design, Housing and Diets
2.2. Data Collection
2.2.1. Blood Sampling
2.2.2. Herd Monitoring
2.3. Serum Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
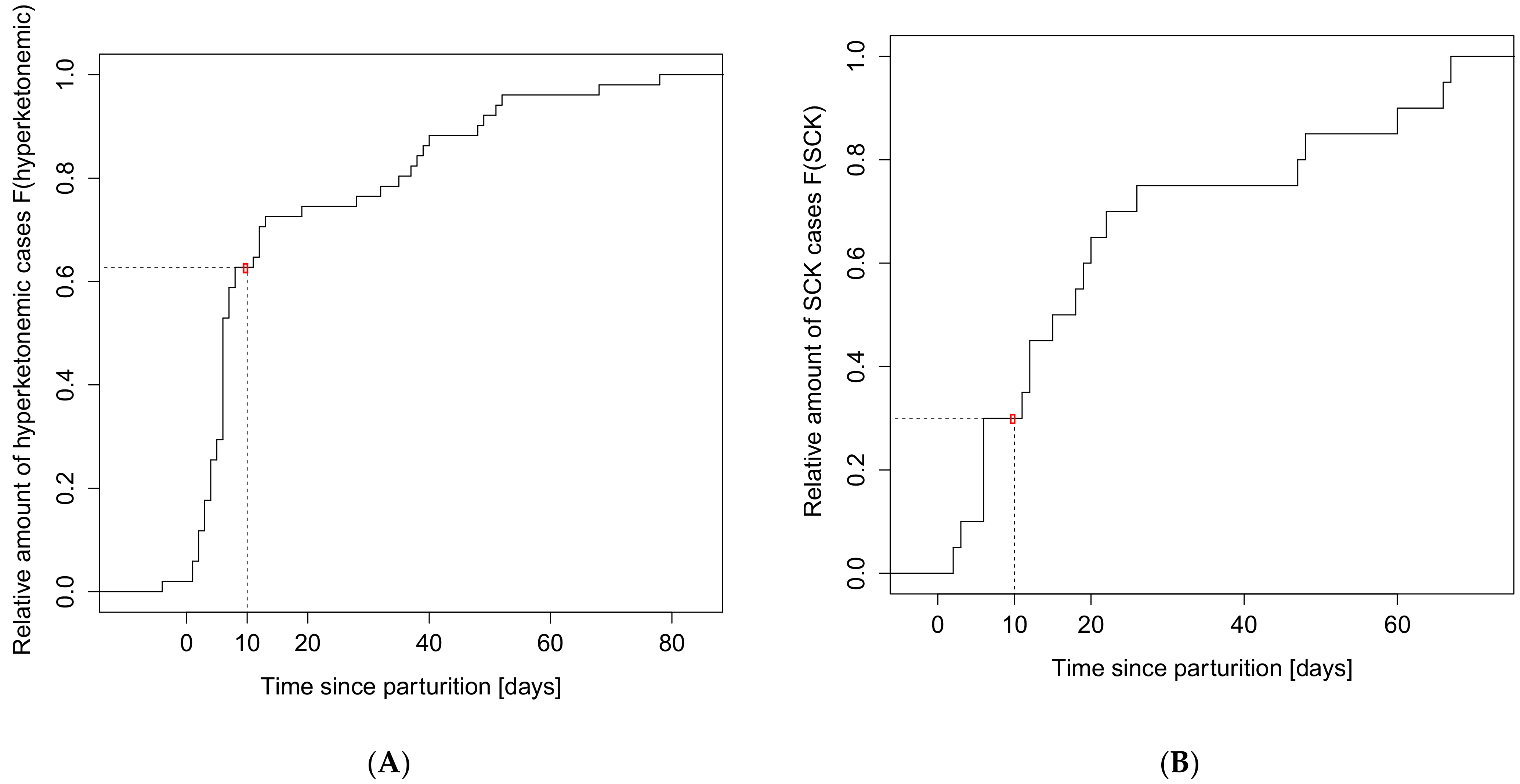
| Hyperketonemia | Subclinical Ketosis | |||||||
|---|---|---|---|---|---|---|---|---|
| BHB | NEFA | tBIL | AST | BHB | NEFA | tBIL | AST | |
| 15–9 days a.p. | - | - | p = 0.02 | - | - | - | p = 0.04 | - |
| 9–3 days a.p. | p = 0.02 | - | - | - | p = 0.04 | - | - | - |
| 2–8 days p.p. | p < 0.001 | - | p < 0.01 | - | p < 0.001 | p = 0.02 | p = 0.001 | - |
| 8–14 days p.p. | p < 0.001 | - | p < 0.001 | - | p < 0.001 | p < 0.01 | p < 0.001 | - |
| 14–20 days p.p. | p < 0.001 | p < 0.01 | p < 0.001 | p = 0.04 | p < 0.001 | - | p = 0.001 | - |
| 20–26 days p.p. | p < 0.001 | - | p < 0.01 | - | p < 0.001 | p < 0.001 | p < 0.01 | - |
| 26–32 days p.p. | p < 0.001 | - | p = 0.01 | - | p < 0.001 | p = 0.01 | p = 0.01 | - |
| 32–38 days p.p. | p < 0.001 | - | - | - | p < 0.001 | p < 0.001 | p < 0.01 | - |
| 38–44 days p.p. | p < 0.001 | - | p = 0.02 | - | p = 0.001 | p < 0.01 | p = 0.02 | - |
| 44–50 days p.p. | p < 0.001 | - | - | - | p < 0.001 | p < 0.001 | p < 0.01 | - |
| 50–56 days p.p. | p < 0.001 | - | - | - | p < 0.01 | p < 0.001 | p < 0.01 | - |
| 56–62 days p.p. | p < 0.001 | - | - | - | p < 0.001 | - | p = 0.04 | - |
| t0 (day of partum) | p < 0.001 | - | - | - | p < 0.001 | p = 0.04 | p < 0.05 | - |
| t10 (10 days p.p.) | p < 0.001 | p < 0.01 | p < 0.001 | - | p < 0.001 | p < 0.01 | p < 0.001 | - |
References
- Sundrum, A. Metabolic disorders in the transition period indicate that the dairy cows’ ability to adapt is overstressed. Animals 2015, 5, 978–1020. [Google Scholar] [CrossRef] [PubMed]
- Rauw, W.; Kanis, E.; Noordhuizen-Stassen, E.; Grommers, F. Undesirable side effects of selection for high production efficiency in farm animals: A review. Livest. Prod. Sci. 1998, 56, 15–33. [Google Scholar] [CrossRef]
- Herdt, T.H. Ruminant Adaptation to Negative Energy Balance: Influences on the Etiology of Ketosis and Fatty Liver. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 215–230. [Google Scholar] [CrossRef]
- Ingvartsen, K.L. Feeding- and management-related diseases in the transition cow: Physiological adaptations around calving and strategies to reduce feeding-related diseases. Anim. Feed Sci. Technol. 2006, 126, 175–213. [Google Scholar] [CrossRef]
- Humer, E.; Khol-Parisini, A.; Metzler-Zebeli, B.U.; Gruber, L.; Zebeli, Q. Alterations of the lipid metabolome in dairy cows experiencing excessive lipolysis early postpartum. PLoS ONE 2016, 11, 1–18. [Google Scholar] [CrossRef]
- Reist, M.; Erdin, D.; von Euw, D.; Tschuemperlin, K.; Leuenberger, H.; Chilliard, Y.; Hammon, H.M.; Morel, C.; Philipona, C.; Zbinden, Y.; et al. Estimation of Energy Balance at the Individual and Herd Level Using Blood and Milk Traits in High-Yielding Dairy Cows. J. Dairy Sci. 2002, 85, 3314–3327. [Google Scholar] [CrossRef]
- LeBlanc, S. Managing critical periods—Transition dairy cows. In Book of Abstracts Proceedings of the 15th Conference on Production Diseases in Farm Animals, Uppsala, Sweden, 24–28 June 2013; Dalin, G., Ed.; Swedish University of Agricultural Science (SLU): Uppsala, Sweden, 2013; pp. 62–65. [Google Scholar]
- Fürll, M. Stoffwechselüberwachung bei Rindern. In Klinische Labordiagnostik in der Tiermedizin; Moritz, A., Ed.; Schattauer: Stuttgart, Germany, 2005; pp. 748–767. ISBN 978-3794527373. [Google Scholar]
- Duffield, T. Subclinical Ketosis in Lactating Dairy Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 231–253. [Google Scholar] [CrossRef]
- Duffield, T.F.; Lissemore, K.D.; McBride, B.W.; Leslie, K.E. Impact of hyperketonemia in early lactation dairy cows on health and production. J. Dairy Sci. 2009, 92, 571–580. [Google Scholar] [CrossRef]
- Geishauser, T.; Leslie, K.; Duffield, T.; Sandais, D.; Edge, V. The Association Between Selected Metabolic Parameters and Left Abomasal Displacement in Dairy Cows. J. Vet. Med. Ser. A 1998, 45, 499–511. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S. Monitoring Metabolic Health of Dairy Cattle in the Transition Period Introduction-Metabolic Challenges in Peripartum Dairy Cows and Their Associations with Reproduction. J. Reprod. Dev. 2010, 56, 29–35. [Google Scholar] [CrossRef]
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 2014, 144, 60–71. [Google Scholar] [CrossRef]
- Shin, E.K.; Jeong, J.K.; Choi, I.S.; Kang, H.G.; Hur, T.Y.; Jung, Y.H.; Kim, I.H. Relationships among ketosis, serum metabolites, body condition, and reproductive outcomes in dairy cows. Theriogenology 2015, 84, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Van Saun, R.J.; Sniffen, C.J. Transition Cow Nutrition and Feeding Management for Disease Prevention. Vet. Clin. N. Am. Food Anim. Pract. 2014, 30, 689–719. [Google Scholar] [CrossRef]
- McArt, J.A.A.; Nydam, D.V.; Overton, M.W. Hyperketonemia in early lactation dairy cattle: A deterministic estimate of component and total cost per case. J. Dairy Sci. 2015, 98, 2043–2054. [Google Scholar] [CrossRef] [PubMed]
- Stöber, M. Fütterungs- und stoffwechselbedingte Krankheiten der Leber. In Innere Medizin und Chirurgie des Rindes; Dirksen, G., Gründer, H.-D., Stöber, M., Eds.; Parey-Verlag: Stuttgart, Germany, 2006; pp. 649–664. [Google Scholar]
- Kelton, D.F.; Lissemore, K.D.; Martin, R.E. Recommendations for Recording and Calculating the Incidence of Selected Clinical Diseases of Dairy Cattle. J. Dairy Sci. 1998, 81, 2502–2509. [Google Scholar] [CrossRef]
- Fürll, M. Das Fettmobilisationssyndrom. Großtierpraxis 2000, 11, 24–34. [Google Scholar]
- Vernon, R.G. Lipid metabolism during lactation: A review of adipose tissue-liver interactions and the development of fatty liver. J. Dairy Res. 2005, 72, 460–469. [Google Scholar] [CrossRef]
- Bobe, G.; Young, J.W.; Beitz, D.C. Invited Review: Pathology, Etiology, Prevention, and Treatment of Fatty Liver in Dairy Cows. J. Dairy Sci. 2004, 87, 3105–3124. [Google Scholar] [CrossRef]
- González, F.D.; Muiño, R.; Pereira, V.; Campos, R.; Benedito, J.L. Relationship among blood indicators of lipomobilization and hepatic function during early lactation in high-yielding dairy cows. J. Vet. Sci. 2011, 12, 251. [Google Scholar] [CrossRef] [PubMed]
- Fürll, M. Entwicklung und Grundlagen der Stoffwechselüberwachung bei Milchkühen. Tierärztliche Praxis Ausgabe G Großtiere/Nutztiere 2016, 44, 107–117. [Google Scholar] [CrossRef]
- Iwersen, M.; Falkenberg, U.; Voigtsberger, R.; Forderung, D.; Heuwieser, W. Evaluation of an electronic cowside test to detect subclinical ketosis in dairy cows. J. Dairy Sci. 2009, 92, 2618–2624. [Google Scholar] [CrossRef]
- Benedet, A.; Manuelian, C.L.; Zidi, A.; Penasa, M.; De Marchi, M. Invited review: β-hydroxybutyrate concentration in blood and milk and its associations with cow performance. Animal 2019, 13, 1676–1689. [Google Scholar] [CrossRef]
- Durrleman, S.; Simon, R. Flexible regression models with cubic splines. Stat. Med. 1989, 8, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Suthar, V.S.; Canelas-Raposo, J.; Deniz, A.; Heuwieser, W. Prevalence of subclinical ketosis and relationships with postpartum diseases in European dairy cows. J. Dairy Sci. 2013, 96, 2925–2938. [Google Scholar] [CrossRef]
- Compton, C.W.R.; Young, L.; McDougall, S. Subclinical ketosis in post-partum dairy cows fed a predominantly pasture-based diet: Defining cut-points for diagnosis using concentrations of beta-hydroxybutyrate in blood and determining prevalence. N. Z. Vet. J. 2015, 63, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Ospina, P.A.; Nydam, D.V.; Stokol, T.; Overton, T.R. Associations of elevated nonesterified fatty acids and β-hydroxybutyrate concentrations with early lactation reproductive performance and milk production in transition dairy cattle in the northeastern United States. J. Dairy Sci. 2010, 93, 1596–1603. [Google Scholar] [CrossRef]
- Djoković, R.; Kurćubić, V.; Ilić, Z.; Cincović, M.; Fratrić, N.; Stanimirović, Z.; Petrović, M.D.; Petrović, M.P. Evaluation of the metabolic status of Simmental dairy cows in early and mid lactation. Anim. Sci. Pap. Rep. 2013, 31, 101–110. [Google Scholar]
- Roche, J.R.; Bell, A.W.; Overton, T.R.; Loor, J.J. Nutritional management of the transition cow in the 21st century—A paradigm shift in thinking. Anim. Prod. Sci. 2013, 53, 1000. [Google Scholar] [CrossRef]
- Li, P.; Li, X.B.; Fu, S.X.; Wu, C.C.; Wang, X.X.; Yu, G.J.; Long, M.; Wang, Z.; Liu, G.W. Alterations of fatty acid β-oxidation capability in the liver of ketotic cows. J. Dairy Sci. 2012, 95, 1759–1766. [Google Scholar] [CrossRef]
- LeBlanc, S.J.; Leslie, K.E.; Duffield, T.F. Metabolic Predictors of Displaced Abomasum in Dairy Cattle. J. Dairy Sci. 2005, 88, 159–170. [Google Scholar] [CrossRef]
- Schulz, K.; Frahm, J.; Meyer, U.; Kersten, S.; Reiche, D.; Rehage, J.; Dänicke, S. Effects of prepartal body condition score and peripartal energy supply of dairy cows on postpartal lipolysis, energy balance and ketogenesis: An animal model to investigate subclinical ketosis. J. Dairy Res. 2014, 81, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Yameogo, N.; Ouedraogo, G.A.; Kanyandekwe, C.; Sawadogo, G.J. Relationship between ketosis and dairy cows’ blood metabolites in intensive production farms of the periurban area of Dakar. Trop. Anim. Health Prod. 2008, 40, 483–490. [Google Scholar] [CrossRef]
- Ropstad, E.; Halse, K.; Refsdal, A.O. Variations in parameters of liver function and plasma progesterone related to underfeeding and ketosis in a dairy herd. Acta Vet. Scand. 1989, 30, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hailemariam, D.; Dervishi, E.; Goldansaz, S.A.; Deng, Q.; Dunn, S.M.; Ametaj, B.N. Dairy cows affected by ketosis show alterations in innate immunity and lipid and carbohydrate metabolism during the dry off period and postpartum. Res. Vet. Sci. 2016, 107, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Benedet, A.; Franzoi, M.; Manuelian, C.L.; Penasa, M.; De Marchi, M. Variation of blood metabolites of brown swiss, holstein-friesian, and simmental cows. Animals 2020, 10, 271. [Google Scholar] [CrossRef]
- Pralle, R.S.; Weigel, K.W.; White, H.M. Predicting blood β-hydroxybutyrate using milk Fourier transform infrared spectrum, milk composition, and producer-reported variables with multiple linear regression, partial least squares regression, and artificial neural network. J. Dairy Sci. 2018, 101, 4378–4387. [Google Scholar] [CrossRef] [PubMed]
- McArt, J.A.A.; Nydam, D.V.; Oetzel, G.R. Epidemiology of subclinical ketosis in early lactation dairy cattle. J. Dairy Sci. 2012, 95, 5056–5066. [Google Scholar] [CrossRef] [PubMed]
- Geishauser, T.; Leslie, K.; Kelton, D.; Duffield, T. Monitoring for Subclinical Ketosis in Dairy Herds. Food Anim. Compend. 2001, 23, 65–71. [Google Scholar]
- Duffield, T.F.; Sandals, D.; Leslie, K.E.; Lissemore, K.; McBride, B.W.; Lumsden, J.H.; Dick, P.; Bagg, R. Efficacy of Monensin for the Prevention of Subclinical Ketosis in Lactating Dairy Cows. J. Dairy Sci. 1998, 81, 2866–2873. [Google Scholar] [CrossRef]
- Oetzel, G.R. Monitoring and testing dairy herds for metabolic disease. Vet. Clin. N. Am. Food Anim. Pract. 2004, 20, 651–674. [Google Scholar] [CrossRef]
- Nielsen, N.I.; Ingvartsen, K.L. Propylene glycol for dairy cows: A review of the metabolism of propylene glycol and its effects on physiological parameters, feed intake, milk production and risk of ketosis. Anim. Feed Sci. Technol. 2004, 115, 191–213. [Google Scholar] [CrossRef]
- Piantoni, P.; Allen, M.S. Evaluation of propylene glycol and glycerol infusions as treatments for ketosis in dairy cows. J. Dairy Sci. 2015, 98, 5429–5439. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Peinhopf, W.; Gottschalk, J.; Einspanier, A.; Koeller, G.; Wittek, T. Effects of different dosages of propylene glycol in dry cows and cows in early lactation. J. Dairy Res. 2017, 84, 375–384. [Google Scholar] [CrossRef] [PubMed]



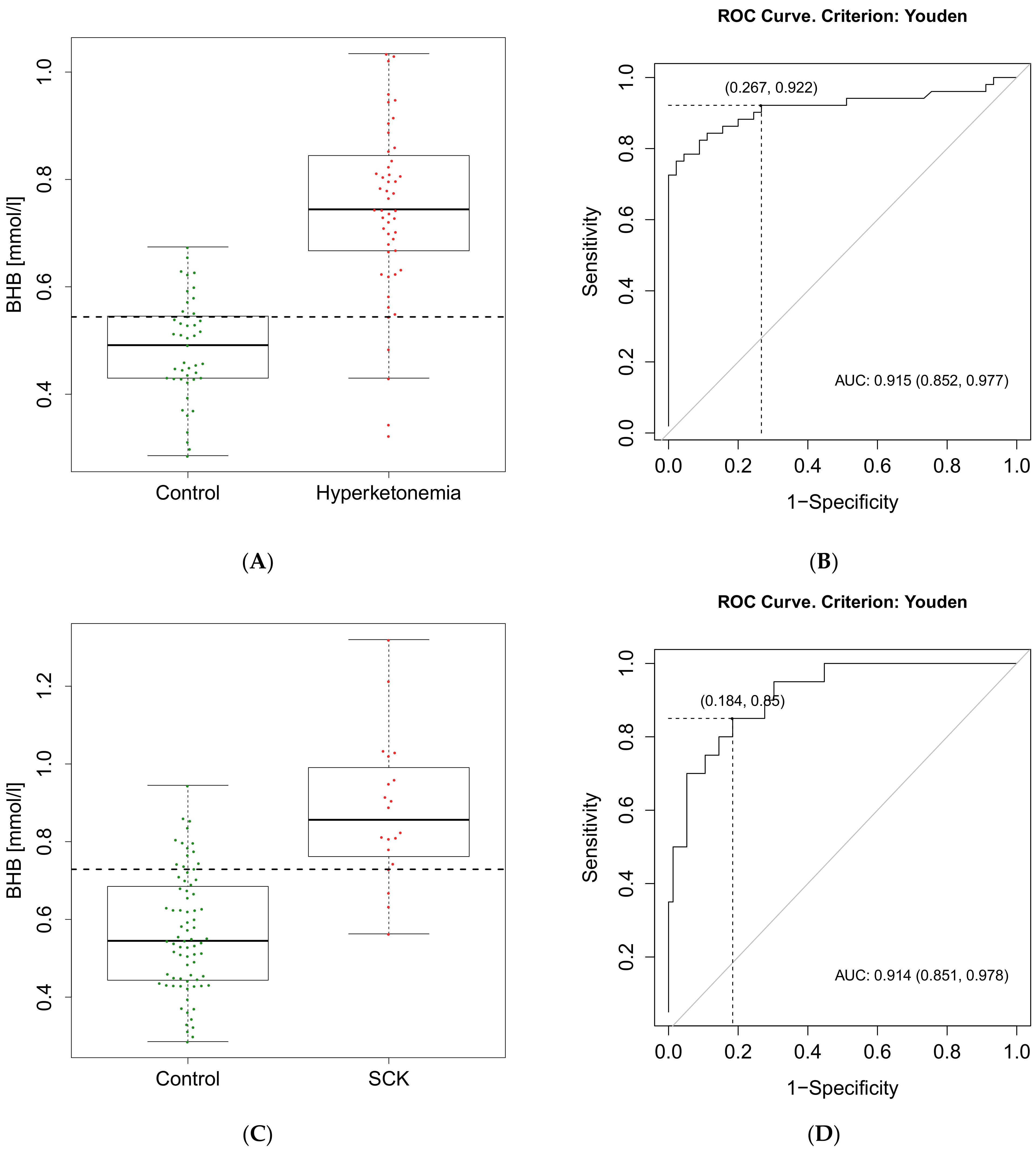
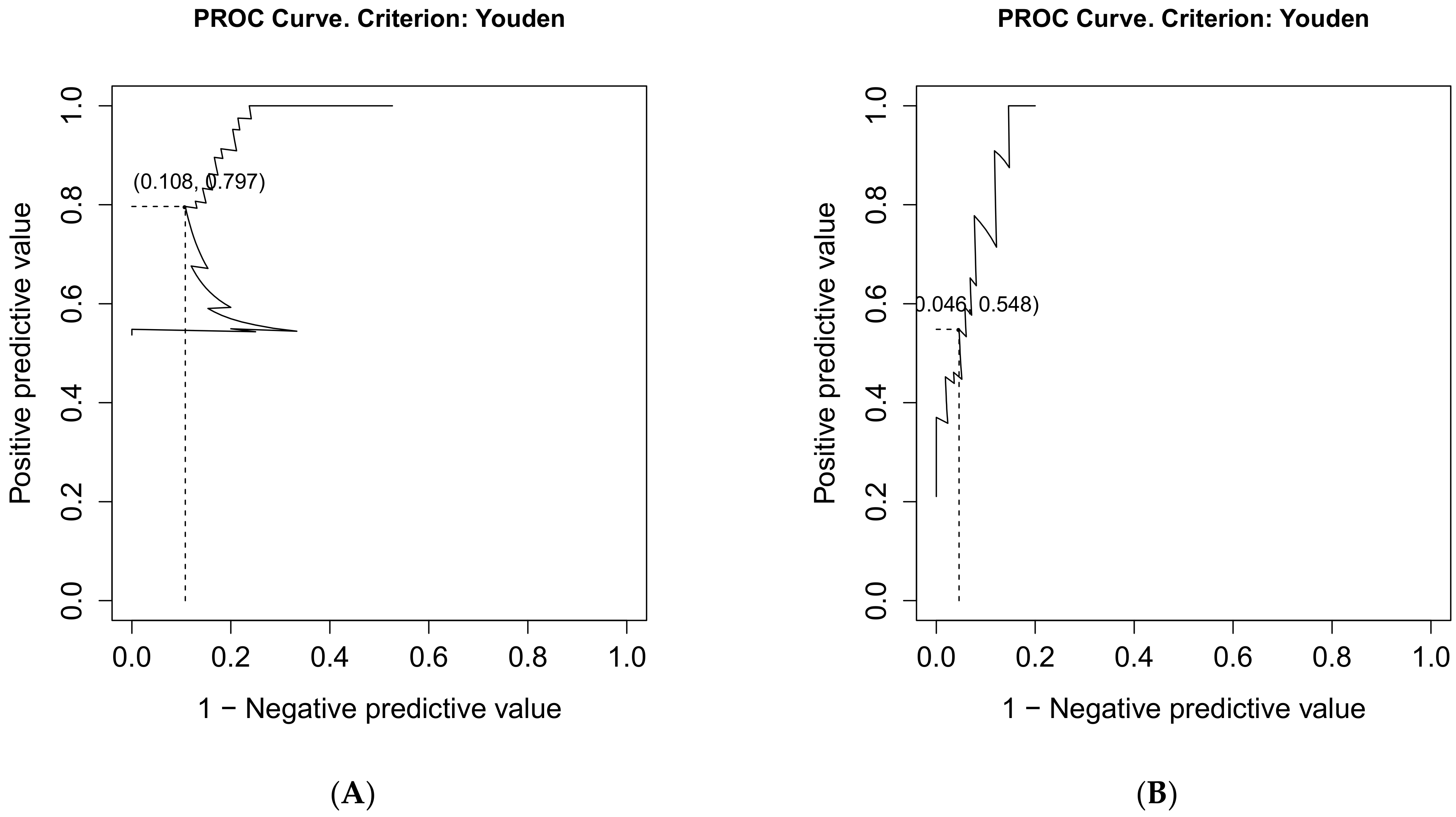
| Hyperketonemia | SCK | |
|---|---|---|
| Calculated cut-off value of BHB | 0.54 mmol/L | 0.73 mmol/L |
| Se (95% CI) | 0.92 (0.81–0.98) | 0.85 (0.62–0.97) |
| Sp (95% CI) | 0.73 (0.58–0.85) | 0.82 (0.71–0.90) |
| PPV (95% CI) | 0.80 (0.66–0.94) | 0.55 (0.42–0.87) |
| NPV (95% CI) | 0.89 (0.75–0.95) | 0.95 (0.86–0.98) |
| FP | 12 | 14 |
| FN | 4 | 3 |
| TP | 47 | 17 |
| TN | 36 | 65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couperus, A.M.; Schroeder, F.; Hettegger, P.; Huber, J.; Wittek, T.; Peham, J.R. Longitudinal Metabolic Biomarker Profile of Hyperketonemic Cows from Dry-Off to Peak Lactation and Identification of Prognostic Classifiers. Animals 2021, 11, 1353. https://doi.org/10.3390/ani11051353
Couperus AM, Schroeder F, Hettegger P, Huber J, Wittek T, Peham JR. Longitudinal Metabolic Biomarker Profile of Hyperketonemic Cows from Dry-Off to Peak Lactation and Identification of Prognostic Classifiers. Animals. 2021; 11(5):1353. https://doi.org/10.3390/ani11051353
Chicago/Turabian StyleCouperus, Anna Mareike, Fabian Schroeder, Peter Hettegger, Johann Huber, Thomas Wittek, and Johannes R. Peham. 2021. "Longitudinal Metabolic Biomarker Profile of Hyperketonemic Cows from Dry-Off to Peak Lactation and Identification of Prognostic Classifiers" Animals 11, no. 5: 1353. https://doi.org/10.3390/ani11051353
APA StyleCouperus, A. M., Schroeder, F., Hettegger, P., Huber, J., Wittek, T., & Peham, J. R. (2021). Longitudinal Metabolic Biomarker Profile of Hyperketonemic Cows from Dry-Off to Peak Lactation and Identification of Prognostic Classifiers. Animals, 11(5), 1353. https://doi.org/10.3390/ani11051353






