The “Sandwich” System: A Potential Solution for Protecting Overwintering Cornu aspersum Snails Reared in Semi-Intensive Heliciculture Farms in Colder Climates
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
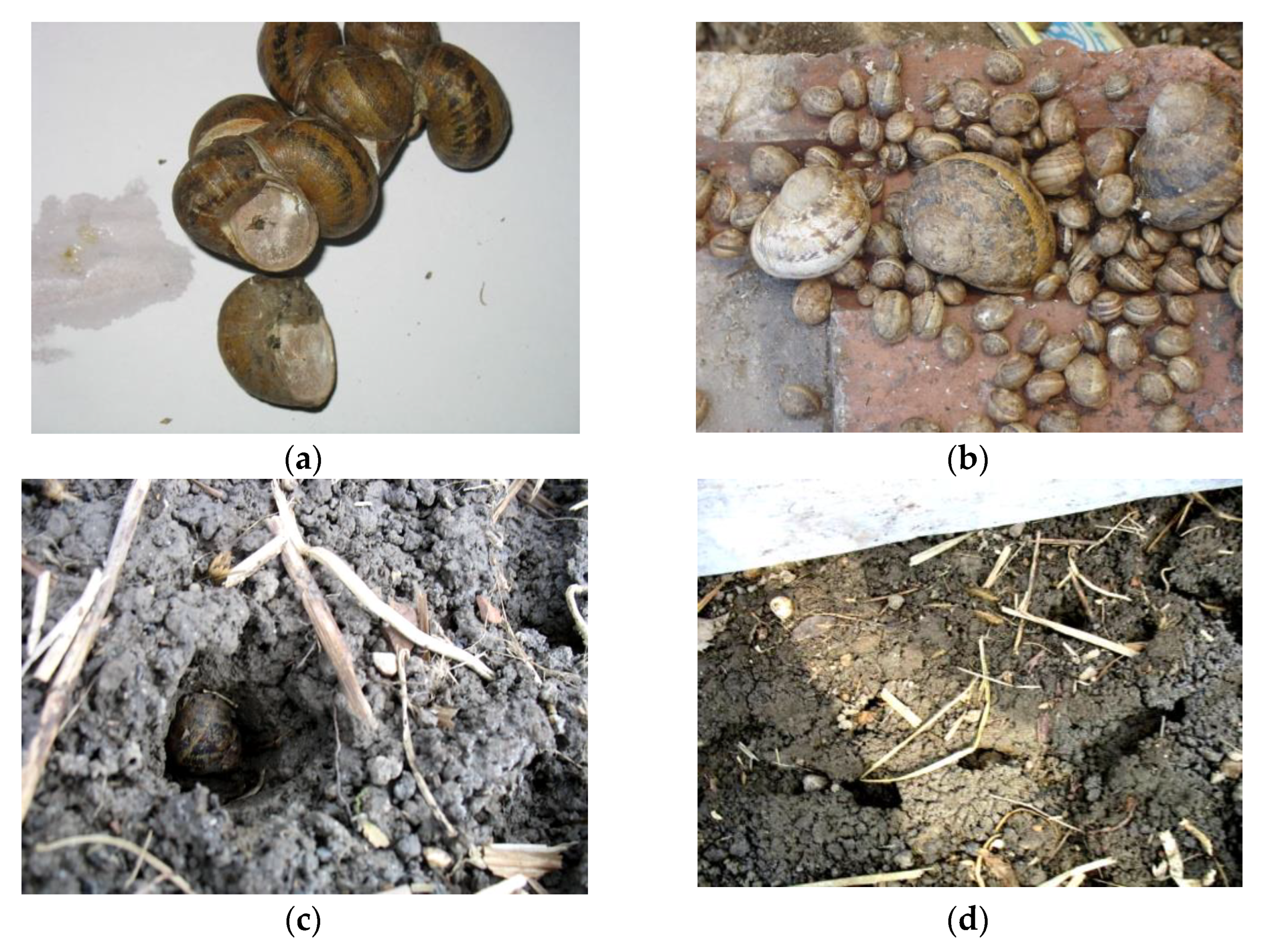
2.1. C. aspersum (Pre)hibernal Behavior
2.2. Identification of Protection System (Pilot Level)
2.3. Testing of Protective Structure under Realistic Farm Conditions
2.4. Statistical Analysis
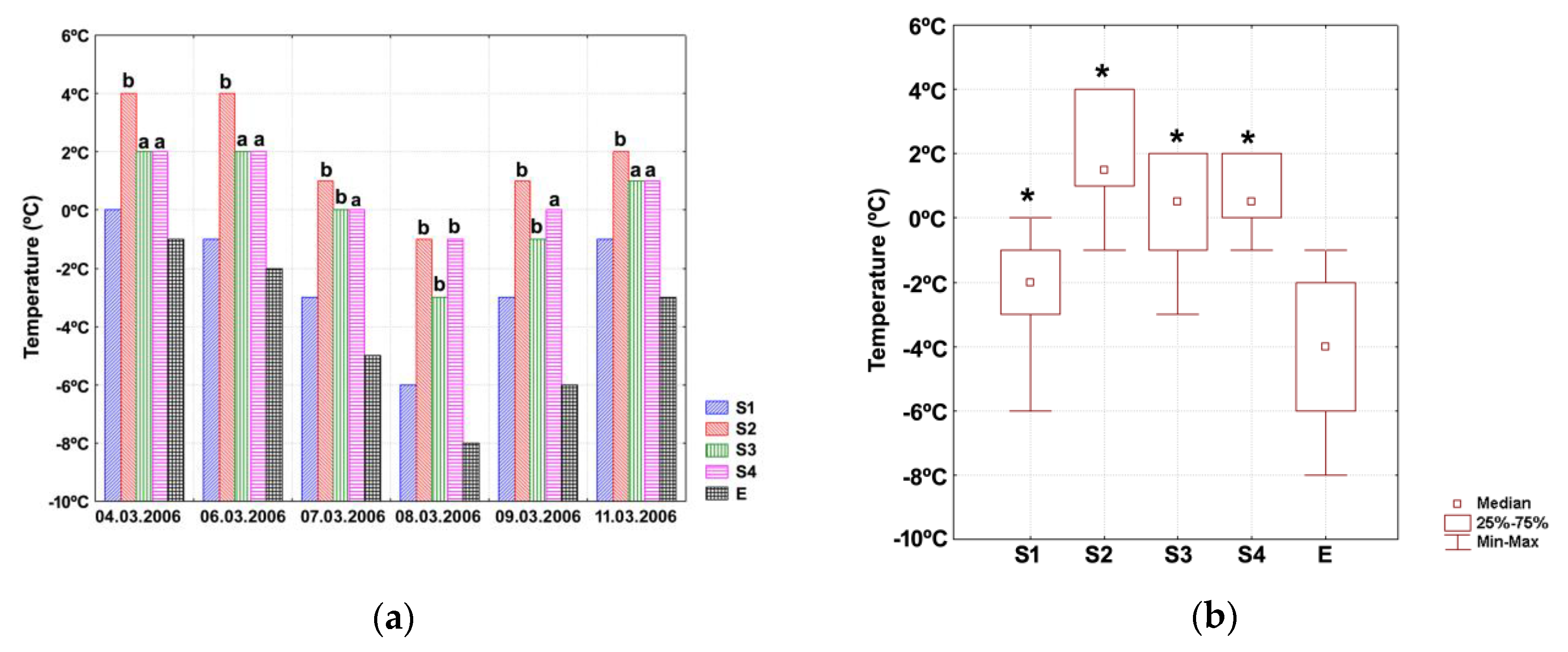
3. Results
3.1. C. aspersum (Pre)hibernal Behavior
3.2. Identification of Protection System (Pilot Level)
3.3. Testing of Protective Structure under Realistic Farm Conditions
4. Discussion
4.1. C. aspersum (Pre)hibernal Behavior
4.2. Identification of Protection System (Pilot Level)
4.3. Testing of Protective Structure under Realistic Farm Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Łozicki, A.; Niemiec, T.; Pietrasik, R.; Pawęta, S.; Rygało-Galewska, A.; Zglińska, K. The Effect of Ag Nanoparticles and Multimicrobial Preparation as Factors Stabilizing the Microbiological Homeostasis of Feed Tables for Cornu aspersum (Müller) Snails on Snail Growth and Quality Parameters of Carcasses and Shells. Animals 2020, 10, 2260. [Google Scholar] [CrossRef] [PubMed]
- Engmann, F.N.; Afoakwah, N.A.; Darko, P.O.; Sefah, W. Proximate and mineral composition of snail (Achatina achatina) meat; Any nutritional justification for acclaimed health benefits. JBASR 2013, 3, 8–15. [Google Scholar]
- Bura, M. Creşterea Melcilor o Activitate Profitabilă, 1st ed.; Eurobit: Timişoara, Romania, 2004; pp. 5–50. [Google Scholar]
- Nica, D. Researches Regarding the Morphophysiology of the Gastropods and the Farming of the Little Grey Snail (Helix Aspersa Muller) in the Pedoclimatic Conditions of Our Country. Ph.D. Thesis, Universitatea de Stiinte Agricole si Medicina Veterinara a Banatului Timisoara, Timisoara, Romania, 2009. [Google Scholar]
- Draghici, G.A.; Deheleana, C.; Susan, R.; Berceanu-Văduva, D.; Nica, D. Indoor Hibernation of Helix aspersa Juveniles. Invertebr. Ecophysiol. Manag. 2020, 3. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, P.S.; Sørensen, N. The Invasive Potential of the Brown Garden Snail (Cantareus Aspersus): A Future Invasive Species in Denmark? Master’s Thesis, Section for Ecology and Evolution, Department of Biology, University of Copenhagen, Copenhagen, Denmark, 2008. [Google Scholar]
- Toader-Williams, A.; Şara, A. Studies on the Edible Terrestrial Snails Helix aspersa Muller Food Conversion Ratio in a Confined Microclimate System. Sci. Pap. Anim. Sci. Biotechnol. 2010, 43, 133–142. [Google Scholar]
- Avagnina, G. Snail Breeding, 4th ed.; International Snail Breeding Institute: Cherasco, Italy, 2012; pp. 12–70. [Google Scholar]
- Barker, G.M.; Watts, C.H. Management of the Invasive Alien Snail Cantareus Aspersus on Conservation Land; Department of Conservation: Wellington, New Zealand, 2002.
- Zucaro, A.; Forte, A.; De Vico, G.; Fierro, A. Environmental loading of Italian semi-intensive snail farming system evaluated by means of life cycle assessment. J. Clean. Prod. 2016, 125, 56–67. [Google Scholar] [CrossRef]
- Thompson, R. Raising Snails; US Department of Agriculture, National Agricultural Library: Beltsville, MD, USA, 1996. Available online: https://pubs.nal.usda.gov/sites/pubs.nal.usda.gov/files/srb96-05_0.html (accessed on 10 March 2021).
- Gheoca, V. Can heliciculture act as a tool for edible land snails’natural populations’management in Romania? Manag. Sustain. Dev. 2013, 5, 21–25. [Google Scholar] [CrossRef]
- Çelik, M.Y.; Duman, M.B.; Sarıipek, M.; Gören, G.U.; Karayücel, S. Growth and Mortality Rates of Cornu aspersum: Organic Snail Culture System, Black Sea Region. J. Agric. Sci. 2018, 25, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Nicolai, A.; Ansart, A. Conservation at a slow pace: Terrestrial gastropods facing fast-changing climate. Conserv. Physiol. 2017, 5. [Google Scholar] [CrossRef] [Green Version]
- Ansart, A.; Vernon, P.; Daguzan, J. Effects of a freezing event during hibernation on further survival, reproduction and growth in the partially freezing tolerant land snail Helix aspersa Müller (Gastropoda: Helicidae). Cryo Lett. 2002, 23, 269–274. [Google Scholar]
- Falkner, G. The Europeanisation of Austria: Misfit, adaptation and controversies. Eur. Integr. Online Pap. (EIoP) 2001, 5. [Google Scholar] [CrossRef] [Green Version]
- Rabitsch, W. AW: RFI Cantareus aspersa (syn: Helix aspersa) (Helicidae) Native and Non-Native Status; Biodiversity and Nature Conservation: Wien, Austria, 2008. [Google Scholar]
- Juřičková, L.; Kapounek, F. Helix (Cornu) aspersa (OF Müller, 1774)(Gastropoda: Helicidae) in the Czech Republic. Malacol. Bohemoslov. 2009, 8, 53–55. [Google Scholar]
- Grilla, A.; LaJeunesse, C.; McMaster, D.; Morgan, D. Feasibility of Snail Farming as a Model for Small Urban Farms to Expand into Niche Markets for Increased Profitability. Bachelor’s Thesis, Worchester Polytechnic Institute, Worchester, UK, 2016. [Google Scholar]
- Outeiro, A.; Agüera, C.; Parejo, C. Use of ecological profilesin a study of the relationship of terrestrial gastropods and environmental factors. J. Conchol. 1993, 34, 365–375. [Google Scholar]
- Ondina, P.; Hermida, J.; Outeiro, A.; Mato, S. Relationships between terrestrial gastropod distribution and soil properties in Galicia (NW Spain). Appl. Soil Ecol. 2004, 26, 1–9. [Google Scholar] [CrossRef]
- Çerçi, K.N.; Daş, M. Modeling of heat transfer coefficient in solar greenhouse type drying systems. Sustainability 2019, 11, 5127. [Google Scholar] [CrossRef] [Green Version]
- Rojas, C.; Cea, M.; Iriarte, A.; Valdés, G.; Navia, R.; Cárdenas-R, J.P. Thermal insulation materials based on agricultural residual wheat straw and corn husk biomass, for application in sustainable buildings. Sustain. Mater. Technol. 2019, 20, e00102. [Google Scholar] [CrossRef]
- Ajmeri, J.; Ajmeri, C. Developments in nonwovens as agrotextiles. In Advances in Technical Nonwovens, 1st ed.; Kellie, G., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 365–384. [Google Scholar]
- Micu, D.M.; Dumitrescu, A.; Cheval, S.; Birsan, M.-V. Climate of the Romanian Carpathians, 1st ed.; Springer: Cham, Switzerland, 2016; pp. 3–14. [Google Scholar]
- Carney, W.P. Mortality and aperture orientation in Allogona ptychophora during winter hibernation in Montana. Nautilus 1966, 79, 134–136. [Google Scholar]
- Jeppesen, L.L. Photoperiodic control of hibernation in Helix pomatia L. (Gastropoda: Pulmonata). Behav. Process. 1977, 2, 373–382. [Google Scholar] [CrossRef]
- Bailey, S.E.R. Circannual and circadian rhythms in the snail Helix aspersa Müller and the photoperiodic control of annual activity and reproduction. J. Comp. Physiol. 1981, 142, 89–94. [Google Scholar] [CrossRef]
- Heller, J.; Dolev, A. Biology and population dynamics of a crevice-dwelling landsnail, Cristataria genezarethana (Clausiliidae). J. Molluscan Stud. 1994, 60, 33–46. [Google Scholar] [CrossRef]
- Nicolai, A.; Filser, J.; Lenz, R.; Bertrand, C.; Charrier, M. Adjustment of metabolite composition in the haemolymph to seasonal variations in the land snail Helix pomatia. J. Comp. Physiol. B 2011, 181, 457–466. [Google Scholar] [CrossRef]
- Terhivuo, J. Growth, reproduction and hibernation of Arianta arbustorum (L.) (Gastropoda, Helicidae) in southern Finland. Ann. Zool. Fenn. 1978, 15, 8–16. [Google Scholar]
- Baur, A.; Baur, B. The effect of hibernation position on winter survival of the rock-dwelling land snails Chondrina clienta and Balea perversa on Öland, Sweden. J. Molluscan Stud. 1991, 57, 331–336. [Google Scholar] [CrossRef]
- Ansart, A.; Guiller, A.; Moine, A.; Martin, M.C.; Madec, L. Is cold hardiness size-constrained? A comparative approach in land snails. Evol. Ecol. 2014, 28, 471–493. [Google Scholar] [CrossRef] [Green Version]
- Ansart, A.; Vernon, P. Cold hardiness abilities vary with the size of the land snail Cornu aspersum. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 139, 205–211. [Google Scholar] [CrossRef]
- Kerney, M.P.; Cameron, R.A.D. Field Guide to the Land Snails of Britain and North-West Europe, 1st ed.; William Collins Sons & Co Ltd.: London, UK, 1979; pp. 5–30. [Google Scholar]
- Bolton, W. Engineering Materials; Butterworth-Heinemann: Oxford, UK, 2014; Volume 2, pp. 24–40. [Google Scholar]
- Potts, D. Persistence and extinction of local populations of the garden snail Helix aspersa in unfavorable environments. Oecologia 1975, 21, 313–334. [Google Scholar] [CrossRef]
- Dekle, G.W.; Fasulo, T.R. Brown Garden Snail, Cornu Aspersum (Müller, 1774) (Gastropoda: Helicidae). Institute of Food and Agricultural Sciences Extension, University of Florida: Gainesville, FL, USA, 2001; Available online: https://edis.ifas.ufl.edu/pdffiles/IN/IN39600.pdf (accessed on 11 March 2021).
- Pearce, T.A.; Örstan, A. Terrestrial gastropoda. In The Mollusks: A Guide to their Study, Collection, and Preservation, 1st ed.; Sturm, C., Pearce, T.A., Valdes, A., Eds.; American Malagological Society: Pittsburgh, PA, USA, 2006; pp. 262–285. [Google Scholar]
- Dees, L.T. Edible Land Snails in the United States; US Department of the Interior, Fish and Wildlife Service, Bureau of Sport Fisheries and Wildlife: Washington, DC, USA, 1970; Volume 91.
- Green Snail Fact Sheet. Available online: https://www.pir.sa.gov.au/__data/assets/pdf_file/0004/167098/Green_Snail_Fact_Sheet_-_July_2019.pdf (accessed on 11 March 2021).
- Staikou, A.; Lazaridou-Dimitriadou, M.; Kattoulas, M.E. Behavioural patterns of the edible snail Helix lucoru L. in the different seasons of the year. Haliotis 1989, 19, 129–136. [Google Scholar]
- Nicolai, A. The Impact of Diet Treatment on Reproduction and Thermophysiological Processes in the Land Snails Cornu Aspersum and Helix Pomatia. Ph.D. Thesis, Université Rennes 1, Rennes, France, 2010. [Google Scholar]
- Bailey, R.G. Ecoregions: The Ecosystem Geography of the Oceans and Continents, 2nd ed.; Springer: Berlin, Germany, 2014; pp. 27–43. [Google Scholar]
- Charlson, J.A. Straw Insulation Materials to Address Heating Fuel Requirements, Thermal Comfort, and Natural Resource Depletion in Developing Regions. Master’s Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1997. [Google Scholar]
- Draghici, G.A.; Dehelean, C.; Susan, R.; Berceanu-Vaduva, D.; Nica, D. Indoor Hibernation of Helix aspersa Juveniles. In Invertebrates-Ecophysiology and Management; Ray, S., Diarte-Plata, G., Escamilla-Montes, R., Eds.; IntechOpen: London, UK, 2020; p. 3. [Google Scholar]
- Bonnefoy-Claudet, R.; Deray, A. Influence de la durée d’hibernation sur l’activité reproductrice de l’escargot Helix aspersa Müller. Comptes Rendus Séances Soc. Biol. Fil. 1984, 178, 442–449. [Google Scholar]
- Driss, Z.; Brahim, N.; Hao-Chun, Z. CFD Techniques and Thermo-Mechanics Applications, 1st ed.; Springer: Berlin, Germany, 2014; p. 27. [Google Scholar]
- Cowie, R.H.; Cain, A.J. Laboratory maintenance and breeding of land snails with example from Helix aspersa. J. Moll. Stud. 1983, 49, 176–179. [Google Scholar]
- Peake, J.F. Distribution and ecology of the Stylommatophora. In Pulmonates: Systematic, Evolution and Ecology; Fretter, V., Peake, J.F., Eds.; Academic Press: London, UK, 1978; Volume 2A, pp. 429–526. [Google Scholar]
- Charrier, M.; Daguzan, J. Food consumption, production and energy evaluation in Helix aspersa müller (a terrestrial pulmonated gasteropod). Proc. Ann. Nutr. Aliment. 1980, 34, 147–166. [Google Scholar]
- Madec, L.; Desbuquois, C.; Coutellec-Vreto, M.-A. Phenotypic plasticity in reproductive traits: Importance in the life history of Helix aspersa (Mollusca: Helicidae) in a recently colonized habitat. Biol. J. Linn. Soc. 2000, 69, 25–39. [Google Scholar] [CrossRef]
- Reischütz, A.; Moog, O.; Reischütz, P.L.; Duda, M. Die Schnecken-und Muschelfauna Tattendorfs Quellschnecken und Trockenrasen-Spezialisten auf engstem Raum. Arianta 2020, 8, 20–37. [Google Scholar]
- Reischütz, P. Zwei eingeschleppte Schneckenarten in Wien-Simmering. Mitt. Zool. Ges. Braunau 1978, 3, 9. [Google Scholar]
- Staikou, A.; Lazaridou-Dimitriadou, M.; Farmakis, N. Aspects of the life cycle, population dynamics, growth and secondary production of the edible snail Helix lucorum Linnaeus,1758 (Gastropoda, Pulmonata) in Greece. J. Moll. Stud. 1988, 54, 139–155. [Google Scholar] [CrossRef]
- Staikou, A. Aspects of life cycle, population dynamics, growth and secondary production of the pulmonate snail Cepaea vindobonensis (Ferussac, 1821) in northern Greece. J. Moll. Stud. 1998, 64, 297–308. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Hidalgo, M.; Rodriguez-Zaldua, I.; Txurruka, J. Hibernation and latitude in Helix (Cornu) aspersa: An attempt to integrate environmental restrictions to active life, length of stasis endurance and metabolic costs. IOBC/WPRS Bull. 2011, 64, 173–177. [Google Scholar]
- Machin, J. Distribution and ecology of the Stylommatophora. InPulmonates: Systematic, Evolution and Ecology; Fretter, V., Peake, J.F., Eds.; Academic Press: London, UK, 1975; Volume 1, pp. 105–163. [Google Scholar]
- Speakman, J.R. The cost of living: Field metabolic rates of small mammals. Adv. Ecol. Res. 1999, 30, 177–297. [Google Scholar]



| Snail Class | Type of Micro Shelter | ||
|---|---|---|---|
| Roofing Sheets | Ridge Tiles | Wooden Pallets | |
| Attached snails | 22% (11) | 56% (28) *** | 50% (25) ** |
| Unattached snails | 78% (39) | 44% (22) | 50% (25) |
| Snail Class | Site ST1 | Site ST2 |
|---|---|---|
| Burrowed snails | 34% (34) ** | 17% (17) |
| Non-burrowed snails | 66% (66) | 83% (83) |
| Snails burrowed within the top 5 cm soil layer | 90.20% (30.66) | 94.12% (16) |
| Snails burrowed deeper than 5 cm in the soil | 9.80% (3.33) | 5.88% (1) |
| Snails attached to micro shelters | 62.12% (41) | 57.83% (48) |
| Snails lying on the ground | 37.88% (25) | 42.17% (35) |
| Snail Class | Site ST3 | Site ST4 | Site ST5 | Reference Pen |
|---|---|---|---|---|
| Alive snails | 70.99% (6283) *** | 68.07% (5675) *** | 67.81% (8011) *** | 47.60% (476) |
| Dead snails | 29.01% (2567) | 31.93% (2662) | 32.91% (3803) | 52.40% (524) |
| Shell condition | ||||
| Intact shell | 64.43% (1654) *** | 47.60% (1267) * | 67.95% (2584) *** | 53.05% (278) |
| Large hole | 13.21% (339) *** | 20.40% (543) | 21.33% (811) | 23.66% (124) |
| Smashed | 13.79% (354) | 15.93% (424) | 8.73% (332) *** | 15.26% (80) |
| Small holes | 8.57% (220) | 16.08% (428) *** | 2.01% (7) *** | 8.01% (42) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manea, D.; Ienciu, A.A.; Ștef, R.; Peț, I.; Șmuleac, L.; Grozea, I.; Cărăbeț, A.; Drăghici, G.A.; Nica, D.V. The “Sandwich” System: A Potential Solution for Protecting Overwintering Cornu aspersum Snails Reared in Semi-Intensive Heliciculture Farms in Colder Climates. Animals 2021, 11, 1420. https://doi.org/10.3390/ani11051420
Manea D, Ienciu AA, Ștef R, Peț I, Șmuleac L, Grozea I, Cărăbeț A, Drăghici GA, Nica DV. The “Sandwich” System: A Potential Solution for Protecting Overwintering Cornu aspersum Snails Reared in Semi-Intensive Heliciculture Farms in Colder Climates. Animals. 2021; 11(5):1420. https://doi.org/10.3390/ani11051420
Chicago/Turabian StyleManea, Dan, Anișoara Aurelia Ienciu, Ramona Ștef, Ioan Peț, Laura Șmuleac, Ioana Grozea, Alin Cărăbeț, George Andrei Drăghici, and Dragoș Vasiles Nica. 2021. "The “Sandwich” System: A Potential Solution for Protecting Overwintering Cornu aspersum Snails Reared in Semi-Intensive Heliciculture Farms in Colder Climates" Animals 11, no. 5: 1420. https://doi.org/10.3390/ani11051420
APA StyleManea, D., Ienciu, A. A., Ștef, R., Peț, I., Șmuleac, L., Grozea, I., Cărăbeț, A., Drăghici, G. A., & Nica, D. V. (2021). The “Sandwich” System: A Potential Solution for Protecting Overwintering Cornu aspersum Snails Reared in Semi-Intensive Heliciculture Farms in Colder Climates. Animals, 11(5), 1420. https://doi.org/10.3390/ani11051420





