Muscle and Subcutaneous Fatty Acid Composition and the Evaluation of Ageing Time on Meat Quality Parameters of Hispano-Bretón Horse Breed
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Handling
2.2. Experimental Design and Sampling
2.3. Chemical and Fatty Acid Composition
2.4. pH Measurements
2.5. Myoglobin Content Determinations
2.6. Instrumental Color Measurements
2.7. Cook Loss Determinations
2.8. Instrumental Texture Measurements
2.9. Statistical Analysis
3. Results and Discussion
3.1. Chemical and Fatty Acid Composition
3.2. Effect of Ageing on Horse Meat Quality Parameters
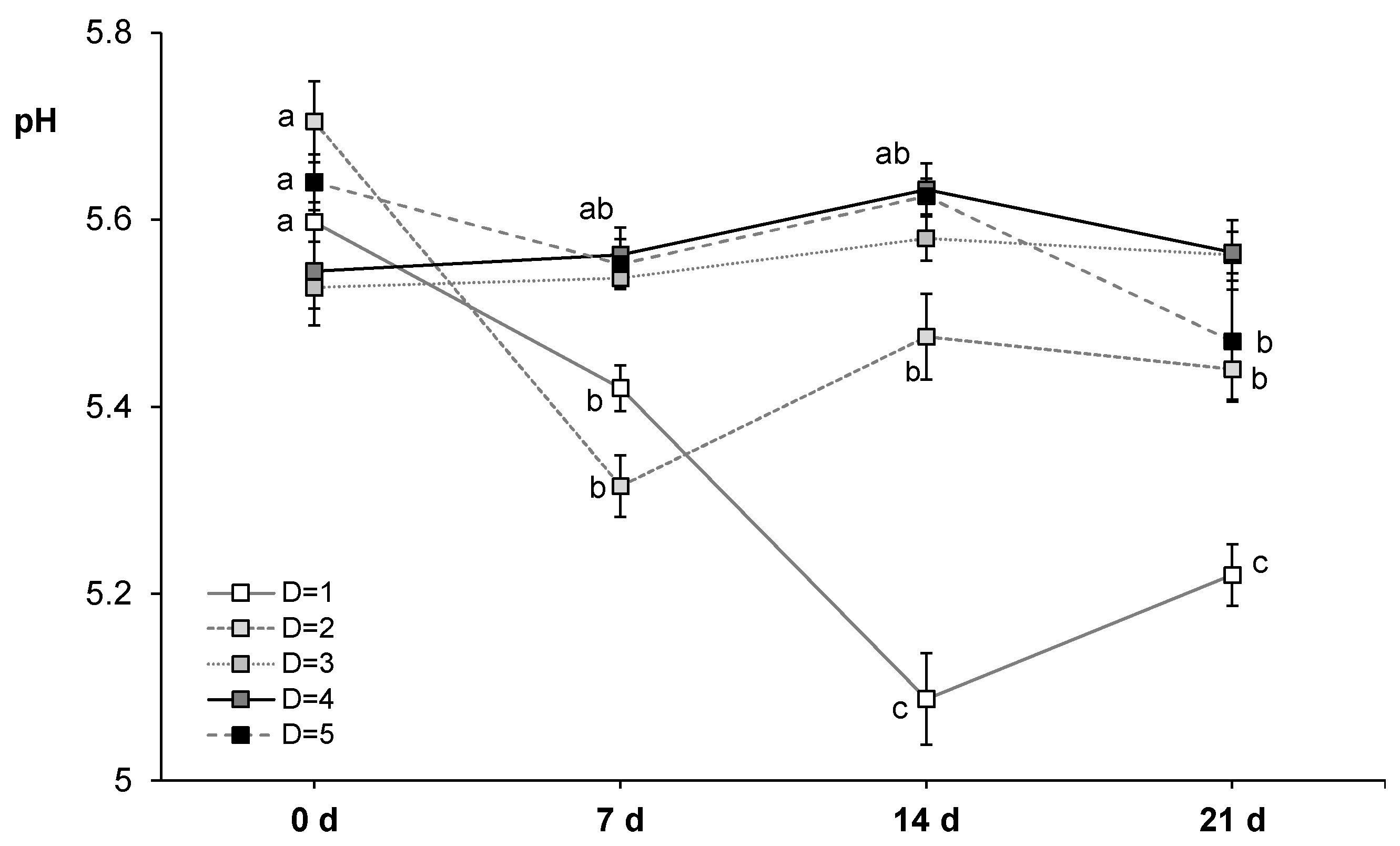
3.2.1. Measurement of pH
3.2.2. Myoglobin Content and Instrumental Color
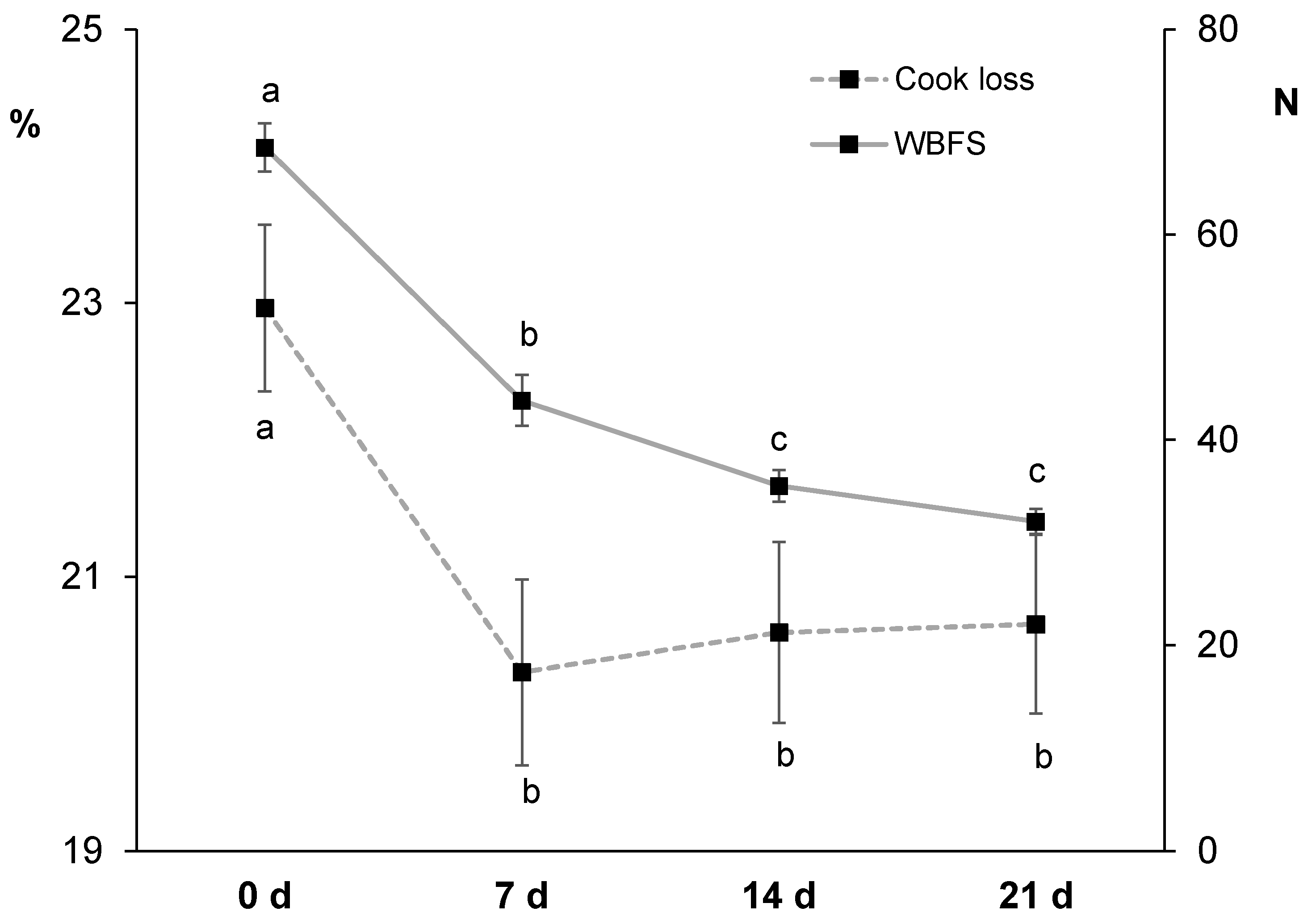
3.2.3. Cook Loss and Instrumental Texture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rossier, E. Horse meat. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Trugo, L., Finglas, P., Eds.; Academic Press: Cambridge, UK, 2003; pp. 3174–3178. [Google Scholar]
- Ursin, L. The Ethics of the Meat Paradox. Environ. Ethics 2016, 38, 131–144. [Google Scholar] [CrossRef]
- Belaunzaran, X.; Bessa, R.J.B.; Lavín, P.; Mantecón, A.R.; Kramer, J.K.G.; Aldai, N. Horse-meat for human con-sumption-Current research and future opportunities. Meat Sci. 2015, 108, 74–81. [Google Scholar] [CrossRef]
- Del Bò, C.; Simonetti, P.; Gardana, C.; Riso, P.; Lucchini, G.; Ciappellano, S. Horse meat consumption affects iron status, lipid profile and fatty acid composition of red blood cells in healthy volunteers. Int. J. Food Sci. Nutr. 2012, 64, 147–154. [Google Scholar] [CrossRef]
- Belaunzaran, X.; Lavín, P.; Barron, L.J.; Mantecón, A.R.; Kramer, J.K.; Aldai, N. An assessment of the fatty acid composition of horse-meat available at the retail level in northern Spain. Meat Sci. 2017, 124, 39–47. [Google Scholar] [CrossRef] [PubMed]
- He, M.L.; Ishikawa, S.; Hidari, H. Fatty Acid Profiles of Various Muscles and Adipose Tissues from Fattening Horses in Comparison with Beef Cattle and Pigs. Asian-Australas. J. Anim. Sci. 2005, 18, 1655–1661. [Google Scholar] [CrossRef]
- Lanza, M.; Landi, C.; Scerra, M.; Galofaro, V.; Pennisi, P. Meat quality and intramuscular fatty acid composition of Sanfrantellano and Haflinger foals. Meat Sci. 2009, 81, 142–147. [Google Scholar] [CrossRef]
- Pinto, F.; Schiavone, M.; Marisco, G. Effects of diets containing ω3 fatty acids on productive performances and meat quality of “Murguese” foals. Prog. Nutr. 2004, 6, 122–131. [Google Scholar]
- Sahaka, M.; Amara, S.; Wattanakul, J.; Gedi, M.; Aldai, N.; Parsiegla, G.; Lecomte, J.; Christeller, J.T.; Gray, D.; Gontero, B.; et al. The digestion of galactolipids and its ubiquitous function in nature for the uptake of the essential α-linoleic acid. Food Funct. 2020, 11, 6710–6744. [Google Scholar] [CrossRef]
- Santos, A.S.; Rodrigues, M.A.M.; Bessa, R.J.B.; Ferreira, L.M.; Martin-Rosset, W. Understanding the equine ce-cum-colon ecosystem: Current knowledge and future perspectives. Animal 2011, 5, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Clauss, M.; Grum, C.; Hatt, J. Polyunsaturated fatty acid content in adipose tissue in foregut and hindgut ferment-ing mammalian herbivores: A literature survey. Mamm. Biol. 2009, 74, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Hartman, L.; Shorland, F.B.; Moir, R.J.; Hartman, F.B.S.L. Occurrence of trans-Unsaturated Fatty Acids in Horse Fæces. Nat. Cell Biol. 1956, 178, 1057–1058. [Google Scholar] [CrossRef]
- McKain, N.; Shingfield, K.J.; Wallace, R.J. Metabolism of Conjugated Linoleic Acids and 18:1 fatty acids by rumi-nal bacteria: Products and mechanism. Microbiology 2010, 156, 579–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlaeminck, B.; Fievez, V.; Cabrita, A.; Fonseca, A.; Dewhurst, R. Factors affecting odd- and branched-chain fatty acids in milk: A review. Anim. Feed. Sci. Technol. 2006, 131, 389–417. [Google Scholar] [CrossRef]
- Delmonte, P.; Belaunzaran, X.; Ridge, C.D.; Aldai, N.; Kramer, J.K. Separation and characterization of products from acidic methanolysis of plasmalogenic lipids by two-dimensional gas chromatography with online reduction. J. Chromatogr. A 2020, 1619, 460955. [Google Scholar] [CrossRef]
- Nagan, N.; Zoeller, R.A. Plasmalogens: Biosynthesis and functions. Prog. Lipid Res. 2001, 40, 199–229. [Google Scholar] [CrossRef]
- Van der Veen, J.N.; Kenelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidyl-choline and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophisica Acta 2017, 1859, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Sarriés, M.V.; Murray, B.E.; Troy, D.; Beriain, M.J. Intramuscular and subcutaneous lipid fatty acid profile compo-sition in male and female foals. Meat Sci. 2006, 72, 475–485. [Google Scholar] [CrossRef] [PubMed]
- De Palo, P.; Maggiolino, A.; Centoducati, P.; Tateo, A. Slaughtering Age Effect on Carcass Traits and Meat Quality of Italian Heavy Draught Horse Foals. Asian-Australas. J. Anim. Sci. 2013, 26, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Duffey, P.A. Sensory and physico-chemical properties of meat form horses of different age groups. In Proceedings from the 42nd International Congress of Meat Science and Technology; ICoMST: Lillehammer, Norway, 1996; pp. 556–557. [Google Scholar]
- Franco, D.; Rodriguez, E.; Purriños, L.; Crecente, S.; Bermúdez, R.; Lorenzo, J.M. Meat quality of “Galician moun-tain” foals breed. Effect of sex, slaughter age and livestock production system. Meat Sci. 2011, 88, 292–298. [Google Scholar] [CrossRef]
- Litwińczuk, A.; Florek, M.; Skałecki, P. Chemical composition and physicochemical properties of horse meat from the longissimus lumborum and semitendinosus muscle. J. Muscle Foods 2008, 19, 223–236. [Google Scholar] [CrossRef]
- Seong, P.; Park, K.M.; Cho, S.; Kang, G.H.; Chae, H.S.; Park, B.Y.; Ba, H.V. Effect of cut type and post mortem ageing on technological quality, textural profile and sensory characteristics of foal meat. Anim. Prod. Sci. 2016, 56, 1551–1559. [Google Scholar] [CrossRef]
- Warren, K.; Kastner, C. A comparison of dry-aged and vacuum-aged beef strip loins. J. Muscle Foods 1992, 3, 151–157. [Google Scholar] [CrossRef]
- Laster, M.A.; Smith, R.D.; Nicholson, K.L.; Nicholson JD, W.; Harris, K.B.; Miller, R.K.; Griffin, D.B.; Savell, J.W. Dry versus wet aging of beef: Retail cutting yields and consumer sensory attribute evaluations of steaks from ri-beyes, strip loins, and top sirloins from two quality grade groups. Meat Sci. 2008, 80, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Kim YH, B.; Ma, D.; Setyabrata, D.; Farouk, M.M.; Lonergan, S.M.; Hyff-Lonergan, E.; Hunt, M.C. Understanding post-mortem biochemical processes and post-harvest ageing factors to develop novel smart-aging strategies. Meat Sci. 2018, 144, 74–90. [Google Scholar] [CrossRef]
- Jeremiah, L. Packaging alternatives to deliver fresh meats using short- or long-term distribution. Food Res. Int. 2001, 34, 749–772. [Google Scholar] [CrossRef]
- MacDougall, D.B. Principles of colour measurement for food. In Instru-Mentation and Sensors for the Food Industry; Kress-Rogers, E., Brimelow, C.J.B., Eds.; Woodhead Printing: Cambridge, UK, 2001; pp. 63–84. [Google Scholar]
- Koohmaraie, M. Muscle proteinases and meat aging. Meat Sci. 1994, 36, 93–104. [Google Scholar] [CrossRef]
- Ouali, A.; Gagaoua, M.; Boudida, Y.; Becila, S.; Boudjellal, A.; Herrera-Mendez, C.H.; Sentandreu, M.A. Biomarkers of meat tenderness: Present knowledge and perspectives in regards to our current understanding of the mechanisms in-volved. Meat Sci. 2013, 95, 854–870. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cerezo, S.; Sañudo, C.; Panea, B.; Medel, I.; Delfa, R.; Sierra, I.; Beltrán, J.; Cepero, R.; Olleta, J. Breed, slaughter weight and ageing time effects on physico-chemical characteristics of lamb meat. Meat Sci. 2005, 69, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Gill, C. Extending the storage life of raw chilled meats. Meat Sci. 1996, 43, 99–109. [Google Scholar] [CrossRef]
- Suman, S.P.; Joseph, P. Myoglobin Chemistry and Meat Color. Annu. Rev. Food Sci. Technol. 2013, 4, 79–99. [Google Scholar] [CrossRef] [Green Version]
- Nair, M.N.; Canto, A.C.; Rentfrow, G.; Suman, S.P. Muscle-specific effect of aging on beef tenderness. LWT 2019, 100, 250–252. [Google Scholar] [CrossRef]
- Hwang, S.; Hong, G. Effects of high pressure in combination with the type of ageing on the eating quality and bi-ochemical changes in pork loin. Meat Sci. 2020, 162, 108028. [Google Scholar] [CrossRef] [PubMed]
- Callejas-Cárdenas, A.R.; Caro, I.; Blanco, C.; Villalobos-Delgado, L.H.; Prieto, N.; Bodas, R.; Giráldez, F.J.; Mateo, J.; García, J.G. Effect of vacuum ageing on quality changes of lamb steaks from early fattening lambs during aerobic display. Meat Sci. 2014, 98, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Della Malva, A.; De Palo, P.; Lorenzo, J.M.; Maggiolino, A.; Albenzio, M.; Marino, R. Application of proteomic to investigate the post-mortem tenderization rate of different horse muscles. Meat Sci. 2019, 157, 107885. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.; Lorenzo, J.M. Effect of packaging conditions on shelf-life of fresh foal meat. Meat Sci. 2012, 91, 513–520. [Google Scholar] [CrossRef]
- Kaić, A.; Žgur, S.; Luštrek, B.; Potočnik, K. Physicochemical properties of horse meat as affected by breed, sex, age, muscle type and aging period. Anim. Prod. Sci. 2018, 58, 2352–2357. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Gómez, M. Shelf life of fresh foal meat under MAP, overwrap and vacuum packaging conditions. Meat Sci. 2012, 92, 610–618. [Google Scholar] [CrossRef]
- Ruiz, M. Effect of Production Factors on Foal Carcass and Meat Quality. Consumers Preferences. Colour and Texture Evolution of Foal Meat during the Storage Time as Quality Attributes, from Animals Reared under Sustainable Conditions. Ph.D. Thesis, Public University of Navarre, Navarre, Spain, 2008. [Google Scholar]
- Council Regulation. No 1099/2009 of 24 September 2009 on the protection of animals at the time of killing. Off. J. Eur. Union L 2009, 303, 1–30. [Google Scholar]
- Beldarrain, L.R.; Etaio, I.; Morán, L.; Sentandreu, M.; Sentandreu, M.Á.; Barron, L.J.R.; Aldai, N. Effect of ageing time on consumer preference and sensory description of foal meat. Food Res. Int. 2020, 129, 108871. [Google Scholar] [CrossRef]
- Council Regulation. No 1208/81 of 28 April 1981 determining the community scale for the classification of carcasses of adult bovine animals. Off. J. Eur. Union L 1981, 123, 3–6. [Google Scholar]
- International Organization for Standardization. Animal Feeding Stuffs: Determination of Moisture and Other Volatile Matter Content; Standard 6496:1999; International Organization for Standardization: Geneva, Switzerland, 1999. [Google Scholar]
- International Organization for Standardization. Animal Feeding Stuffs-Determination of Nitrogen Content and Calculation of Crude Protein Content-Part 2: Block Digestion and Steam Distillation Method; International Standard ISO 5983-2:2005; International Organization for Standardization: Geneva, Switzerland, 2005. [Google Scholar]
- American Oil Chemists’ Society. Official Methods and Recommended Practices of the American Oil Chemists´ Society; American Oil Chemists’ Society: Champaign, IL, USA, 2008. [Google Scholar]
- International Organization for Standardization. Animal Feeding Stuffs- Determination of Crude Ash; Standard 5984:2002; International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Kramer, J.K.; Hernandez, M.; Cruz-Hernandez, C.; Kraft, J.; Dugan ME, R. Combining results of two GC separa-tions partly achieves determination of all cis and trans 16:1, 18:1, 18:2 and 18:3 except CLA isomers of milk fat as demon-strated using Ag-ion SPE fractionation. Lipids 2008, 43, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Belaunzaran, X.; Bravo-Lamas, L.; Kramer JK, G.; Aldai, N. Limitation of using silver ion solid-phase extraction for animal lipids with a low trans content. Eur. J. Lipid Sci. Technol. 2014, 116, 1621–1625. [Google Scholar] [CrossRef]
- Belaunzaran, X.; Bravo-Lamas, L.; Kramer JK, G.; Morales, R.; Aldai, N. Silver ion solid-phase extraction car-tridges employing glass housings overcome the limitations observed in the GC analysis of animal lipids with low trans fatty acid content. Eur. J. Lipid Sci. Technol. 2017, 119, 1600124. [Google Scholar] [CrossRef]
- Bravo-Lamas, L.; Barron LJ, R.; Kramer JK, G.; Etaio, I.; Aldai, N. Characterization of the fatty acid composition of lam commercially available in northern Spain: Emphasis on the trans-18:1 and CLA content and profile. Meat Sci. 2016, 117, 108–116. [Google Scholar] [CrossRef]
- Wolff, R.L.; Bayard, C.C.; Fabien, R.J. Evaluation of sequential methods for the determination of butterfat fatty acid composition with emphasis on trans-18:1 acid. Application to the study of seasonal variations in French butters. J. Am. Oil Chem. Soc. 1995, 72, 1471–1483. [Google Scholar] [CrossRef]
- Faustman, C.; Philips, A.L. Measurement of discoloration in fresh meat. In Current protocols in Food Analytical Chemistry; Wrostlad, R.E., Ed.; Wiley & Sonspp: New York, NY, USA, 2001; pp. F3.3.1–F3.3.13. [Google Scholar]
- Bowen, W.J. The absorption expectra and extinction coefficients of myoglobin. J.Biol. Chem. 1949, 179, 235–245. [Google Scholar] [CrossRef]
- Comision Internationale de l´Eclairage. Colorimetry: Official Recommendations of the International Commission on Illumintion; Comision Internationale de l´Eclairage: Paris, France, 1976. [Google Scholar]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sarriés, M.V.; Franco, D. Sex effect on meat quality and carcass traits of foals slaughtered at 15 months of age. Animal 2013, 7, 1199–1207. [Google Scholar] [CrossRef] [Green Version]
- Sarriés, M.; Beriain, M. Carcass characteristics and meat quality of male and female foals. Meat Sci. 2005, 70, 141–152. [Google Scholar] [CrossRef]
- Belaunzaran, X.; Lavín, P.; Mantecón, A.R.; Kramer, J.K.G.; Aldai, N. Effect of slaughter age and feeding system on the neutral and polar lipid composition of horse meat. Animal 2018, 12, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Juarez, M.; Polvillo, O.; Gómez, M.D.; Alcalde, M.J.; Romero, F.; Valera, M. Breed effect on carcass and meat quali-ty of foals slaughtered at 24 months of age. Meat Sci. 2009, 83, 224–228. [Google Scholar] [CrossRef]
- Tateo, A.; De Palo, P.; Ceci, E.; Centoducati, P. Physicochemical properties of meat of Italian Heavy Draft horses slaughtered at the age of eleven months1. J. Anim. Sci. 2008, 86, 1205–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pannier, L.; Pethick, D.W.; Boyce, M.D.; Ball, A.; Jacob, R.H.; Gardner, G.E. Associations of genetic and non-genetic factors with concentrations of iron and zink in the longissimus muscle of lamb. Meat Sci. 2014, 96, 1111–1119. [Google Scholar] [CrossRef]
- Leiber, F.; Meier, J.S.; Burger, B.; Wettstein, H.-R.; Kreuzer, M.; Hatt, J.-M.; Clauss, M. Significance of Coprophagy for the Fatty Acid Profile in Body Tissues of Rabbits Fed Different Diets. Lipids 2008, 43, 853–865. [Google Scholar] [CrossRef] [Green Version]
- Guil-Guerrero, J.L.; Rincón-Cervera, M.A.; Venegas-Venegas, C.E.; Ramos-Bueno, R.P.; Suarez, M.D. Highly bi-oavailable α-linolenic acid from the subcutaneous fat of the Palaeolithic Relict “Galician Horse”. Int. Food Res. J. 2013, 20, 3249–3258. [Google Scholar]
- Ferjak, E.; Cavinder, C.; Sukumaran, A.; Burnett, D.; Lemley, C.; Dinh, T. Fatty acid composition of mesenteric, cardiac, abdominal, intermuscular, and subcutaneous adipose tissues from horses of three body condition scores. Livest. Sci. 2019, 223, 116–123. [Google Scholar] [CrossRef]
- Lorenzo, J.; Crecente, S.; Franco, D.; Sarriés, M.; Gómez, M. The effect of livestock production system and concentrate level on carcass traits and meat quality of foals slaughtered at 18 months of age. Animal 2014, 8, 494–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo, J.M.; Fuciños, C.; Purriños, L.; Franco, D. Intramuscular fatty acid composition of “Galician mountain” foals breed. Effect of sex, slaughtered age and livestock production system. Meat Sci. 2010, 86, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Ribiero, T.; Lordelo, M.M.; Alves, S.P.; Bessa, R.J.B.; Costa, P.; Lemos, J.P.C.; Ferreira, L.M.A.; Fontes, C.M.G.A.; Prates, J.A.M. Direct supplementation of diet is the most efficient way of enriching broiler meat with n-3 long chain poly-unsaturated fatty acid. Br. Poult. Sci. 2014, 54, 753–765. [Google Scholar] [CrossRef]
- Kouba, M.; Benatmane, F.; Blochet, J.; Mourot, J. Effect of a linseed diet on lipid oxidation, fatty acid composition of muscle, perirenal fat, and raw and cooked rabbit meat. Meat Sci. 2008, 80, 829–834. [Google Scholar] [CrossRef]
- Dugan, M.E.; Vahmani, P.; Turner, T.D.; Mapiye, C.; Juárez, M.; Prieto, N.; Beaulieu, A.D.; Zijlstra, R.T.; Patience, J.F.; Aalhus, J.L. Pork as a Source of Omega-3 (n-3) Fatty Acids. J. Clin. Med. 2015, 4, 1999–2011. [Google Scholar] [CrossRef]
- Aldai, N.; Lavín, P.; Kramer JK, G.; Jaroso, R.; Mantecón, A.R. Breed effect on quality veal production in moun-tain areas: Emphasis on meat fatty acid composition. Meat Sci. 2012, 92, 687–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enomoto, H.; Furukawa, T.; Takeda, S.; Hatta, H.; Zaima, N. Unique distribution of diacyl-, alkylacyl- and alkenylacyl-phosphatidylcholine species visualized in pork chop tissues by matrix-assisted laser desorp-tion/ionization-mass spectrometry imaging. Foods 2020, 9, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delosiere, M.; Durand, D.; Bourget, C.; Terlow EM, C. Lipid oxidation, pre-slaughter animal stress and meat packaging: Can dietary supplementation of vitamin E and plant extracts come to rescue? Food Chem. 2020, 309, 125668. [Google Scholar] [CrossRef]
- Ferguson, D.M.; Warner, R.D. Have we underestimated the impact of pre-slaughter stress on meat quality in ru-minants? Meat Sci. 2008, 80, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Young, J.F.; Rosenvold, K.; Stagsted, J.; Steffensen, C.L.; Nielsen, J.H.; Andersen, H.N. Significance of preslaugh-ter stress and different tissue PUFA levels in the oxidative status and stability of porcine muscle and meat. J. Agric. Chem. 2003, 51, 6877–6881. [Google Scholar] [CrossRef]
- Gill, C. Safety and storage stability of horse meat for human consumption. Meat Sci. 2005, 71, 506–513. [Google Scholar] [CrossRef]
- Lawrie, R.D. Metabolic stress which affect muscle. In The Physiology and Biochemistry of Muscle as a Food; Briskey, E.J., Cassens, R.G., Trautman, J.D., Eds.; University of Wisconsin Press: Madison, WI, USA, 1966; pp. 137–164. [Google Scholar]
- Jelenikova, J.; Pipek PStaruch, L. The influence of ante-mortem treatment on relationship between pH and ten-derness of beef. Meat Sci. 2008, 80, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Farouk, M.; Clerens, S.; Rosenvold, K. Effect of beef ultimate pH and large structural protein changes with aging on meat tenderness. Meat Sci. 2014, 98, 637–645. [Google Scholar] [CrossRef]
- American Meat Science Association. Meat Colour Measurement Guidelines, Revised December 2012; American Meat Science Association: Champaign, IL, USA, 2012. [Google Scholar]
- Badiani, A.; Manfredini, M. The production of horse meat. Ital. J. Anim. Sci. 1994, 19, 23–31. [Google Scholar]
- Sarriés, M.; Beriain, M. Colour and texture characteristics in meat of male and female foals. Meat Sci. 2006, 74, 738–745. [Google Scholar] [CrossRef]
- De Palo, P.; Maggiolino, A.; Centoducati, P.; Tateo, A. Colour Changes in Meat of Foals as Affected by Slaughtering Age and Post-thawing Time. Asian-Australas. J. Anim. Sci. 2012, 25, 1775–1779. [Google Scholar] [CrossRef] [PubMed]
- Salueña, B.H.; Gamasa, C.S.; Rubial, J.M.D.; Odriozola, C.A. CIELAB color paths during meat shelf life. Meat Sci. 2019, 157, 107889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, J.; Oiseth, S.; Purslow, P.; Warner, R. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci. 2014, 98, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Warner, R.D. The eating quality of meat- IV Water-Holding Capacity and juiciness. In Lawrie´s Meat Science; Toldrá, F., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 419–459. [Google Scholar]
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—A review. Meat Sci. 2011, 89, 111–124. [Google Scholar] [CrossRef]
- Oillic, S.; Lemoine, E.; Gros, J.-B.; Kondjoyan, A. Kinetic analysis of cooking losses from beef and other animal muscles heated in a water bath—Effect of sample dimensions and prior freezing and ageing. Meat Sci. 2011, 88, 338–346. [Google Scholar] [CrossRef]
- Shanks, B.C.; Wulf, D.M.; Maddok, R.J. Technical note: The effect of freezing on Warner-Bratzler shear force val-ues of beef longissimus steaks across several post-mortem ageing periods. J. Anim. Sci. 2002, 80, 2122–2125. [Google Scholar]
- Shackelford, S.; Morgan, J.; Cross, H.; Savell, J. Identification of threshold levels for Warner-Bratzler shear force in beef top loin steaks. J. Muscle Foods 1991, 2, 289–296. [Google Scholar] [CrossRef]


| Mean | Min | Max | SEM | |
|---|---|---|---|---|
| Chemical composition | ||||
| Moisture | 75.3 | 72.9 | 78.1 | 0.3 |
| Crude protein | 20.4 | 19.1 | 22.3 | 0.2 |
| Fat (ether extract) | 3.31 | 1.87 | 5.13 | 0.19 |
| Ash | 1.03 | 0.750 | 1.17 | 0.02 |
| Fatty acid composition | ||||
| Total FAME | 2427 | 893 | 4238 | 199 |
| SFA | 956 | 333 | 1743 | 85 |
| BCFA | 4.98 | 2.32 | 7.97 | 0.35 |
| MUFA | 1054 | 380 | 1877 | 93 |
| cis-MUFA | 1051 | 378 | 1872 | 92 |
| trans-MUFA | 3.32 | 1.07 | 5.42 | 0.29 |
| CLA (18:2) | 2.75 | 1.42 | 4.36 | 0.21 |
| NC-dienes (18:2) | 1.40 | 0.814 | 2.91 | 0.11 |
| Trienes (18:3) | 0.522 | 0.105 | 1.14 | 0.05 |
| PUFA | 345 | 147 | 546 | 22 |
| n-6 | 266 | 113 | 390 | 15 |
| 18:2n-6 | 231 | 96 | 350 | 14 |
| n-3 | 78 | 25 | 177 | 8 |
| 18:3n-3 | 56.1 | 15.6 | 154 | 8 |
| DMA | 60.3 | 26.6 | 84.7 | 3.3 |
| Muscle | Subcutaneous | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Mean | Min | Max | SEM | Mean | SEM | ||
| SFA | 38.9 | 36.8 | 43.8 | 0.3 | 39.5 | 0.5 | 0.383 |
| 12:0 | 0.206 | 0.151 | 0.256 | 0.006 | 0.224 | 0.008 | 0.079 |
| 14:0 | 3.65 | 3.00 | 4.58 | 0.09 | 4.33 | 0.09 | <0.001 |
| 15:0 | 0.274 | 0.186 | 0.429 | 0.015 | 0.410 | 0.017 | <0.001 |
| 16:0 | 29.3 | 26.8 | 31.3 | 0.3 | 29.9 | 0.4 | 0.256 |
| 17:0 | 0.357 | 0.238 | 0.489 | 0.015 | 0.505 | 0.020 | <0.001 |
| 18:0 | 4.79 | 4.38 | 5.50 | 0.09 | 3.85 | 0.14 | <0.001 |
| 20:0 | 0.0590 | 0.0450 | 0.0700 | 0.0020 | 0.0496 | 0.0017 | <0.001 |
| 22:0 | 0.0665 | 0.0370 | 0.106 | 0.0039 | 0.0138 | 0.0018 | <0.001 |
| BCFA | 0.211 | 0.176 | 0.260 | 0.006 | 0.285 | 0.012 | <0.001 |
| i-16:0 | 0.0890 | 0.068 | 0.107 | 0.0023 | 0.0844 | 0.0037 | 0.297 |
| Muscle | Subcutaneous | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Min | Max | SEM | Mean | SEM | |||
| MUFA | 42.9 | 37.6 | 47.7 | 0.6 | 44.3 | 0.4 | 0.074 | |
| cis-MUFA | 42.8 | 37.4 | 47.5 | 0.6 | 44.2 | 0.4 | 0.076 | |
| 9c-14:1 | 0.412 | 0.297 | 0.511 | 0.013 | 0.389 | 0.016 | 0.295 | |
| 7c-16:1 | 0.182 | 0.138 | 0.272 | 0.008 | 0.328 | 0.014 | <0.001 | |
| 9c-16:1 | 8.09 | 5.602 | 9.693 | 0.27 | 7.58 | 0.23 | 0.174 | |
| 9c-18:1 | 31.4 | 27.4 | 34.1 | 0.4 | 32.7 | 0.4 | 0.025 | |
| 11c-18:1 | 1.93 | 1.57 | 2.22 | 0.04 | 1.51 | 0.03 | <0.001 | |
| 13c-18:1 | 0.0965 | 0.0740 | 0.119 | 0.0027 | 0.0531 | 0.0017 | <0.001 | |
| 11c-19:1 | 0.0675 | 0.0491 | 0.0880 | 0.0027 | 0.0738 | 0.0042 | 0.201 | |
| 11c-20:1 | 0.335 | 0.283 | 0.416 | 0.008 | 0.470 | 0.016 | <0.001 | |
| trans-MUFA | 0.136 | 0.101 | 0.237 | 0.006 | 0.149 | 0.005 | 0.143 | |
| 9t-18:1 | 0.0970 | 0.0680 | 0.117 | 0.0026 | 0.0625 | 0.0026 | <0.001 | |
| Muscle | Subcutaneous | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Mean | Min | Max | SEM | Mean | SEM | ||
| CLA (18:2) | 0.118 | 0.0884 | 0.198 | 0.007 | 0.0931 | 0.0050 | 0.008 |
| 9c,11t- | 0.0535 | 0.0394 | 0.0712 | 0.0022 | 0.0581 | 0.0043 | 0.318 |
| NC-dienes (18:2) | 0.0605 | 0.0372 | 0.1002 | 0.0033 | 0.0475 | 0.0030 | 0.008 |
| Trienes (18:3) | 0.0220 | 0.0073 | 0.0371 | 0.0016 | 0.0263 | 0.0020 | 0.099 |
| PUFA | 15.0 | 11.1 | 21.6 | 0.7 | 15.6 | 0.5 | 0.455 |
| 20:3n-9 | 0.0263 | 0.0174 | 0.0441 | 0.0019 | 0.0125 | 0.0014 | <0.001 |
| n-6 | 11.7 | 8.76 | 19.38 | 0.7 | 12.5 | 0.6 | 0.424 |
| 18:2n-6 | 10.1 | 7.55 | 16.6 | 0.6 | 12.0 | 0.5 | 0.026 |
| 20:2n-6 | 0.206 | 0.141 | 0.335 | 0.012 | 0.281 | 0.012 | <0.001 |
| 20:3n-6 | 0.240 | 0.124 | 0.387 | 0.015 | 0.0338 | 0.0022 | <0.001 |
| 20:4n-6 | 0.946 | 0.491 | 1.74 | 0.071 | 0.0631 | 0.0053 | <0.001 |
| 22:4n-6 | 0.0705 | 0.0381 | 0.160 | 0.0067 | 0.0194 | 0.0021 | <0.001 |
| 22:5n-6 | 0.0470 | 0.266 | 0.793 | 0.0054 | ND | - | - |
| n-3 | 3.19 | 2.05 | 5.31 | 0.21 | 3.18 | 0.23 | 0.975 |
| 18:3n-3 | 2.26 | 1.11 | 4.14 | 0.19 | 2.98 | 0.21 | 0.018 |
| 20:3n-3 | 0.110 | 0.0720 | 0.188 | 0.007 | 0.106 | 0.008 | 0.714 |
| 20:5n-3 | 0.125 | 0.0480 | 0.296 | 0.0134 | 0.0119 | 0.0010 | <0.001 |
| 22:5n-3 | 0.503 | 0.266 | 0.793 | 0.027 | 0.0561 | 0.0034 | <0.001 |
| 22:6n-3 | 0.115 | 0.0460 | 0.187 | 0.0084 | 0.0120 | 0.0010 | <0.001 |
| n-6/n-3 | 3.97 | 2.06 | 8.77 | 0.38 | 4.30 | 0.45 | 0.565 |
| P/S | 0.386 | 0.284 | 0.587 | 0.020 | 0.399 | 0.016 | 0.609 |
| DMA | 2.63 | 1.531 | 3.472 | 0.11 | ND | - | - |
| AT | D | AT*D | ||||
|---|---|---|---|---|---|---|
| p-Value | ɳ2 | p-Value | ɳ2 | p-Value | ɳ2 | |
| pH | < 0.001 | 0.583 | < 0.001 | 0.758 | < 0.001 | 0.796 |
| L* | < 0.001 | 0.884 | 0.001 | 0.348 | < 0.001 | 0.586 |
| a* | < 0.001 | 0.665 | 0.015 | 0.251 | 0.319 | 0.254 |
| b* | < 0.001 | 0.858 | 0.067 | 0.185 | 0.025 | 0.394 |
| C* | < 0.001 | 0.718 | 0.024 | 0.230 | 0.230 | 0.278 |
| h* | < 0.001 | 0.923 | 0.041 | 0.207 | < 0.001 | 0.558 |
| Cook loss | 0.007 | 0.246 | 0.004 | 0.297 | 0.577 | 0.200 |
| WBSF | < 0.001 | 0.926 | < 0.001 | 0.684 | 0.114 | 0.321 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beldarrain, L.R.; Morán, L.; Sentandreu, M.Á.; Insausti, K.; R. Barron, L.J.; Aldai, N. Muscle and Subcutaneous Fatty Acid Composition and the Evaluation of Ageing Time on Meat Quality Parameters of Hispano-Bretón Horse Breed. Animals 2021, 11, 1421. https://doi.org/10.3390/ani11051421
Beldarrain LR, Morán L, Sentandreu MÁ, Insausti K, R. Barron LJ, Aldai N. Muscle and Subcutaneous Fatty Acid Composition and the Evaluation of Ageing Time on Meat Quality Parameters of Hispano-Bretón Horse Breed. Animals. 2021; 11(5):1421. https://doi.org/10.3390/ani11051421
Chicago/Turabian StyleBeldarrain, Lorea R., Lara Morán, Miguel Ángel Sentandreu, Kizkitza Insausti, Luis Javier R. Barron, and Noelia Aldai. 2021. "Muscle and Subcutaneous Fatty Acid Composition and the Evaluation of Ageing Time on Meat Quality Parameters of Hispano-Bretón Horse Breed" Animals 11, no. 5: 1421. https://doi.org/10.3390/ani11051421






