Three Manual Noncommercial Methods to Prepare Equine Platelet-Rich Plasma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.2.1. Method 1
2.2.2. Method 2
2.2.3. Method 3
2.3. Cell Counts
2.4. Platelet Viability
2.5. Enrichment Factor and Platelet Recovery and Loss
2.6. Data Analyses
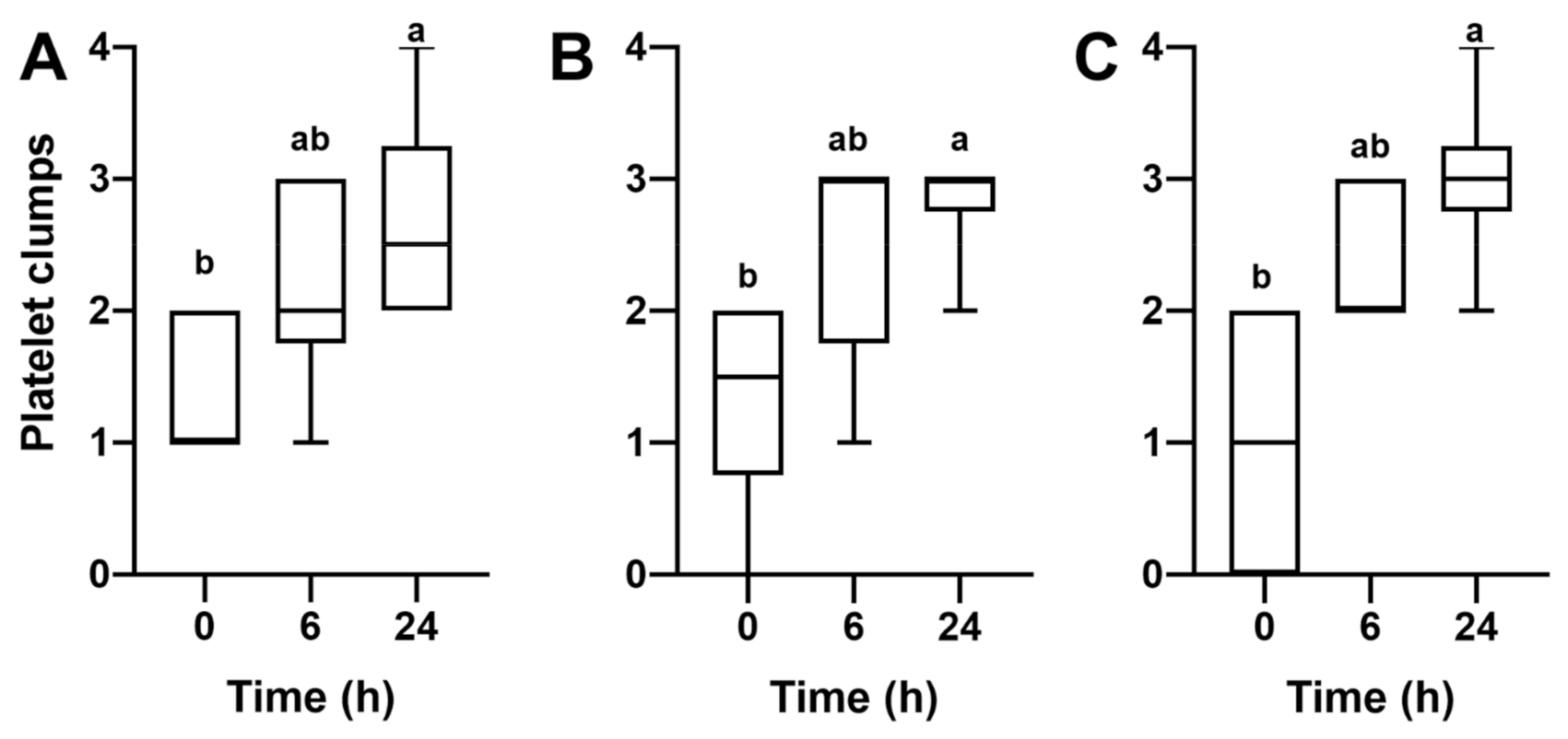
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soares, C.S.; Babo, P.S.; Reis, R.L.; Carvalho, P.P.; Gomes, M.E. Platelet-Derived Products in Veterinary Medicine: A New Trend or an Effective Therapy? Trends Biotechnol. 2020, 20, 30206–30207. [Google Scholar] [CrossRef] [PubMed]
- Bos-Mikich, A.; De Oliveira, R.; Frantz, N. Platelet-rich plasma therapy and reproductive medicine. J. Assist. Reprod. Genet. 2018, 35, 753–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segabinazzi, L.G.; Friso, A.M.; Correal, S.B.; Crespilho, A.M.; Dell’Aqua, J.A.; Miró, J.; Papa, F.O.; Alvarenga, M.A. Uterine clinical findings, fertility rate, leucocyte migration, and COX-2 protein levels in the endometrial tissue of susceptible mares treated with platelet-rich plasma before and after AI. Theriogenology 2017, 104, 120–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segabinazzi, L.G.T.M.; Canisso, I.F.; Podico, G.; Cunha , L.L.; Novello, G.; Rosser, M.F.; Loux , S.C.; Lima, F.S.; Alvarenga, M.A. Intrauterine blood plasma platelet-therapy mitigates persistent breeding-induced endometritis, reduces uterine infections, and improves embryo recovery in mares. Antibiotics 2021, 2021, 490. [Google Scholar]
- Reghini, M.F.S.; Ramires Neto, C.; Segabinazzi, L.G.; Castro Chaves, M.M.B.; Dell’Aqua, C.d.P.F.; Bussiere, M.C.C.; Dell’Aqua, J.A.; Papa, F.O.; Alvarenga, M.A. Inflammatory response in chronic degenerative endometritis mares treated with platelet-rich plasma. Theriogenology 2016, 86, 516–522. [Google Scholar] [CrossRef] [Green Version]
- Metcalf, E.S.; Scoggin, K.; Troedsson, M.H.T. The effect of platelet-rich plasma on endometrial pro-inflammatory cytokines in susceptible mares following semen deposition. J. Equine Vet. Sci. 2012, 32, 498. [Google Scholar] [CrossRef]
- Metcalf, E.S. The effect of Platelet-Rich Plasma (PRP) on intraluminal fluid and pregnancy rates in mares susceptible to persistent mating-induced endometritis (PMIE). J. Equine Vet. Sci. 2014, 34, 128. [Google Scholar] [CrossRef]
- Pasch, L.; Schmidt, A.; King, W. Clinical observations after pre breeding intrauterine plasma infusion in 18 mares inseminated with thawed frozen semen. J. Equine Vet. Sci. 2021, 99, 103389. [Google Scholar] [CrossRef]
- Bendinelli, P.; Matteucci, E.; Dogliotti, G.; Corsi, M.M.; Banfi, G.; Maroni, P.; Desiderio, M.A. Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: Mechanisms of NF-κB inhibition via HGF. J. Cell. Physiol. 2010, 225, 757–766. [Google Scholar] [CrossRef] [Green Version]
- Bos-Mikich, A.; Ferreira, M.O.; de Oliveira, R.; Frantz, N. Platelet-rich plasma or blood-derived products to improve endometrial receptivity? J. Assist. Reprod. Genet. 2019, 36, 613–620. [Google Scholar] [CrossRef] [Green Version]
- Drago, L.; Bortolin, M.; Vassena, C.; Taschieri, S.; Del Fabbro, M. Antimicrobial activity of pure platelet-rich plasma against microorganisms isolated from oral cavity. BMC Microbiol. 2013, 13, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anitua, E.; Alonso, R.; Girbau, C.; Aguirre, J.J.; Muruzabal, F.; Orive, G. Antibacterial effect of plasma rich in growth factors (PRGF®-Endoret®) against Staphylococcus aureus and Staphylococcus epidermidis strains. Clin. Exp. Dermatol. 2012, 37, 652–657. [Google Scholar] [CrossRef]
- Álvarez, M.; López, C.; Giraldo, C.; Samudio, I.; Carmona, J. In vitro bactericidal activity of equine platelet concentrates, platelet poor plasma, and plasma against methicillin-resistant Staphylococcus aureus. Arch. Med. Vet. 2011, 43, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.C.d.F.; De La Côrte, F.D.; Brass, K.E.; da Silva Azevedo, M.; Gallio, M.; Cantarelli, C.; Dau, S.L.; Cezar, A.S.; Inkelmann, M.A. Evaluation of three methods of platelet-rich plasma for treatment of equine distal limb skin wounds. J. Equine Vet. Sci. 2019, 72, 1–7. [Google Scholar] [CrossRef]
- Lee, E.-B.; Kim, J.-W.; Seo, J.-P. Comparison of the methods for platelet rich plasma preparation in horses. J. Anim. Sci. Technol. 2018, 60, 20. [Google Scholar] [CrossRef] [Green Version]
- Rinnovati, R.; Romagnoli, N.; Gentilini, F.; Lambertini, C.; Spadari, A. The influence of environmental variables on platelet concentration in horse platelet-rich plasma. Acta Vet. Scand. 2016, 58, 45. [Google Scholar] [CrossRef] [Green Version]
- Bozorgmanesh, R.; Magdesian, K.G.; Sutton-Burges, J.W.; Owens, S.D.; Tablin, F. Equine platelet concentrate preparation and validation. J. Vet. Intern. Med. 2019, 33, 1500–1506. [Google Scholar] [CrossRef]
- Hessel, L.N.; Bosch, G.; van Weeren, P.R.; Ionita, J.C. Equine autologous platelet concentrates: A comparative study between different available systems. Equine Vet. J. 2015, 47, 319–325. [Google Scholar] [CrossRef]
- Maia, L.; de Souza, M.V.; Ribeiro Júnior, J.I.; de Oliveira, A.C.; Alves, G.E.S.; dos Anjos Benjamin, L.; Silva, Y.F.R.S.; Zandim, B.M.; do Carmo LopesMoreira, J. Platelet-Rich Plasma in the Treatment of Induced Tendinopathy in Horses: Histologic Evaluation. J. Equine Vet. Sci. 2009, 29, 618–626. [Google Scholar] [CrossRef]
- Canisso, I.F.; Segabinazzi, L.G.T.M.; Fedorka, C.E. Persistent Breeding-Induced Endometritis in mares—A multifaceted challenge: From clinical aspects to immunopathogenesis and pathobiology. Int. J. Mol. Sci. 2020, 21, 1432. [Google Scholar] [CrossRef] [Green Version]
- Mack, J.P.; Miles, J.; Stolla, M. Cold-Stored Platelets: Review of Studies in Humans. Transfus. Med. Rev. 2020, 34, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. 121st Blood Products Advisory Committee Meeting. In Proceedings of the Tommy Douglas Conference Center, Silver Spring, MD, USA, 22 November 2019. [Google Scholar]
- Levy, J.H.; Neal, M.D.; Herman, J.H. Bacterial contamination of platelets for transfusion: Strategies for prevention. Crit. Care 2018, 22, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AAEP. Vaccination Guidelines; American Association of Equine Practitioners: Lexington, KY, USA, 2020. [Google Scholar]
- AAEP. Internal Parasite Control Guidelines; American Association of Equine Practitioners: Lexington, KY, USA, 2020. [Google Scholar]
- Pennell, E.N.; Wagner, K.H.; Mosawy, S.; Bulmer, A.C. Acute bilirubin ditaurate exposure attenuates ex vivo platelet reactive oxygen species production, granule exocytosis and activation. Redox Biol. 2019, 26, 101250. [Google Scholar] [CrossRef]
- O’Shea, C.M.; Werre, S.R.; Dahlgren, L.A. Comparison of Platelet Counting Technologies in Equine Platelet Concentrates. Vet. Surg. 2015, 44, 304–313. [Google Scholar] [CrossRef] [Green Version]
- Argüelles, D.; Carmona, J.U.; Pastor, J.; Iborra, A.; Viñals, L.; Martínez, P.; Bach, E.; Prades, M. Evaluation of single and double centrifugation tube methods for concentrating equine platelets. Res. Vet. Sci. 2006, 81, 237–245. [Google Scholar] [CrossRef]
- Pichereau, F.; Décory, M.; Cuevas Ramos, G. Autologous platelet concentrate as a treatment for horses with refractory fetlock osteoarthritis. J. Equine Vet. Sci. 2014, 34, 489–493. [Google Scholar] [CrossRef]
- Textor, J.A.; Tablin, F. Intra-articular use of a platelet-rich product in normal horses: Clinical signs and cytologic responses. Vet. Surg. 2013, 42, 499–510. [Google Scholar] [CrossRef]
- Tyrnenopoulou, P.; Diakakis, N.; Karayannopoulou, M.; Savvas, I.; Koliakos, G. Evaluation of intra-articular injection of autologous platelet lysate (PL) in horses with osteoarthritis of the distal interphalangeal joint. Vet. Q. 2016, 36, 56–62. [Google Scholar] [CrossRef]
- Romero, A.; Barrachina, L.; Ranera, B.; Remacha, A.R.; Moreno, B.; de Blas, I.; Sanz, A.; Vázquez, F.J.; Vitoria, A.; Junquera, C.; et al. Comparison of autologous bone marrow and adipose tissue derived mesenchymal stem cells, and platelet rich plasma, for treating surgically induced lesions of the equine superficial digital flexor tendon. Vet. J. 2017, 224, 76–84. [Google Scholar] [CrossRef]
- Carmona, J.U.; López, C. Autologous platelet concentrates as a treatment for shoulder injury in a horse. J. Equine Vet. Sci. 2011, 31, 506–510. [Google Scholar] [CrossRef]
- Carmona, J.U.; López, C.; Samudio, I.J. Autologous platelet concentrates as an adjunctive treatment for chronic laminitis in a mare with pituitary pars intermedia dysfunction. J. Equine Vet. Sci. 2013, 33, 191–195. [Google Scholar] [CrossRef]
- López, C.; Carmona, J.U. Platelet-rich plasma as an adjunctive therapy for the management of a severe chronic distal limb wound in a foal. J. Equine Vet. Sci. 2014, 34, 1128–1133. [Google Scholar] [CrossRef]
- Mirza, M.H.; Bommala, P.; Richbourg, H.A.; Rademacher, N.; Kearney, M.T.; Lopez, M.J. Gait changes vary among horses with naturally occurring osteoarthritis following intra-articular administration of autologous platelet-rich plasma. Front. Vet. Sci. 2016, 3, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, T.E.; Puskas, B.L.; Mandelbaum, B.R.; Gerhardt, M.B.; Rodeo, S.A. Platelet-rich plasma: From basic science to clinical applications. Am. J. Sports Med. 2009, 37, 2259–2272. [Google Scholar] [CrossRef]
- Argüelles, D.; Carmona, J.U.; Climent, F.; Muñoz, E.; Prades, M. Autologous platelet concentrates as a treatment for musculoskeletal lesions in five horses. Vet. Rec. 2008, 162, 208–211. [Google Scholar] [CrossRef]
- Carmona, J.U.; Argüelles, D.; Climent, F.; Prades, M. Autologous platelet concentrates as a treatment of horses with osteoarthritis: A preliminary pilot clinical study. J. Equine Vet. Sci. 2007, 27, 167–170. [Google Scholar] [CrossRef]
- Scoggin, C.F. Not just a number: Effect of age on fertility, pregnancy and offspring vigour in thoroughbred brood-mares. Reprod. Fertil. Dev. 2015, 27, 872–879. [Google Scholar] [CrossRef]
- Woodward, E.M.; Christoffersen, M.; Campos, J.; Squires, E.L.; Troedsson, M.H.T. Susceptibility to persistent breeding-induced endometritis in the mare: Relationship to endometrial biopsy score and age, and variations between seasons. Theriogenology 2012, 78, 495–501. [Google Scholar] [CrossRef]
- Bozorgmanesh, R.; Sutton-Burges, J.W.; Tablin, F. Comparison of equine platelet function and survival in whole blood collected in acid-citrate-dextrose solution or citrate-phosphate-dextrose-adenine solution. Vet. Clin. Pathol. 2017, 46, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Giraldo, C.E.; Álvarez, M.E.; Carmona, J.U. Effects of sodium citrate and acid citrate dextrose solutions on cell counts and growth factor release from equine pure-platelet rich plasma and pure-platelet rich gel. BMC Vet. Res. 2015, 11, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gottschall, J.L.; Johnston, V.L.; Rzad, L.; Anderson, A.J.; Aster, R.H. Importance of White Blood Cells in Platelet Storage. Vox Sang. 1984, 47, 101–107. [Google Scholar] [CrossRef]
- Andrade Junior, L.R.P.; Segabinazzi, L.G.T.M.; Oliveira, S.N.; Dell’Aqua, J.A.; Papa, F.O. An approach to rescue the fertility of stallions with a high level of hemospermia. Reprod. Domest. Anim. 2020, 1–5. [Google Scholar] [CrossRef]
- Neves, A.P.; Keller, A.; Trein, C.R.; Möller, G.; Jobim, M.I.M.; Castilho, L.F.F.; de Itapema Cardoso, M.R.; Leibold, W.; Zerbe, H.; Klug, E.; et al. Use of leukocytes as treatment for endometritis in mares experimentally infected with Streptococcus equi subsp. zooepidemicus. Anim. Reprod. Sci. 2007, 97, 314–322. [Google Scholar] [CrossRef]
- McCarrel, T.; Fortier, L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J. Orthop. Res. 2009, 27, 1033–1042. [Google Scholar] [CrossRef]
- Everts, P.A.M.; Hoffmann, J.; Weibrich, G.; Mahoney, C.B.; Schönberger, J.P.A.M.; Van Zundert, A.; Knape, J.T.A. Differences in platelet growth factor release and leucocyte kinetics during autologous platelet gel formation. Transfus. Med. 2006, 16, 363–368. [Google Scholar] [CrossRef]
- Dragoo, J.L.; Braun, H.J.; Durham, J.L.; Ridley, B.A.; Odegaard, J.I.; Luong, R.; Arnoczky, S.P. Comparison of the acute inflammatory response of two commercial platelet-rich plasma systems in healthy rabbit tendons. Am. J. Sports Med. 2012, 40, 1274–1281. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelet concentrates? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101. [Google Scholar] [CrossRef] [PubMed]
- Boswell, S.G.; Schnabel, L.V.; Mohammed, H.O.; Sundman, E.A.; Minas, T.; Fortier, L.A. Increasing platelet concentrations in leukocyte-reduced platelet-rich plasma decrease collagen gene synthesis in tendons. Am. J. Sports Med. 2014, 42, 42–49. [Google Scholar] [CrossRef]
- Carmona, J.U. Use of Autologous Platelet Concentrates for the Treatment Of Musculoskeletal Injuries in the Horse. Ph.D. Thesis, Universitat Autònoma de Barcelona, Bellaterra, Spain, 2006. [Google Scholar]
- Brinsko, S.P.; Varner, D.D.; Blanchard, T.L. The effect of uterine lavage performed four hours post insemination on pregnancy rate in mares. Theriogenology 1991, 35, 1111–1119. [Google Scholar] [CrossRef]
- Currie, L.M.; Harper, J.R.; Allan, H.; Connor, J. Inhibition of cytokine accumulation and bacterial growth during storage of platelet concentrates at 4 °C with retention of in vitro functional activity. Transfusion 1997, 37, 18–24. [Google Scholar] [CrossRef]
- Kwirant, L.A.d.A.; De La Corte, F.D.; Cantarelli, C.; Cargnelutti, J.F.; Martins, M.; Cabral, M.W.; Maciel, N.; Rubin, M.I.B. Cooling and cryopreservation of equine platelet-rich plasma with dimethyl sulfoxide and trehalose. J. Equine Vet. Sci. 2019, 72, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Handigund, M.; Bae, T.W.; Lee, J.; Cho, Y.G. Evaluation of in vitro storage characteristics of cold stored platelet concentrates with N acetylcysteine (NAC). Transfus. Apher. Sci. 2016, 54, 127–138. [Google Scholar] [CrossRef] [PubMed]



| Mare | Age | Breed | Weight (kg) | Blood Counts | ||
|---|---|---|---|---|---|---|
| Platelets (×103/µL) | White Blood Count (×103/µL) | Red Blood Count (×106/µL) | ||||
| 1 | 5 | Quarter Horse | 480 | 120 | 7.0 | 7.2 |
| 2 | 6 | Quarter Horse | 470 | 116 | 6.6 | 6.5 |
| 3 | 9 | Thoroughbred | 540 | 125 | 5.0 | 6.4 |
| 4 | 8 | Arabian | 400 | 150 | 5.2 | 7.6 |
| 5 | 12 | Paint Horse | 520 | 152 | 6.0 | 7.8 |
| 6 | 6 | Paint Horse | 500 | 168 | 5.2 | 8.1 |
| 7 | 9 | Standardbred | 500 | 130 | 5.5 | 8.1 |
| 8 | 8 | Standardbred | 580 | 124 | 6.9 | 9.3 |
| 9 | 12 | Standardbred | 600 | 120 | 6.0 | 6.8 |
| 10 | 8 | Quarter Horse | 450 | 135 | 5.5 | 7.2 |
| 11 | 13 | Thoroughbred | 500 | 102 | 5.0 | 6.2 |
| 12 | 7 | Arabian | 400 | 118 | 6.5 | 6.7 |
| * Subset (n = 6) | ||||||
| 13 | 16 | Quarter Horse | 480 | 172 | 5.1 | 6.1 |
| 14 | 14 | Quarter Horse | 520 | 112 | 5.6 | 7.5 |
| 15 | 10 | Standardbred | 580 | 106 | 5.4 | 6.8 |
| 16 | 11 | Arabian | 430 | 100 | 5.3 | 6.5 |
| 17 | 7 | Saddlebred | 510 | 138 | 5.9 | 6.1 |
| 18 | 8 | Arabian | 400 | 124 | 6.1 | 6.3 |
| Concentration (×103/µL) | Viability (%) | Enrichment Factor (%) | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |||
| Method 1 | WB | 125.8 ± 27.6 d | 92–188 | 94.5 ± 10 | 60–99 | - |
| F-C | 200.6 ± 60.2 c | 110–345 | - | - | 1.59 | |
| PPP | 44.2 ± 31.1 e | 15–130 | 85.9 ± 15 | 57–98 | 0.35 | |
| PRP | 709.4 ± 159.8 a | 425–960 | 92.5 ± 8 | 72–99 | 5.64 | |
| Method 2 | WB | 130.0 ± 18.4 d | 102–168 | 87.9 ± 16 | 50–99 | - |
| PPP | 62.3 ± 23.7 e | 40–125 | 69.6 ± 24 | 53–99 | 0.37 | |
| PRP | 327.5 ± 64.2 b | 230–460 | 87.5 ± 15 | 55–98 | 2.52 | |
| Method 3 | WB | 129.6 ± 238 d | 80–172 | 85.6 ± 15 | 52–99 | - |
| PPP | 105.9 ± 44.0 d | 45–180 | 84.1 ± 17 | 58–99 | 0.82 | |
| PRP | 239.4 ± 64.5 c | 180–435 | 86.8 ± 15 | 59–99 | 1.85 | |
| White Blood Cell | Red Blood Cell | ||||||
|---|---|---|---|---|---|---|---|
| Concentration (×103/µL) | Enrichment Factor (%) | Concentration (×106/µL) | Enrichment Factor (%) | ||||
| Mean ± SD | Range | Mean ± SD | Range | ||||
| Method 1 | WB | 5.9 ± 1.0 a | 4.4–7.1 | - | 6.4 ± 8.7 a | 5.1–7.8 | - |
| F-C | 0.043 ± 0.018 bc | 0.02–0.08 | 0.007 | 0.058 ± 0.05 b | 0.02–0.1 | 0.009 | |
| PPP | 0.014 ± 0.009 c | 0.01–0.04 | 0.002 | 0.020 ± 0.02 b | 0.01–0.07 | 0.003 | |
| PRP | 0.027 ± 0.017 c | 0.01–0.06 | 0.005 | 0.042 ± 0.04 b | 0.03–0.1 | 0.007 | |
| Method 2 | WB | 5.7 ± 1.2 a | 4.5–7.0 | - | 7.3 ± 8.9 a | 6.2–9.3 | - |
| PPP | 0.04 ± 0.03 c | 0.01–0.08 | 0.007 | 0.069 ± 0.07 b | 0.06–0.2 | 0.009 | |
| PRP | 0.05 ± 0.04 c | 0.01–0.2 | 0.009 | 0.083 ± 0.07 b | 0.02–0.2 | 0.011 | |
| Method 3 | WB | 5.5 ± 0.9 a | 4.3–7.2 | - | 6.4 ± 7.4 a | 4770–7500 | - |
| PPP | 0.8 ± 1.1 b | 10–3180 | 0.15 | 0.13 ± 0.12 b | 22–400 | 0.020 | |
| PRP | 1.9 ± 1.7 b | 360–4740 | 0.35 | 0.11 ± 0.1 b | 4–305 | 0.017 | |
| Time (h) | Platelet (×103/µL) | Platelet Viability (%) | WBC (×103/µL) | RBC (×106/µL) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | ||
| Method 1 | 0 | 587.8 ± 133 | 455–825 | 81.7 ± 9 | 70–96 | 2.58 ± 3.2 | 0.5–5.0 | 0.04 ± 0.03 | 0.008–0.10 |
| 6 | 510.3 ± 102 | 392–650 | 55.2 ± 12 | 39–74 | 1.25 ± 1.4 | 0.5–4.0 | 0.05 ± 0.03 | 0.02–0.09 | |
| 24 | 495.7 ± 74 | 412–582 | 52.3 ± 13 | 32–72 | 0.75 ± 0.3 | 0.5–1.0 | 0.04 ± 0.02 | 0.02–0.07 | |
| Method 2 | 0 | 508.7 ± 64 | 450–625 | 85.9 ± 9 | 82–98 | 2.83 ± 1.6 | 1.0–5.0 | 0.20 ± 0.30 | 0.03–0.73 |
| 6 | 474.5 ± 102 | 400–615 | 61.1 ± 15 | 36–88 | 2.83 ± 1.3 | 2.0–5.0 | 0.10 ± 0.07 | 0.04–0.19 | |
| 24 | 448.2 ± 74 | 375–575 | 54.6 ± 19 | 27–83 | 1.25 ± 0.6 | 0.5–2.0 | 0.07 ± 0.05 | 0.03–0.15 | |
| Method 3 | 0 | 451.2 ± 116 | 365–610 | 89.3 ± 6 a | 74–98 | 47.17 ± 25.9 | 22.0–85.0 | 0.12 ± 0.11 | 0.03–0.31 |
| 6 | 424.5 ± 94 | 330–590 | 60.1 ± 19 ab | 36–79 | 48.17 ± 19.0 | 22.0–67.0 | 0.12 ± 0.10 | 0.03–0.27 | |
| 24 | 368.7 ± 33 | 320–417 | 46.9 ± 21 b | 26–78 | 36.50 ± 26.4 | 11.0–88.0 | 0.14 ± 0.13 | 0.01–0.35 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segabinazzi, L.G.T.M.; Podico, G.; Rosser, M.F.; Nanjappa, S.G.; Alvarenga, M.A.; Canisso, I.F. Three Manual Noncommercial Methods to Prepare Equine Platelet-Rich Plasma. Animals 2021, 11, 1478. https://doi.org/10.3390/ani11061478
Segabinazzi LGTM, Podico G, Rosser MF, Nanjappa SG, Alvarenga MA, Canisso IF. Three Manual Noncommercial Methods to Prepare Equine Platelet-Rich Plasma. Animals. 2021; 11(6):1478. https://doi.org/10.3390/ani11061478
Chicago/Turabian StyleSegabinazzi, Lorenzo G. T. M., Giorgia Podico, Michael F. Rosser, Som G. Nanjappa, Marco A. Alvarenga, and Igor F. Canisso. 2021. "Three Manual Noncommercial Methods to Prepare Equine Platelet-Rich Plasma" Animals 11, no. 6: 1478. https://doi.org/10.3390/ani11061478
APA StyleSegabinazzi, L. G. T. M., Podico, G., Rosser, M. F., Nanjappa, S. G., Alvarenga, M. A., & Canisso, I. F. (2021). Three Manual Noncommercial Methods to Prepare Equine Platelet-Rich Plasma. Animals, 11(6), 1478. https://doi.org/10.3390/ani11061478






