The Effect of Horse Shoeing with Egg Bar Shoes and Shoes with Wedge Pads on the Results of Thermal Imaging of the Equine Distal Limb
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
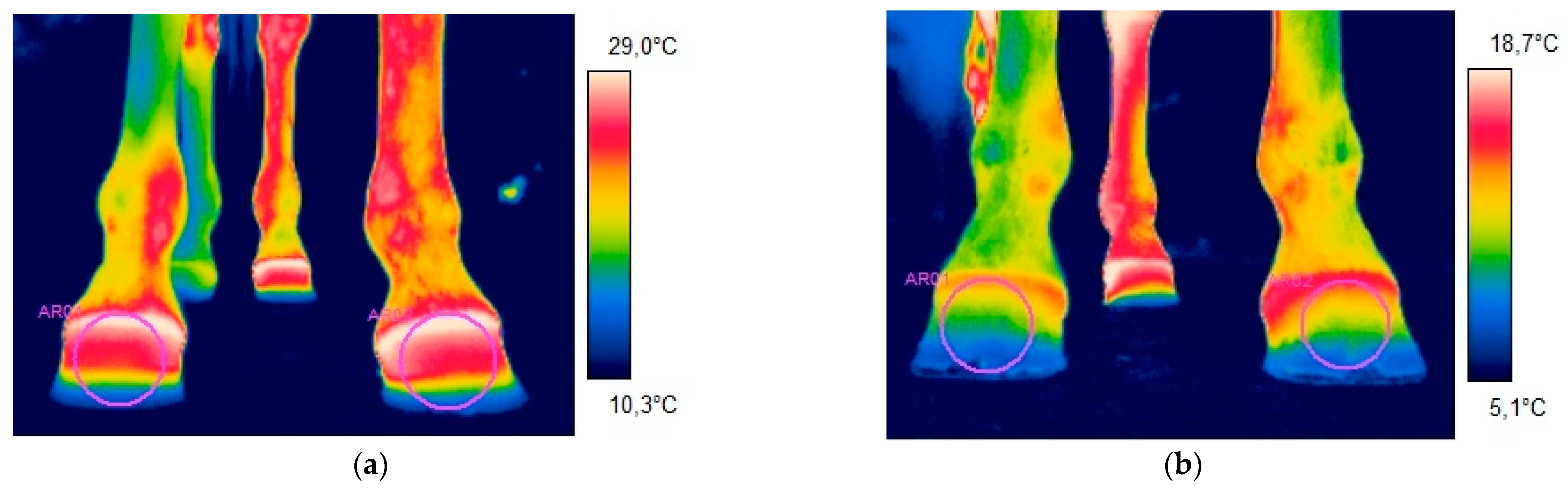
- Group 1—eight horses; the first thermographic examination was performed before shoeing. The animals were then shod with egg bar shoes using the cold technique. Thermography was repeated after a month;
- Group 2—eight horses; the first thermographic examination was performed before shoeing. Subsequently, the horses were shod with wedge pads using the cold technique. After a monthly period of adaptation, thermography was repeated.
3. Results
3.1. Group 1
3.2. Group 2
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Proske, D.K.; Leatherwood, J.L.; Anderson, M.J.; Stutts, K.J.; Hammer, C.J.; Coverdale, J. 0799 Effects of barefoot trimming and shoeing on the lower forelimb: Hoof morphology. J. Anim. Sci. 2016, 94, 384. [Google Scholar] [CrossRef]
- Thomason, J.J. Variation in surface strain on the equine hoof wall at the midstep with shoeing, gait, substrate, direction of travel, and hoof shape. Equine Veter. J. 2010, 30, 86–95. [Google Scholar] [CrossRef]
- Hinterhofer, C.; Stanek, C.; Haider, H. Finite element analysis (FEA) as a model to predict effects of farriery on the equine hoof. Equine Veter. J. 2001, 33, 58–62. [Google Scholar] [CrossRef]
- Roepstorff, L.; Johnston, C.; Drevemo, S. In vivo and in vitro heel expansion in relation to shoeing and frog pressure. Equine Veter. J. 2001, 33, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, E.; Takahashi, T.; Otsuka, N.; Isayama, T.; Tomiyama, T.; Hiraga, A.; Wada, S. Heel movement in horses: Comparison between glued and nailed horse shoes at different speeds. Equine Veter. J. 2010, 42, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Senderska-Płonowska, M.; Zielińska, P.; Żak, A.; Stefaniak, T. Do Metal Shoes Contract Heels?—A Retrospective Study on 114 Horses. J. Equine Veter. Sci. 2020, 95, 103293. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.A. Thermography as an Aid to the Clinical Lameness Evaluation. Veter. Clin. North Am. Equine Pr. 1991, 7, 311–338. [Google Scholar] [CrossRef]
- Ciutacu, O.; Tanase, O.; Miclaus, I. Digital infrared thermography in assessing soft tissues injuries on sport equines. Bull. Univ. Agri. Sci. Vet. 2006, 63, 228–233. [Google Scholar]
- Mogg, K.C.; Pollitt, C.C. Hoof and distal limb surface temperature in the normal pony under constant and changing ambient temperatures. Equine Veter. J. 1992, 24, 134–139. [Google Scholar] [CrossRef]
- Eddy, A.; Van Hoogmoed, L.; Snyder, J. The Role of Thermography in the Management of Equine Lameness. Veter. J. 2001, 162, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Soroko, M.; Henklewski, R.; Filipowski, H.; Jodkowska, E. The Effectiveness of Thermographic Analysis in Equine Orthopedics. J. Equine Veter. Sci. 2013, 33, 760–762. [Google Scholar] [CrossRef]
- Collins, A.J.; Ring, F.; Bacon, P.A.; Brookshaw, J.D. Thermography and radiology complimentary methods for the study of inflammatory diseases. Clin. Radiol. 1976, 27, 237–243. [Google Scholar] [CrossRef]
- Denoix, J.-M.D. Spinal Biomechanics and Functional Anatomy. Veter. Clin. N. Am. Equine Pr. 1999, 15, 27–60. [Google Scholar] [CrossRef]
- Turner, T.A.; Waldsmith, J.K.; Wilson, J.H. How to assess saddle fit in horses. Proc. Am. Assoc. Equine Pract. 2004, 50, 196–201. [Google Scholar]
- Arruda, T.Z.; Brass, K.E.; De La Corte, F.D. Thermographic Assessment of Saddles Used on Jumping Horses. J. Equine Veter. Sci. 2011, 31, 625–629. [Google Scholar] [CrossRef]
- Soroko, M.; Cwynar, P.; Howell, K.; Yarnell, K.; Dudek, K.; Zaborski, D. Assessment of Saddle Fit in Racehorses Using Infrared Thermography. J. Equine Veter. Sci. 2018, 63, 30–34. [Google Scholar] [CrossRef]
- Ahern, T. Reflex sympathetic dystrophy syndrome (RSDS), complex regional pain syndrome-type 1 (CRPS 1), neuropathic pain: An equine perspective. J. Equine Veter. Sci. 1996, 16, 463–468. [Google Scholar] [CrossRef]
- McQueen, E.K.; Urban, S.E.; McQueen, M.T. Equine Performance and Autonomic Nervous System Improvement After Joint Manipulation: A Case Study. J. Equine Veter. Sci. 2017, 56, 80–87. [Google Scholar] [CrossRef]
- Levet, T.; Martens, A.; Devisscher, L.; Duchateau, L.; Bogaert, L.; Vlaminck, L. Distal limb cast sores in horses: Risk factors and early detection using thermography. Equine Veter. J. 2009, 41, 18–23. [Google Scholar] [CrossRef]
- Cetinkya, M.A.; Demirutku, A. Thermography in the assessment of equine lameness. Turk. J. Vet. Anim. Sci. 2012, 36, 43–48. [Google Scholar] [CrossRef]
- Dai, F.; Cogi, N.H.; Heinzl, E.U.L.; Costa, E.D.; Canali, E.; Minero, M. Validation of a fear test in sport horses using infrared thermography. J. Veter. Behav. 2015, 10, 128–136. [Google Scholar] [CrossRef]
- Redaelli, V.; Luzi, F.; Mazzola, S.; Bariffi, G.D.; Zappaterra, M.; Costa, L.N.; Padalino, B. The Use of Infrared Thermography (IRT) as Stress Indicator in Horses Trained for Endurance: A Pilot Study. Animals 2019, 9, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hoogmoed, L.M.; Snyder, J.R. Use of infrared thermography to detect injections and palmar digital neurectomy in horses. Veter. J. 2002, 164, 129–141. [Google Scholar] [CrossRef]
- Maśko, M.; Zdrojkowski, L.; Domino, M.; Jasinski, T.; Gajewski, Z. The Pattern of Superficial Body Temperatures in Leisure Horses Lunged with Commonly Used Lunging Aids. Animals 2019, 9, 1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, C.W.; Back, W. Wedge and Eggbar Shoes Change the Pressure Distribution Under the Hoof of the Forelimb in the Square Standing Horse. J. Equine Veter. Sci. 2003, 23, 306–309. [Google Scholar] [CrossRef]
- Hall, L.; Clarke, K.; Trim, C. Principles of sedation, analgesia and premedication. In Veterinary Anaesthesia; Elsevier BV: Amsterdam, The Netherlands, 2001; pp. 75–112. [Google Scholar]
- Soroko, M.; Howell, K.; Dudek, K. The effect of ambient temperature on infrared thermographic images of joints in the distal forelimbs of healthy racehorses. J. Therm. Biol. 2017, 66, 63–67. [Google Scholar] [CrossRef]
- Gloster, J.; Ebert, K.; Gubbins, S.; Bashiruddin, J.; Paton, D.J. Normal variation in thermal radiated temperature in cattle: Implications for foot-and-mouth disease detection. BMC Veter. Res. 2011, 7, 73. [Google Scholar] [CrossRef] [Green Version]
- Heel, M.C.V.; Moleman, M.; Barneveld, A.; Weeren, P.R.; Back, W. Changes in location of centre of pressure and hoof-unrollment pattern in relation to an 8-week shoeing interval in the horse. Equine Veter. J. 2010, 37, 536–540. [Google Scholar] [CrossRef]
- Thompson, K.; Cheung, T.; Silverman, M. The effect of toe angle on tendon, ligament and hoof wall strains in vitro. J. Equine Veter. Sci. 1993, 13, 651–654. [Google Scholar] [CrossRef]
- Willemen, M.A.; Savelberg, H.H.C.M.; Barneveld, A. The effect of orthopaedic shoeing on the force exerted by the deep digital flexor tendon on the navicular bone in horses. Equine Veter. J. 1999, 31, 25–30. [Google Scholar] [CrossRef]
- Østblom, L.C.; Lund, C.; Melsen, F. Navicular bone disease: Results of treatment using egg-bar shoeing technique. Equine Veter. J. 1984, 16, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Kummer, M.; Geyer, H.; Imboden, I.; Auer, J.; Lischer, C. The effect of hoof trimming on radiographic measurements of the front feet of normal Warmblood horses. Veter. J. 2006, 172, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Page, B.T.; Hagen, T.L. Breakover of the hoof and its effect on stuctures and forces within the foot. J. Equine Veter. Sci. 2002, 22, 258–264. [Google Scholar] [CrossRef]
- Willemen, M.A.; Lanovaz, J.L.; Schamhardt, H.C.; Clayton, H. Effects of a Heel Wedge in Horses with Superficial Digital Flexor Tendinitis. Veter. Comp. Orthop. Traumatol. 2000, 13, 01–08. [Google Scholar] [CrossRef]
- Ritmeester, A.M.; Blevins, W.E.; Ferguson, D.W.; Adams, S.B. Digital perfusion, evaluated scintigraphically, and hoof wall growth in horses with chronic laminitis treated with egg bar-heart bar shoeing and coronary grooving. Equine Veter. J. 2010, 30, 111–118. [Google Scholar] [CrossRef] [PubMed]
| Table | N | Median Barefoot/Shod | Mean Barefoot/Shod | Standard Deviation Barefoot/Shod | Minimum Barefoot/Shod | Maximum Barefoot/Shod |
|---|---|---|---|---|---|---|
| Avg, dorsal | 8 | 22.4/15.2 | 19.56/13.68 | 6.81/7.8 | 8.1/2.75 | 25.2/23.0 |
| Min, dorsal | 8 | 13.7/7.9 | 11.61/9.37 | 4.73/10.52 | 3.5/5.75 | 15.15/13.0 |
| Max, dorsal | 8 | 27.75/24.25 | 24.7/23.02 | 7.13/6.21 | 13.3/13.15 | 30.45/29.8 |
| Avg, palmar | 8 | 22.25/19.22 | 18.57/17.71 | 9.59/5.21 | 3.63/11.8 | 27.45/23.57 |
| Min, palmar | 8 | 14.07/7.8 | 15.12/7.9 | 8.26/2.86 | 4.47/5.3 | 30.0/12.6 |
| Max, palmar | 8 | 27.26/25.72 | 25.92/24.12 | 6.41/5.92 | 14.12/15.02 | 31.82/29.5 |
| Table | N | Median Barefoot/Shod | Mean Barefoot/Shod | Standard Deviation Barefoot/Shod | Minimum Barefoot/Shod | Maximum Barefoot/Shod |
|---|---|---|---|---|---|---|
| Avg, dorsal | 8 | 22.95/20.4 | 20.94/19.27 | 5.17/2.89 | 8.7/12.3 | 25.2/21.55 |
| Min, dorsal | 8 | 13/9.12 | 12.09/9.22 | 2.92/1.96 | 4.5/5.05 | 14.4/12.55 |
| Max, dorsal | 8 | 29.02/27.42 | 26.3/26.44 | 6.53/3.47 | 11.3/17.1 | 31/28.95 |
| Avg, palmar | 8 | 25.58/22.23 | 23.79/21.53 | 5.47/2.82 | 10.55/14.6 | 28.07/23.9 |
| Min, palmar | 8 | 12.81/7.72 | 12.69/7.25 | 2.65/1.86 | 6.5/3.87 | 16.5/9.97 |
| Max, palmar | 8 | 30.16/28.31 | 28.28/28.11 | 5.45/2.72 | 14.12/21.52 | 31.85/30.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mieszkowska, M.; Adamiak, Z.; Holak, P.; Głodek, J.; Jastrzębska, E.; Wolińska, K.; Mieszkowski, M. The Effect of Horse Shoeing with Egg Bar Shoes and Shoes with Wedge Pads on the Results of Thermal Imaging of the Equine Distal Limb. Animals 2021, 11, 1479. https://doi.org/10.3390/ani11061479
Mieszkowska M, Adamiak Z, Holak P, Głodek J, Jastrzębska E, Wolińska K, Mieszkowski M. The Effect of Horse Shoeing with Egg Bar Shoes and Shoes with Wedge Pads on the Results of Thermal Imaging of the Equine Distal Limb. Animals. 2021; 11(6):1479. https://doi.org/10.3390/ani11061479
Chicago/Turabian StyleMieszkowska, Marta, Zbigniew Adamiak, Piotr Holak, Joanna Głodek, Ewa Jastrzębska, Katarzyna Wolińska, and Marcin Mieszkowski. 2021. "The Effect of Horse Shoeing with Egg Bar Shoes and Shoes with Wedge Pads on the Results of Thermal Imaging of the Equine Distal Limb" Animals 11, no. 6: 1479. https://doi.org/10.3390/ani11061479
APA StyleMieszkowska, M., Adamiak, Z., Holak, P., Głodek, J., Jastrzębska, E., Wolińska, K., & Mieszkowski, M. (2021). The Effect of Horse Shoeing with Egg Bar Shoes and Shoes with Wedge Pads on the Results of Thermal Imaging of the Equine Distal Limb. Animals, 11(6), 1479. https://doi.org/10.3390/ani11061479






