The Influence of Chicken Egg Lysozyme or Zinc-Bacitracin Antibiotic on the Growth Performance, Antibacterial Capacity, Blood Profiles, and Antioxidative Status of Rabbits: A Comparative Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Microbial Enumerations
2.3. Blood Sampling and Biochemistry Examination
2.4. Gene Expression
2.5. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Cecum Microbial Load
3.3. Blood Biochemistry
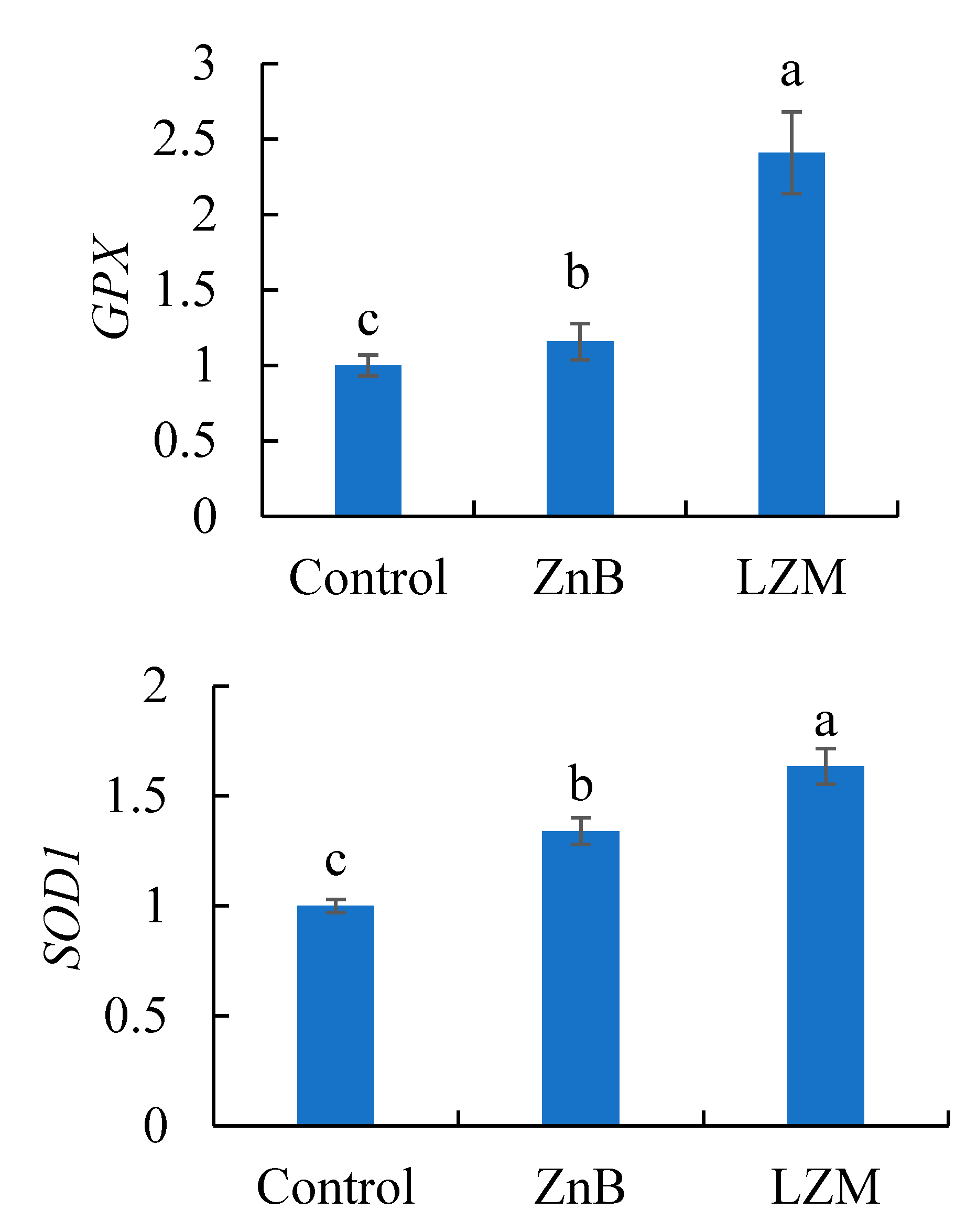
3.4. Antioxidant Related Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalle Zotte, A.; Celia, C.; Cullere, M.; Szendrő, Z.; Kovács, M.; Gerencsér, Z.; Dal Bosco, A.; Giaccone, V.; Matics, Z. Effect of an in-vivo and/or in-meat application of a liquorice (Glycyrrhiza glabra L.) extract on fattening rabbits live performance, carcass traits and meat quality. Anim. Feed Sci. Technol. 2020, 260, 114333. [Google Scholar] [CrossRef]
- Marai, I.F.M.; Habeeb, A.A.M.; Gad, A.E. Rabbits’ productive, reproductive and physiological performance traits as affected by heat stress: A review. Livest. Prod. Sci. 2002, 78, 71–90. [Google Scholar] [CrossRef]
- El-Deep, M.H.; Amber, K.A.; Eid, Y.Z.; Alrashood, S.T.; Khan, H.A.; Sakr, M.S.; Dawood, M.A.O. The influence of dietary chicken egg lysozyme on the growth performance, blood health, and resistance against Escherichia coli in the growing rabbits’ cecum. Front. Vet. Sci. 2020, 7, 579576. [Google Scholar] [CrossRef] [PubMed]
- Castanon, J.I.R. History of the use of antibiotic as growth promoters in european poultry feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef] [PubMed]
- Thema, K.; Mlambo, V.; Snyman, N.; Mnisi, C.M. Evaluating alternatives to zinc-bacitracin antibiotic growth promoter in broilers: Physiological and meat quality responses. Animals 2019, 9, 1160. [Google Scholar] [CrossRef] [Green Version]
- Engberg, R.M.; Hedemann, M.S.; Leser, T.D.; Jensen, B.B. Effect of zinc bacitracin and salinomycin on intestinal microflora and performance of broilers. Poult. Sci. 2000, 79, 1311–1319. [Google Scholar] [CrossRef]
- Crisol-Martínez, E.; Stanley, D.; Geier, M.S.; Hughes, R.J.; Moore, R.J. Understanding the mechanisms of zinc bacitracin and avilamycin on animal production: Linking gut microbiota and growth performance in chickens. Appl. Microbiol. Biotechnol. 2017, 101, 4547–4559. [Google Scholar] [CrossRef]
- El-Deep, M.H.; Amber, K.A.; Elgendy, S.; Dawood, M.A.O.; Elwakeel, E.M.; Paray, B.A. Oxidative stress, hemato-immunological, and intestinal morphometry changes induced by ochratoxin A in apri rabbits and the protective role of probiotics. Environ. Sci. Pollut. Res. 2020, 27, 35439–35448. [Google Scholar] [CrossRef]
- El-Deep, M.H.; Dawood, M.A.O.; Assar, M.H.; Ahamad Paray, B. Aspergillus awamori positively impacts the growth performance, nutrient digestibility, antioxidative activity and immune responses of growing rabbits. Vet. Med. Sci. 2021, 7, 226–235. [Google Scholar] [CrossRef]
- Zhao, Y.; Balasubramanian, B.; Guo, Y.; Qiu, S.-J.; Jha, R.; Liu, W.-C. Dietary Enteromorpha polysaccharides supplementation improves breast muscle yield and is associated with modification of mrna transcriptome in broiler chickens. Front. Vet. Sci. 2021, 8, 337. [Google Scholar] [CrossRef]
- Liu, W.; Yuan, Y.; Sun, C.; Balasubramanian, B.; Zhao, Z.; An, L. Effects of dietary betaine on growth performance, digestive function, carcass traits, and meat quality in indigenous yellow-feathered broilers under long-term heat stress. Animals 2019, 9, 506. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Latif, M.A.; El-Far, A.H.; Elbestawy, A.R.; Ghanem, R.; Mousa, S.A.; Abd El-Hamid, H.S. Exogenous dietary lysozyme improves the growth performance and gut microbiota in broiler chickens targeting the antioxidant and non-specific immunity mRNA expression. PLoS ONE 2017, 12, e0185153. [Google Scholar] [CrossRef]
- Syngai, G.G.; Ahmed, G. Chapter 11—lysozyme: A natural antimicrobial enzyme of interest in food applications. In Enzymes in Food Biotechnology; Kuddus, M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 169–179. [Google Scholar]
- Lee, M.; Kovacs-Nolan, J.; Yang, C.; Archbold, T.; Fan, M.Z.; Mine, Y. Hen egg lysozyme attenuates inflammation and modulates local gene rxpression in a porcine model of dextran sodium sulfate (dss)-induced colitis. J. Agric. Food Chem. 2009, 57, 2233–2240. [Google Scholar] [CrossRef]
- Nyachoti, C.M.; Kiarie, E.; Bhandari, S.K.; Zhang, G.; Krause, D.O. Weaned pig responses to Escherichia coli k88 oral challenge when receiving a lysozyme supplement. J. Anim. Sci. 2012, 90, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, K.D.; Wells, J.E.; Maxwell, C.V.; Oliver, W.T. Granulated lysozyme as an alternative to antibiotics improves growth performance and small intestinal morphology of 10-day-old pigs. J. Anim. Sci. 2012, 90, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Lin, S.; Zhu, J.; Pang, X.; Fang, Z.; Lin, Y.; Che, L.; Xu, S.; Li, J.; Huang, Y.; et al. Effects of dietary lysozyme levels on growth performance, intestinal morphology, non-specific immunity and mRNA expression in weanling piglets. Anim. Sci. J. 2016, 87, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.; Wells, J. Lysozyme as an alternative to antibiotics improves growth performance and small intestinal morphology in nursery pigs. J. Anim. Sci. 2013, 91, 3129–3136. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, S.; Pan, L.; Piao, X. Effects of lysozyme on the growth performance, nutrient digestibility, intestinal barrier, and microbiota of weaned pigs fed diets containing spray-dried whole egg or albumen powder. Can. J. Anim. Sci. 2017, 97, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Rymuszka, A.; Studnicka, M.; Siwicki, A.K.; Sierosławska, A.; Bownik, A. The immunomodulatory effects of the dimer of lysozyme (klp-602) in carp (Cyprinus carpio l)—in vivo study. Ecotoxicol. Environ. Saf. 2005, 61, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Siwicki, A.K.; Klein, P.; Morand, M.; Kiczka, W.; Studnicka, M. Immunostimulatory effects of dimerized lysozyme (klp-602) on the nonspecific defense mechanisms and protection against furunculosis in salmonids. Vet. Immunol. Immunopathol. 1998, 61, 369–378. [Google Scholar] [CrossRef]
- Deng, J.; Bi, B.; An, Q.; Kong, L.; Wang, Q.; Tao, L.; Zhang, X. Effect of dietary inclusion of lysozyme on growth performance and plasma biochemical parameters of rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2012, 18, 332–339. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, X.; Yang, Y.; Han, D.; Jin, J.; Xie, S. Effect of dietary lysozyme on growth, immune response, intestine microbiota, intestine morphology and resistance to Aeromonas hydrophilia in gibel carp (Carassius auratus gibelio). Aquac. Nutr. 2014, 20, 229–241. [Google Scholar] [CrossRef]
- Shakoori, M.; Hoseinifar, S.H.; Paknejad, H.; Jafari, V.; Safari, R.; Van Doan, H.; Torfi Mozanzadeh, M. Enrichment of rainbow trout (Oncorhynchus mykiss) fingerlings diet with microbial lysozyme: Effects on growth performance, serum and skin mucus immune parameters. Fish Shellfish. Immunol. 2019, 86, 480–485. [Google Scholar] [CrossRef]
- Agnoletti, F.; Bacchin, C.; Bano, L.; Passera, A.; Favretti, M.; Mazzolini, E. Antimicrobial susceptibility to zinc bacitracin of Clostridium perfringens of rabbit origin. World Rabbit Sci. 2007, 15, 19–22. [Google Scholar] [CrossRef] [Green Version]
- NRC. National research council. In Nutrient Requirements of Rabbits, 6th ed.; National Academy Press: Washington, DC, USA, 1997. [Google Scholar]
- AOAC. Official Methods of Analysis, 13th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2007. [Google Scholar]
- Ibrahim, H.R.; Imazato, K.; Ono, H. Human lysozyme possesses novel antimicrobial peptides within its n-terminal domain that target bacterial respiration. J. Agric. Food Chem. 2011, 59, 10336–10345. [Google Scholar] [CrossRef]
- Collins, C.H.; Lyne, P.M. Microbiological Methods; University Park Press: London, UK, 1970. [Google Scholar]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Clinical Biochemistry of Domestic Animals; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Abdel-Wareth, A.A.A.; Metwally, A.E. Productive and physiological response of male rabbits to dietary supplementation with thyme essential oil. Animals 2020, 10, 1844. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Koshio, S.; Esteban, M.Á. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2018, 10, 950–974. [Google Scholar] [CrossRef]
- Liu, W.-C.; Guo, Y.; Zhihui, Z.; Jha, R.; Balasubramanian, B. Algae-derived polysaccharides promote growth performance by improving antioxidant capacity and intestinal barrier function in broiler chickens. Front. Vet. Sci. 2020, 7, 990. [Google Scholar] [CrossRef]
- Dalle Zotte, A. Perception of rabbit meat quality and major factors influencing the rabbit carcass and meat quality. Livest. Prod. Sci. 2002, 75, 11–32. [Google Scholar] [CrossRef]
- Abo Ghanima, M.M.; Abd El-Aziz, A.H.; Noreldin, A.E.; Atta, M.S.; Mousa, S.A.; El-Far, A.H. Β-glucan administration improves growth performance and gut health in New Zealand white and apri rabbits with different breed responses. PLoS ONE 2020, 15, e0234076. [Google Scholar] [CrossRef] [PubMed]
- Falcão-e-Cunha, L.; Castro-Solla, L.; Maertens, L.; Marounek, M.; Pinheiro, V.; Freire, J.; Mourão, J.L. Alternatives to antibiotic growth promoters in rabbit feeding: A review. World Rabbit Sci. 2007, 15. [Google Scholar] [CrossRef] [Green Version]
- Assan, N. Plant based feed additives (phytogenic) as a primary solution to an antibiotic free nutritional program and feeding strategy in rabbit production. Sci. J. Anim. Sci. 2018, 7, 493–503. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Kim, I.H. Efficacy of phytogenic feed additive on performance, production and health status of monogastric animals—A review. Ann. Anim. Sci. 2017, 17, 929–948. [Google Scholar] [CrossRef] [Green Version]
- Fang, S.; Chen, X.; Ye, X.; Zhou, L.; Xue, S.; Gan, Q. Effects of gut microbiome and short-chain fatty acids (scfas) on finishing weight of meat rabbits. Front. Microbiol. 2020, 11, 1835. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Brundige, D.R.; Maga, E.A.; Klasing, K.C.; Murray, J.D. Lysozyme transgenic goats’ milk influences gastrointestinal morphology in young pigs. J. Nutr. 2008, 138, 921–926. [Google Scholar] [CrossRef] [Green Version]
- Monteils, V.; Cauquil, L.; Combes, S.; Godon, J.-J.; Gidenne, T. Potential core species and satellite species in the bacterial community within the rabbit caecum. FEMS Microbiol. Ecol. 2008, 66, 620–629. [Google Scholar] [CrossRef]
- Gidenne, T.; Jehl, N.; Lapanouse, A.; Segura, M. Inter-relationship of microbial activity, digestion and gut health in the rabbit: Effect of substituting fibre by starch in diets having a high proportion of rapidly fermentable polysaccharides. Br. J. Nutr. 2004, 92, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Gong, M. Efficacy of Lysozyme as an Alternative to Antibiotics for Broiler Chickens. Master’s Thesis, Faculty of Science, Dalhousie University, Halifax, NS, Canada, 2014. [Google Scholar]
- Zhao, H.; Zhang, F.; Chai, J.; Wang, J. Effect of lactic acid bacteria on listeria monocytogenes infection and innate immunity in rabbits. Czech J. Anim. Sci. 2020, 65, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Attia, Y.A.; Al-Hanoun, A.; Tag El- Din, A.E.; Bovera, F.; Shewika, Y.E. Effect of bee pollen levels on productive, reproductive and blood traits of NZW rabbits. J. Anim. Physiol. Anim. Nutr. 2011, 95, 294–303. [Google Scholar] [CrossRef]
- Liu, W.C.; Pi, S.H.; Kim, I.H. Effects of Scutellaria baicalensis and Lonicera japonica extract mixture supplementation on growth performance, nutrient digestibility, blood profiles and meat quality in finishing pigs. Ital. J. Anim. Sci. 2016, 15, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Puppel, K.; Kapusta, A.; Kuczyńska, B. The etiology of oxidative stress in the various species of animals, a review. J. Sci. Food Agric. 2015, 95, 2179–2184. [Google Scholar] [CrossRef]
- Guo, Y.; Balasubramanian, B.; Zhao, Z.-H.; Liu, W.-C. Marine algal polysaccharides alleviate aflatoxin B1-induced bursa of fabricius injury by regulating redox and apoptotic signaling pathway in broilers. Poult. Sci. 2021, 100, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Veskoukis, A.S.; Nikolaidis, M.G.; Kyparos, A.; Kouretas, D. Blood reflects tissue oxidative stress depending on biomarker and tissue studied. Free Radic. Biol. Med. 2009, 47, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-C.; Ou, B.-H.; Liang, Z.-L.; Zhang, R.; Zhao, Z.-H. Algae-derived polysaccharides supplementation ameliorates heat stress-induced impairment of bursa of fabricius via modulating nf-κb signaling pathway in broilers. Poult. Sci. 2021, 101139. [Google Scholar] [CrossRef]
- Casas-Grajales, S.; Muriel, P. Antioxidants in liver health. World J. Gastrointest. Pharm. 2015, 6, 59–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Wang, Z.; Li, J.-J.; Chen, C.; Zhang, P.-C.; Dong, L.; Chen, J.-H.; Chen, Q.; Zhang, X.-T.; Wang, Z.-L. Protective effects of selenium on oxidative damage and oxidative stress related gene expression in rat liver under chronic poisoning of arsenic. Food Chem. Toxicol. 2013, 58, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bera, A.K.; Rana, T.; Bhattacharya, D.; Das, S.; Pan, D.; Das, S.K. Sodium arsenite-induced alteration in hepatocyte function of rat with special emphasis on superoxide dismutase expression pathway and its prevention by mushroom lectin. Basic Clin. Pharmacol. Toxicol. 2011, 109, 240–244. [Google Scholar] [CrossRef]
- Bacova, K.; Zitterl-Eglseer, K.; Chrastinova, L.; Laukova, A.; Madarova, M.; Gancarcikova, S.; Sopkova, D.; Andrejcakova, Z.; Placha, I. Effect of thymol addition and withdrawal on some blood parameters, antioxidative defence system and fatty acid profile in rabbit muscle. Animals 2020, 10, 1248. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, M.; Al-Homidan, I.; Ebeid, T.; Abou-Emera, O.; Mostafa, M.; Abd El-Razik, M.; Shehab-El-Deen, M.; Abdel Ghani, S.; Fathi, M. Effect of silver nanoparticle administration on productive performance, blood parameters, antioxidative status, and silver residues in growing rabbits under hot climate. Animals 2019, 9, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortun-Lamothe, L.; Boullier, S. A review on the interactions between gut microflora and digestive mucosal immunity. Possible ways to improve the health of rabbits. Livest. Sci. 2007, 107, 1–18. [Google Scholar] [CrossRef]
| Ingredients | % | Analysis | % |
|---|---|---|---|
| Berseem hay | 30 | Dry matter | 89.7 |
| Barley | 23 | Crude protein | 17.2 |
| Soybean meal | 17.5 | Crude fiber | 13.1 |
| Wheat bran | 15 | Ether extract | 3.4 |
| Yellow corn | 10 | Nitrogen free extract | 56. |
| Molasses | 3 | Ash | 10.3 |
| Di. Ca. phosphate | 0.8 | Digestible energy (Kcal/kg) | 2519 |
| Sodium chloride | 0.3 | ||
| Vitamin and mineral premix * | 0.3 | ||
| DL-Methionine | 0.1 |
| Gene | Sequence Primer (5’–3’) | Accession Number |
|---|---|---|
| GAPDH | F: GGAGAAAGCTGCTAA | L23961 |
| R: ACGACCTGGTCCTCGGTGTA | ||
| SOD1 | F: CGCCGCTCGGAAGTCAT | XM_017347219 |
| R: TTATGCGTCCCTTGACCACC | ||
| GPX1 | F: TTCGAGCCCAACTTCATGCT | NM_001085444 |
| R: TCGAAGCTCCAGGAAACGTC |
| Control | ZnB | LZM | |
|---|---|---|---|
| Initial BW (g) | 612 ± 0.43 a | 611.25 ± 0.26 a | 611 ± 0.45 a |
| Final BW (g) | 2256 ± 0.33 c | 2273 ± 0.91 b | 2343 ± 0.71 a |
| BWG (g) | 1644 ± 0.80 c | 1662 ± 0.24 b | 1732 ± 0.30 a |
| FI (g, as feed) | 5490.96 ± 32.55 a | 4853.04 ± 41.69 b | 4676.4 ± 65.22 c |
| Total FCR | 3.34 ± 0.02 a | 2.92 ± 0.11 b | 2.70 ± 0.05 c |
| Control | ZnB | LZM | |
|---|---|---|---|
| Clostridium spp. | 6.11 ± 0.05 a | 4.58 ± 0.01 b | 4.14 ± 0.05 b |
| Escherichia coli (×104) | 1.61 ± 0.02 a | 1.02 ± 0.02 b | 1.11 ± 0.11 b |
| Lactobacillus (×105) | 5.08 ± 0.04 c | 9.84 ± 0.02 b | 10.76 ± 0.02 a |
| Total bacterial count (×106) | 16.5 ± 0.01 c | 19.84 ± 0.02 b | 24.7 ± 0.02 a |
| Control | ZnB | LZM | |
|---|---|---|---|
| Total protein (g/dL) | 6.30 ± 0.25 c | 7.03 ± 0.03 b | 7.46 ± 0.32 a |
| Albumin (g/dL) | 4.05 ± 0.24 a | 3.71 ± 0.11 a | 3.7 ± 0.12 a |
| Globulin (g/dL) | 2.25 ± 0.06 c | 3.33 ± 0.14 b | 3.76 ± 0.12 a |
| Albumin/globulin ratio | 1.80 ± 0.15 a | 0.89 ± 0.17 a | 0.98 ± 0.10 a |
| Creatinine (mg/dL) | 1.18 ± 0.20 a | 1.10 ± 0.04 a | 0.87 ± 0.12 b |
| Urea (mg/dL) | 4.40 ± 3.29 a | 3.67 ± 1.45 b | 3.53 ± 0.67 b |
| Aspartate aminotransferase (AST) (IU/L) | 55.0 ± 1.53 a | 53.67 ± 1.20 b | 51.3 ± 0.88 c |
| Alanine aminotransferase (ALT) (IU/L) | 47.0 ± 1.53 a | 35.67 ± 1.20 b | 34.6 ± 0.88 b |
| Cholesterol (mg/dL) | 28.3 ± 1.77 a | 26.00 ± 1.53 a | 28.7 ± 0.58 a |
| Triglyceride (mg/dL) | 74.7 ± 0.88 a | 83.67 ± 0.88 a | 73.3 ± 1.73 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
EL-Deep, M.H.; Amber, K.A.; Eid, Y.Z.; Aboelenin, S.M.; Soliman, M.M.; Sakr, M.S.; Dawood, M.A.O. The Influence of Chicken Egg Lysozyme or Zinc-Bacitracin Antibiotic on the Growth Performance, Antibacterial Capacity, Blood Profiles, and Antioxidative Status of Rabbits: A Comparative Study. Animals 2021, 11, 1731. https://doi.org/10.3390/ani11061731
EL-Deep MH, Amber KA, Eid YZ, Aboelenin SM, Soliman MM, Sakr MS, Dawood MAO. The Influence of Chicken Egg Lysozyme or Zinc-Bacitracin Antibiotic on the Growth Performance, Antibacterial Capacity, Blood Profiles, and Antioxidative Status of Rabbits: A Comparative Study. Animals. 2021; 11(6):1731. https://doi.org/10.3390/ani11061731
Chicago/Turabian StyleEL-Deep, Mahmoud H., Khairy A. Amber, Yahia Z. Eid, Salama Mostafa Aboelenin, Mohamed Mohamed Soliman, Mohamed S. Sakr, and Mahmoud A. O. Dawood. 2021. "The Influence of Chicken Egg Lysozyme or Zinc-Bacitracin Antibiotic on the Growth Performance, Antibacterial Capacity, Blood Profiles, and Antioxidative Status of Rabbits: A Comparative Study" Animals 11, no. 6: 1731. https://doi.org/10.3390/ani11061731







