Oxytetracycline and Florfenicol Concentrations in Food-Additive Premixes Authorised for Broiler Chickens: Assessing Degree of Agreement with Manufacturers Labelling
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Solvents, Reagents, and Certified Standards
2.2. Commercial Premixes of OTC and FF
2.3. Sample Treatment
2.4. Instrumental Analysis
2.5. Implementation and In-House Validation of the Analytical Method
2.6. Statistical Analysis
3. Results
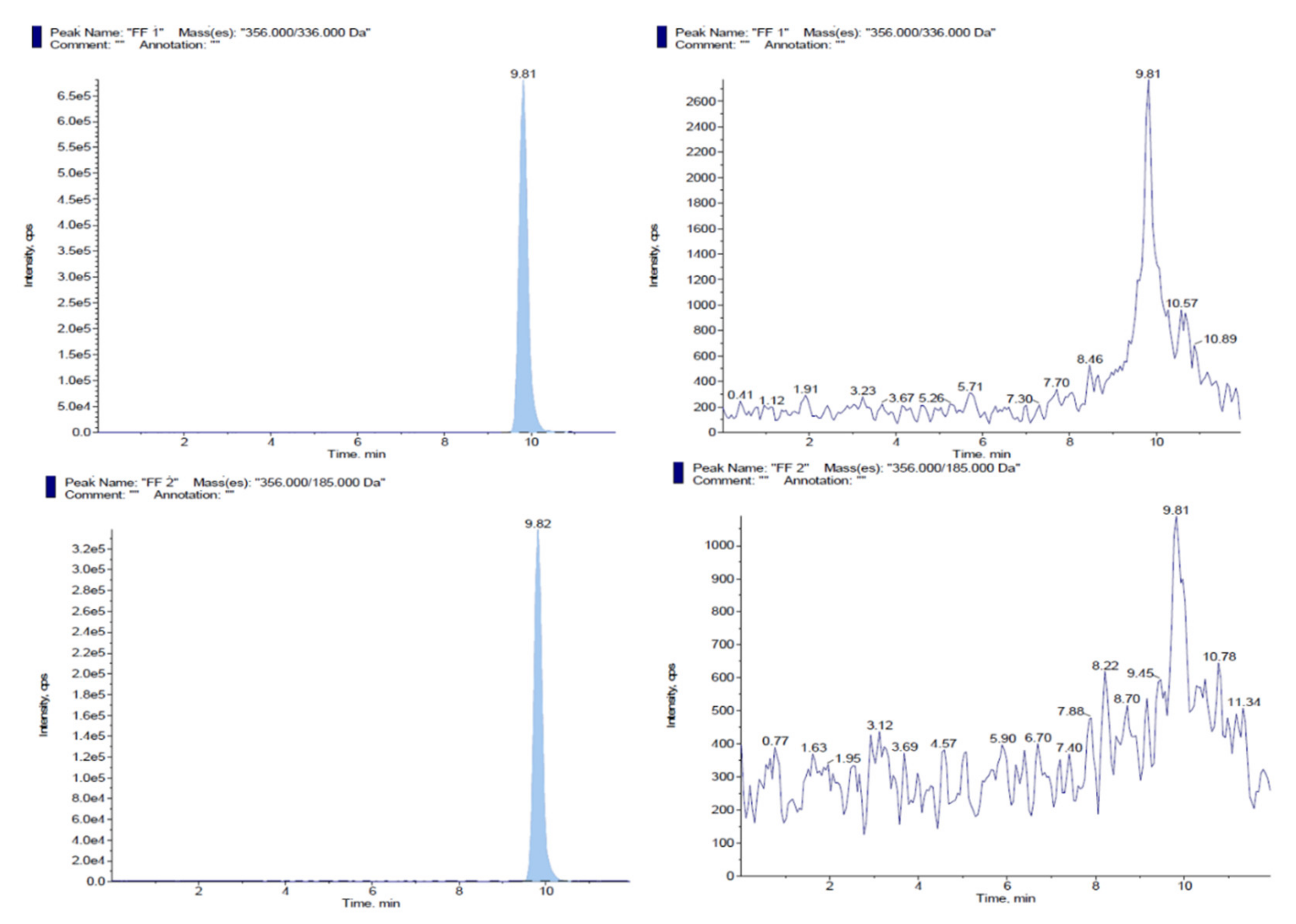
3.1. Implementation of HPLC-MS/MS and UPLC-MS/MS Chromatographic Conditions for Detection of Oxytetracycline or Florfenicol in Antimicrobial Premixes
3.2. In-House Validation of Analytical Methodologies
3.3. Calculation of OTC and FF Concentrations in Antimicrobial Premixes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- European Parliament and the Council of the European Union Directive 2001/82/EC of the European Parliament and of the Council of 6 November 2001 on the Community Code Relating to Veterinary Medicinal Products. Off. J. Eur. Union 2001, 311, 1–66.
- Food and Agriculture Organization. Good Practices for the Feed Industry: Implementing the Codex Alimentarius Code of Practice on Good Animal Feeding. In FAO Animal Production and Health Manual; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010; ISBN 978-92-5-106487-0. [Google Scholar]
- Van Boeckel, T.P.; Glennon, E.E.; Chen, D.; Gilbert, M.; Robinson, T.P.; Grenfell, B.T.; Levin, S.A.; Bonhoeffer, S.; Laxminarayan, R. Reducing Antimicrobial Use in Food Animals. Science 2017, 357, 1350–1352. [Google Scholar] [CrossRef]
- Beyene, T. Veterinary Drug Residues in Food-Animal Products: Its Risk Factors and Potential Effects on Public Health. J. Vet. Sci. Technol. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Food Safety in Poultry Meat Production; Venkitanarayanan, K., Thakur, S., Ricke, S.C., Eds.; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-05010-8. [Google Scholar]
- Servicio Agrícola y Ganadero Declaración de Venta de Antimicrobianos. 2016. Available online: http://www.sag.cl/ambitos-de-accion/declaracion-de-venta-de-antimicrobianos (accessed on 12 December 2020).
- Bryskier, A. Antimicrobial Agents: Antibacterials and Antifungals; André, B., Ed.; ASM Press: Washington, DC, USA, 2005; ISBN 1-55581-237-6. [Google Scholar]
- Ríos Insua, A. Biodisponibilidad y Metabolismo de un Derivado Fluorado del Tianfenicol en Pollos Broiler. Doctoral Thesis, Universidad Complutense de Madrid, Servicio de Publicaciones, Madrid, Spain, 2005. (In Spanish). [Google Scholar]
- Calvo, J.; Martínez-Martínez, L. Mecanismos de acción de los antimicrobianos. Enferm. Infecc. Microbiol. Clínica 2009, 27, 44–52. [Google Scholar] [CrossRef]
- Park, B.-K.; Lim, J.-H.; Kim, M.-S.; Hwang, Y.-H.; Yun, H.-I. Pharmacokinetics of Florfenicol and Its Major Metabolite, Florfenicol Amine, in Rabbits. J. Vet. Pharmacol. Ther. 2007, 30, 32–36. [Google Scholar] [CrossRef]
- American Academy of Veterinary Pharmacology and Therapeutics Veterinary Clinical Drug Information Monographs—Florfenicol. 2007. Available online: https://cdn.ymaws.com/www.aavpt.org/resource/resmgr/imported/florfenicol.pdf (accessed on 16 January 2021).
- Chang, S.K.; Davis, J.L.; Cheng, C.N.; Shien, R.H.; Hsieh, M.K.; Koh, B.W.; Chou, C.C. Pharmacokinetics and Tissue Depletion of Florfenicol in Leghorn and Taiwan Native Chickens. J. Vet. Pharmacol. Ther. 2010, 33, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Patyra, E.; Kwiatek, K. HPLC-DAD Analysis of Florfenicol and Thiamphenicol in Medicated Feedingstuffs. Food Addit. Contam. Part. A 2019, 36, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.L.; Levy, S.B. The History of the Tetracyclines: The History of the Tetracyclines. Ann. N. Y. Acad. Sci. 2011, 1241, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef]
- MacDougall, C.; Chambers, H. Antimicrobianos: Inhibidores de la síntesis de proteína y otros antibacterianos. In Las Bases Farmacológicas de la Terapéutica; McGraw-Hill: New York, NY, USA, 2007. (In Spanish) [Google Scholar]
- Ziółkowski, H.; Madej-Śmiechowska, H.; Grabowski, T.; Jaroszewski, J.J.; Maślanka, T. Hard Water May Increase the Inhibitory Effect of Feed on the Oral Bioavailability of Oxytetracycline in Broiler Chickens. Pol. J. Vet. Sci. 2019, 251–258. [Google Scholar] [CrossRef]
- Yagoub, Y.M.M.; Abdoun, S.; Seri, H.I. In-Use Stability Studies of Two Veterinary Medicinal Products: Albendazole and Oxytetracycline. Assiut Vet. Med. J. 2013, 59, 6. [Google Scholar]
- Abou-Raya Salah, H.; Shalaby Ali, R.; Salama Nadia, A.; Emam Wafaa, H.; Mehaya Fathy, M. Effect of Ordinary Cooking Procedures on Tetracyclin Residues in Chicken Meat. J. Food Drug Anal. 2013, 21, 80–86. [Google Scholar] [CrossRef]
- Alaboudi, A.; Basha, E.A.; Musallam, I. Chlortetracycline and Sulfanilamide Residues in Table Eggs: Prevalence, Distribution between Yolk and White and Effect of Refrigeration and Heat Treatment. Food Control 2013, 33, 281–286. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Pang, M.-D.; Xie, X.; Xie, K.-Z.; Cui, L.-L.; Gao, Q.; Liu, J.-Y.; Wang, B.; Zhang, Y.-Y.; Wang, R.; et al. Residue Depletion of Amoxicillin and Its Major Metabolites in Eggs. J. Vet. Pharmacol. Ther. 2017, 40, 383–391. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. 2010. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:015:0001:0072:EN:PDF (accessed on 21 November 2020).
- Ziółkowski, H.; Jasiecka-Mikołajczyk, A.; Madej-Śmiechowska, H.; Janiuk, J.; Zygmuntowicz, A.; Dąbrowski, M. Comparative Pharmacokinetics of Chlortetracycline, Tetracycline, Minocycline, and Tigecycline in Broiler Chickens. Poult. Sci. 2020, 99, 4750–4757. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Cheng, Y.; Samia, A.C.; Meyers, J.D.; Panagopoulos, I.; Fei, B.; Burda, C. Highly Efficient Drug Delivery with Gold Nanoparticle Vectors for in Vivo Photodynamic Therapy of Cancer. J. Am. Chem. Soc. 2008, 130, 10643–10647. [Google Scholar] [CrossRef]
- Biju, V. Chemical Modifications and Bioconjugate Reactions of Nanomaterials for Sensing, Imaging, Drug Delivery and Therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef]
- Fernandez-González, R.; García-Falcón, M.S.; Simal-Gándara, J. Quantitative Analysis for Oxytetracycline in Medicated Premixes and Feeds by Second-Derivative Synchronous Spectrofluorimetry. Anal. Chim. Acta 2002, 455, 143–148. [Google Scholar] [CrossRef]
- Krasucka, D.; Mitura, A.; Cybulski, W.; Kos, K.; Pietro, W. Tiamulin Hydrogen Fumarate—Veterinary Uses and HPLC Method of Determination in Premixes and Medicated Feeding Stuffs. Acta Pol. Pharm. 2010, 67, 682–685. [Google Scholar] [PubMed]
- Song, X.; Xie, J.; Zhang, M.; Zhang, Y.; Li, J.; Huang, Q.; He, L. Simultaneous Determination of Eight Cyclopolypeptide Antibiotics in Feed by High Performance Liquid Chromatography Coupled with Evaporation Light Scattering Detection. J. Chromatogr. B 2018, 1076, 103–109. [Google Scholar] [CrossRef]
- Han, J.; Jiang, D.; Chen, T.; Jin, W.; Wu, Z.; Cui, F. Simultaneous Determination of Olaquindox, Oxytetracycline and Chlorotetracycline in Feeds by High Performance Liquid Chromatography with Ultraviolet and Fluorescence Detection Adopting Online Synchronous Derivation and Separation. J. Chromatogr. B 2020, 1152, 122253. [Google Scholar] [CrossRef]
- Yang, P.; Wang, H.; Zhu, M.; Ma, Y. Effects of Choline Chloride, Copper Sulfate and Zinc Oxide on Long-Term Stabilization of Microencapsulated Vitamins in Premixes for Weanling Piglets. Animals 2019, 9, 1154. [Google Scholar] [CrossRef]
- Yang, P.; Wang, H.K.; Zhu, M.; Li, L.X.; Ma, Y.X. Degradation Kinetics of Vitamins in Premixes for Pig: Effects of Choline, High Concentrations of Copper and Zinc, and Storage Time. Asian-Australas. J. Anim. Sci. 2020, 34, 701. [Google Scholar] [CrossRef]
- Saensukjaroenphon, M.; Evans, C.E.; Paulk, C.B.; Gebhardt, J.T.; Woodworth, J.C.; Stark, C.R.; Bergstrom, J.R.; Jones, C.K. Impact of Storage Conditions and Premix Type on Fat-Soluble Vitamin Stability1. Transl. Anim. Sci. 2020, 4, 1–10. [Google Scholar] [CrossRef]
- Pauli, G.F.; Chen, S.-N.; Simmler, C.; Lankin, D.C.; Gödecke, T.; Jaki, B.U.; Friesen, J.B.; McAlpine, J.B.; Napolitano, J.G. Importance of Purity Evaluation and the Potential of Quantitative 1H NMR as a Purity Assay: Miniperspective. J. Med. Chem. 2014, 57, 9220–9231. [Google Scholar] [CrossRef]
- Patel, S.J.; Wellington, M.; Shah, R.M.; Ferreira, M.J. Antibiotic Stewardship in Food-Producing Animals: Challenges, Progress, and Opportunities. Clin. Ther. 2020, 42, 1649–1658. [Google Scholar] [CrossRef]
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Espectrometría de masas molecular. In Principios de Análisis Instrumental; Cengage: Boston, MS, USA, 2019; ISBN 978-607-526-664-0. [Google Scholar]
- Guo, L.; Chen, Y.; Zhang, L.; Yang, W.; He, P. Development and Validation of a Liquid Chromatographic/Tandem Mass Spectrometric Method for Determination of Chlortetracycline, Oxytetracycline, Tetracycline, and Doxycycline in Animal Feeds. J. AOAC Int. 2012, 95, 1010–1015. [Google Scholar] [CrossRef]
- Gavilán, R.E.; Nebot, C.; Patyra, E.; Vazquez, B.; Miranda, J.M.; Cepeda, A. Determination of Florfenicol, Thiamfenicol and Chloramfenicol at Trace Levels in Animal Feed by HPLC–MS/MS. Antibiotics 2019, 8, 59. [Google Scholar] [CrossRef]
- Gavilán, R.E.; Nebot, C.; Veiga-Gómez, M.; Roca-Saavedra, P.; Vazquez Belda, B.; Franco, C.M.; Cepeda, A. A Confirmatory Method Based on HPLC-MS/MS for the Detection and Quantification of Residue of Tetracyclines in Nonmedicated Feed. J. Anal. Methods Chem. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.; Cantley, M.; Walker, M.; Crooks, S.; Kennedy, D.; Elliott, C. Evidence of Non-Extractable Florfenicol Residues: Development and Validation of a Confirmatory Method for Total Florfenicol Content in Kidney by UPLC-MS/MS. Food Addit. Contam. Part A 2016, 33, 983–994. [Google Scholar] [CrossRef]
- European Commission 2002/657/EC: Commission Decision of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results. Off. J. Eur. Union 2002, L221, 0008–0036.
- Infostat® Version 2020I Update 04/30/2020. 2020. Available online: https://www.infostat.com.ar/ (accessed on 12 December 2020).
- Chen, J.; Ying, G.-G.; Deng, W.-J. Antibiotic Residues in Food: Extraction, Analysis, and Human Health Concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.; Andersson, D.I. Selection of Resistance at Lethal and Non-Lethal Antibiotic Concentrations. Curr. Opin. Microbiol. 2012, 15, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Kristiansson, E.; Fick, J.; Janzon, A.; Grabic, R.; Rutgersson, C.; Weijdegård, B.; Söderström, H.; Larsson, D.G.J. Pyrosequencing of Antibiotic-Contaminated River Sediments Reveals High Levels of Resistance and Gene Transfer Elements. PLoS ONE 2011, 6, e17038. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson-Palme, J.; Larsson, D.G.J. Concentrations of Antibiotics Predicted to Select for Resistant Bacteria: Proposed Limits for Environmental Regulation. Environ. Int. 2016, 86, 140–149. [Google Scholar] [CrossRef]
- Tello, A.; Austin, B.; Telfer, T.C. Selective Pressure of Antibiotic Pollution on Bacteria of Importance to Public Health. Environ. Health Perspect. 2012, 120, 1100–1106. [Google Scholar] [CrossRef]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilbäck, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of Resistant Bacteria at Very Low Antibiotic Concentrations. PLoS Pathog. 2011, 7, e1002158. [Google Scholar] [CrossRef]
- Naidong, W.; Hua, S.; Roets, E.; Hoogmartens, J. Assay and Purity Control of Tetracycline, Chlortetracycline and Oxytetracycline in Animal Feeds and Premixes by TLC Densitometry with Fluorescence Detection. J. Pharm. Biomed. Anal. 2003, 33, 85–93. [Google Scholar] [CrossRef]
- Ziółkowski, H.; Grabowski, T.; Jasiecka, A.; Zuśka-Prot, M.; Barski, D.; Jaroszewski, J.J. Pharmacokinetics of Oxytetracycline in Broiler Chickens Following Different Routes of Administration. Vet. J. 2016, 208, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Arriaga, J.M.; Gutierrez-Pabello, J.A.; Chavez-Gris, G.; Hernandez-Castro, R. Efficacy of Florfenicol Premix in Weaning Pigs Experimentally Infected with Actinobacillus Pleuropneumoniae. Rev. Latinoam. Microbiol. 2000, 42, 27–34. [Google Scholar] [PubMed]
- Chiţescu, C.L.; Nicolau, A.I.; Römkens, P.; Van Der Fels-Klerx, H.J. Quantitative Modelling to Estimate the Transfer of Pharmaceuticals through the Food Production System. J. Environ. Sci. Health Part B 2014, 49, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Bernabéu, J.A.; Camacho, M.A.; Gil-Alegre, M.E.; Torres-Suárez, A.I. Procedure to Evaluate the Stability during Processing and Storage of a Medicated Premix and Medicated Farm Feed: Erythromycin Thiocyanate. J. Agric. Food Chem. 2001, 49, 3709–3712. [Google Scholar] [CrossRef]
- Pérez-Lozano, P.; García-Montoya, E.; Orriols, A.; Miñarro, M.; Ticó, J.R.; Suñé-Negre, J.M. Stability Evaluation of Amoxicillin in a Solid Premix Veterinary Formulation by Monitoring the Degradation Products through a New HPLC Analytical Method. J. Pharm. Biomed. Anal. 2006, 42, 192–199. [Google Scholar] [CrossRef]


| Premix | Excipient | Active Product | Labelled Concentration | Manufacturer |
|---|---|---|---|---|
| Product A | 100 g | 10.78 g of Oxytetracycline dihydrate (equivalent to 10 g of Oxytetracycline base) | 10% | 1 |
| Product B | 100 g | 80 g of Oxytetracycline hydrochloride (equivalent to 74.2 g of Oxytetracycline base) | 80% | 2 |
| Product C | 1 kg | 100 g of Oxytetracycline | 10% | 2 |
| Product D | 100 mL | 2 g of Florfenicol | 2% | 1 |
| Product E | 100 mL | 10 g of Florfenicol | 10% | 2 |
| Analyte | Polarity | Precursor Ion (m/z) | Product Ion (m/z) | DP 5 (volts) | EP 6 (volts) | CE 7 (volts) | CXP 8 (volts) |
|---|---|---|---|---|---|---|---|
| OTC 1 | + | 461.00 | 426.00 | 72.00 | 10.00 | 28.00 | 25.00 |
| 381.00 | 36.00 | 22.00 | |||||
| MTC 2 | + | 443.17 | 426.15 | 20.00 | 15.00 | 20.00 | 30.00 |
| FF 3 | – | 356.00 | 336.00 | 30.00 | 10.00 | 28.00 | 25.00 |
| 185.00 | 30.00 | 10.00 | 28.00 | 25.00 | |||
| CAF d5 4 | – | 321.00 | 256.30 | 30.00 | 10.00 | 28.00 | 25.00 |
| Analyte | Retention Time (min) | Mean (min) | SD 5 | RSD 6 (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||||
| OTC 1 (461.19/426.3) | 2.75 | 2.76 | 2.75 | 2.75 | 2.76 | 2.76 | 2.754 | 0.0054 | 0.21 |
| OTC (461.19/337.16) | 2.76 | 2.76 | 2.76 | 2.75 | 2.75 | 2.75 | 2.755 | 0.0055 | 0.20 |
| TC-D6 2 (443.17/426.24) | 3.25 | 3.25 | 3.24 | 3.24 | 3.25 | 3.24 | 3.245 | 0.0055 | 0.17 |
| FF 3 (356.00/336.00) | 9.35 | 9.35 | 9.35 | 9.34 | 9.33 | 9.35 | 9.343 | 0.0082 | 0.09 |
| FF (356.00/185.00) | 9.34 | 9.34 | 9.34 | 9.34 | 9.31 | 9.35 | 9.337 | 0.0137 | 0.15 |
| CAF-D5 4 (321.00/256.80) | 9.77 | 9.77 | 9.77 | 9.76 | 9.75 | 9.78 | 9.767 | 0.0103 | 0.11 |
| Analyte | Linearity | Recovery (%) | LOD 4 (μg/kg) | LOQ 5 (μg/kg) | |
|---|---|---|---|---|---|
| Mean R 2,* ± SD 3 | Slope Mean ± SD | ||||
| OTC 1 | 0.9921 ± 0.003 | 0.4260 ± 0.028 | 98.6 | 1 | 1.022 |
| FF 2 | 0.9957 ± 0.001 | 0.1771 ± 0.011 | 97.1 | 5 | 5.451 |
| Premix | Active Ingredient | Labelled Concentrations (mg/kg) | Average Concentration 3 (mg/kg) ± SD 4 | Difference of Concentration 5 (mg/kg) | Percentage of Difference 6 (%) |
|---|---|---|---|---|---|
| Product A | OTC 1 | 100,000 | 113,279.52 ± 2689.88 | 13,279.52 | 13.28 |
| Product B | OTC | 742,000 | 901,804.57 ± 24,297.15 | 159,804.57 | 21.54 |
| Product C | OTC | 100,000 | 129,678.38 ± 5987.27 | 29,678.38 | 29.68 |
| Product D | FF 2 | 20,000 | 17,388.69 ± 627.02 | −2611.31 | −13.06 |
| Product E | FF | 100,000 | 85,247 ± 2484.82 | −14,752.61 | −14.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maddaleno, A.; Maturana, M.; Pokrant, E.; Martín, B.S.; Cornejo, J. Oxytetracycline and Florfenicol Concentrations in Food-Additive Premixes Authorised for Broiler Chickens: Assessing Degree of Agreement with Manufacturers Labelling. Animals 2021, 11, 1797. https://doi.org/10.3390/ani11061797
Maddaleno A, Maturana M, Pokrant E, Martín BS, Cornejo J. Oxytetracycline and Florfenicol Concentrations in Food-Additive Premixes Authorised for Broiler Chickens: Assessing Degree of Agreement with Manufacturers Labelling. Animals. 2021; 11(6):1797. https://doi.org/10.3390/ani11061797
Chicago/Turabian StyleMaddaleno, Aldo, Matías Maturana, Ekaterina Pokrant, Betty San Martín, and Javiera Cornejo. 2021. "Oxytetracycline and Florfenicol Concentrations in Food-Additive Premixes Authorised for Broiler Chickens: Assessing Degree of Agreement with Manufacturers Labelling" Animals 11, no. 6: 1797. https://doi.org/10.3390/ani11061797
APA StyleMaddaleno, A., Maturana, M., Pokrant, E., Martín, B. S., & Cornejo, J. (2021). Oxytetracycline and Florfenicol Concentrations in Food-Additive Premixes Authorised for Broiler Chickens: Assessing Degree of Agreement with Manufacturers Labelling. Animals, 11(6), 1797. https://doi.org/10.3390/ani11061797







