Sperm Behavior and Response to Melatonin under Capacitating Conditions in Three Sheep Breeds Subject to the Equatorial Photoperiod
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Semen Collection and Processing
2.3. In Vitro Sperm Capacitation
2.4. Evaluation of Motility and Plasma Membrane Integrity
2.5. Detection of Membrane Phosphatidylserine Translocation
2.6. Assessment of Capacitation Status by Chlortetracycline (CTC) Staining
2.7. Tyrosine Phosphorylation as Capacitation Assay
2.8. Statistical Analysis
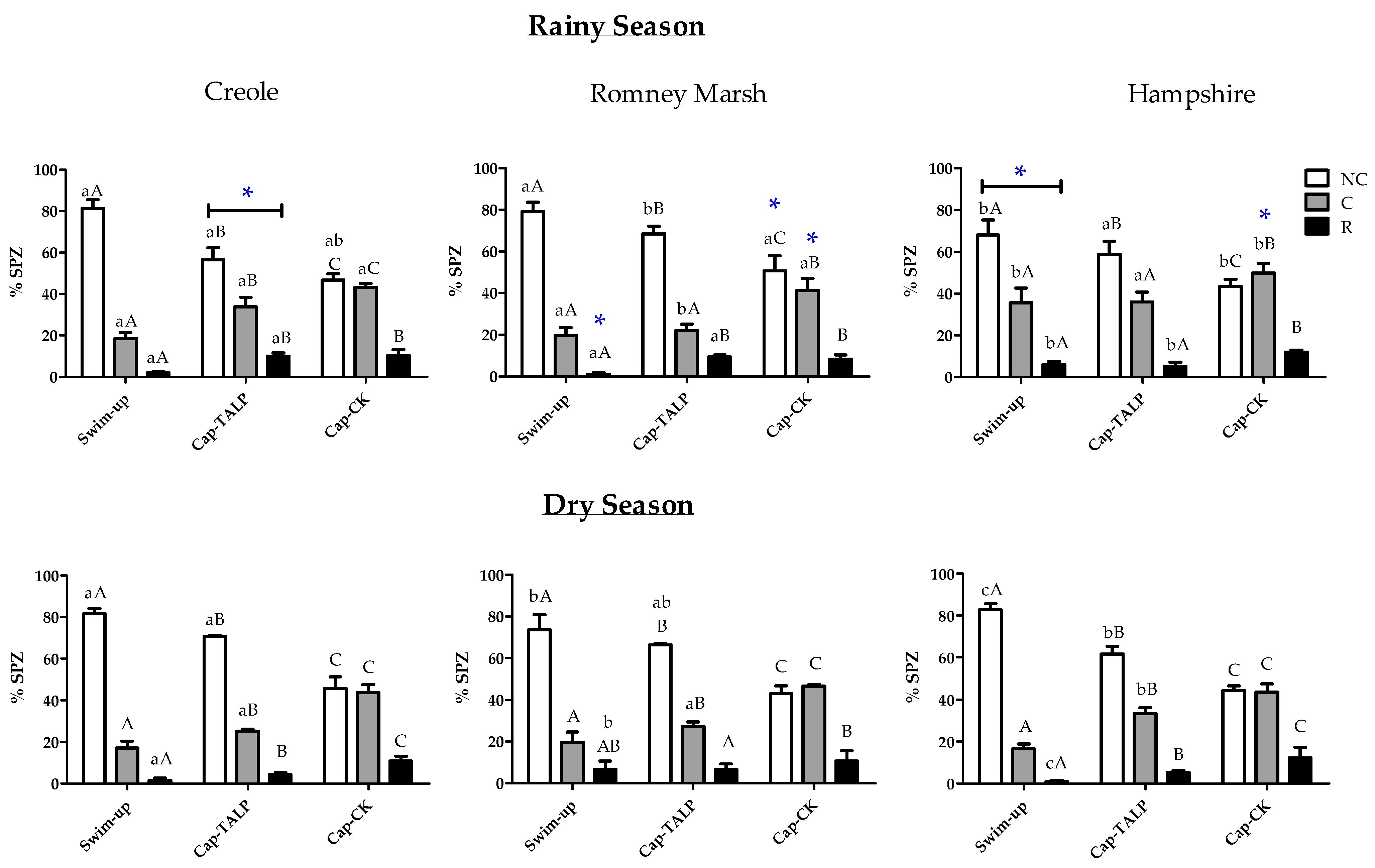
3. Results
3.1. Evaluation of the Changes after In Vitro Capacitation in Spermatozoa from Different Breeds and Seasons
3.1.1. Changes in Motility after In Vitro Capacitation
3.1.2. Changes in Plasma Membrane after In Vitro Capacitation
3.1.3. Changes in Capacitation Status after In Vitro Capacitation
3.1.4. Changes in Phosphorylation in Tyrosine Residues after In Vitro Capacitation
3.2. Evaluation of the Effect of Melatonin on In Vitro Capacitation in Spermatozoa from Different Breeds and Seasons
3.2.1. Effects on Motility during In Vitro Capacitation
3.2.2. Effects on Plasma Membrane during In Vitro Capacitation
3.2.3. Effects on Capacitation Status during In Vitro Capacitation
3.2.4. Effects on Phosphorylation in Tyrosine Residues during In Vitro Capacitation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Austin, C. The “capacitation” of the mammalian sperm. Nature 1952, 170, 326. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 1951, 168, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Harkiss, D.; Knox, W.; Paterson, M.; Irvine, D.S. A novel signal transduction cascade in capacitating human spermatozoa characterised by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation. J. Cell Sci. 1998, 111, 645–656. [Google Scholar] [CrossRef]
- Colas, C.; James, P.; Howes, L.; Jones, R.; Cebrian-Perez, J.A.; Muiño-Blanco, T. Cyclic-AMP initiates protein tyrosine phosphorylation independent of cholesterol efflux during ram sperm capacitation. Reprod. Fertil. Dev. 2008, 20, 649–658. [Google Scholar] [CrossRef]
- Visconti, P.; Galantino-Homer, H.; Moore, G.D.; Bailey, J.L.; Ning, X.; Fornes, M.; Kopf, G.S. The molecular basis of sperm capacitation. J. Androl. 1998, 19, 242–248. [Google Scholar]
- Molina, L.C.P.; Luque, G.M.; Balestrini, P.A.; Marín-Briggiler, C.I.; Romarowski, A.; Buffone, M.G. Molecular basis of human sperm capacitation. Front. Cell Dev. Biol. 2018, 6, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Grasa, P.; Cebrián-Pérez, J.Á.; Muiño-Blanco, T. Signal transduction mechanisms involved in in vitro ram sperm capacitation. Reproduction 2006, 132, 721–732. [Google Scholar] [CrossRef] [Green Version]
- Rickard, J.P.; Leahy, T.; Soleilhavoup, C.; Tsikis, G.; Labas, V.; Harichaux, G.; Lynch, G.W.; Druart, X.; de Graaf, S.P. The identification of proteomic markers of sperm freezing resilience in ram seminal plasma. J. Proteom. 2015, 126, 303–311. [Google Scholar] [CrossRef]
- Colás, C.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. Caffeine induces ram sperm hyperactivation independent of cAMP-dependent protein kinase. Int. J. Androl. 2010, 33. [Google Scholar] [CrossRef] [PubMed]
- Rosa, H.J.D.; Bryant, M.J. Seasonality of reproduction in sheep. Small Rumin. Res. 2003, 48, 155–171. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Paredes, S.D.; Mayo, J.C.; Sainz, R.M. Melatonin and reproduction revisited. Biol. Reprod. 2009, 81, 445–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bittman, E.L.; Dempsey, R.J.; Karsch, F.J. Pineal melatonin secretion drives the reproductive response to daylength in the ewe *. Endocrinology 1983, 113, 2276–2283. [Google Scholar] [CrossRef]
- Ortavant, R.; Bocquier, F.; Pelletier, J.; Ravault, J.P.; Thimonier, J.; Volland-Nail, P. Seasonality of reproduction in sheep and its control by photoperiod. Aust. J. Biol. Sci. 1988, 41, 69–86. [Google Scholar] [CrossRef]
- Malpaux, B.; Vigué, C.; Skinner, D.C.; Thiéry, J.C.; Pelletier, J.; Chemineau, P. Seasonal breeding in sheep: Mechanism of action of melatonin. Anim. Reprod. Sci. 1996, 42, 109–117. [Google Scholar] [CrossRef]
- Chemineau, P.; Guillaume, D.; Migaud, M.; Thiéry, J.C.; Pellicer-Rubio, M.T.; Malpaux, B. Seasonality of reproduction in mammals: Intimate regulatory mechanisms and practical implications. Reprod. Domest. Anim. 2008, 43, 40–47. [Google Scholar] [CrossRef]
- Lincoln, G.A.; Lincoln, C.E.; McNeilly, A.S. Seasonal cycles in the blood plasma concentration of FSH, inhibin and testosterone, and testicular size in rams of wild, feral and domesticated breeds of sheep. J. Reprod. Fertil. 1990, 88, 623–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gastel, T.; Bielli, A.; Perez, R.; Lopez, A.; Castrillejo, A.; Tagle, R.; Laborde, D.; Forsberg, M.; Rodriguez-martinez, H. Seasonal variations in testicular morphology in Uruguayan Corriedale rams. Anim. Reprod. Sci. 1995, 40, 59–75. [Google Scholar] [CrossRef]
- Avdi, M.; Banos, G.; Stefos, K.; Chemineau, P. Seasonal variation in testicular volume and sexual behavior of Chios and Serres rams. Theriogenology 2004, 62, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.L.; Jansen, H.; Kao, C. Continuous exposure of Suffolk ewes to an equatorial photoperiod disrupts expression of the annual breeding season. Biol. Reprod. 1990, 42, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Aguiar, G.V.; van Tilburg, M.F.; Catunda, A.G.V.; Celes, C.K.S.; Lima, I.C.S.; Campos, A.C.N.; Moura, A.A.A.; Araújo, A.A. Sperm parameters and biochemical components of goat seminal plasma in the rainy and dry seasons in the Brazilian northeast: The season’s influence on the cooling of semen. Arq. Bras. Med. Vet. e Zootec. 2013, 65, 6–12. [Google Scholar] [CrossRef] [Green Version]
- Lozano, H.; Carvajal, M.; Manrique, P.; Grajales, H. Seminal quality parameters and its relationship with environmental variables in rams under colombian high tropic conditions. Actas Iberoam. Conserv. Anim. 2016, 8, 55–62. [Google Scholar]
- Goshme, S.; Banerjee, S.; Rekik, M.; Haile, A.; Yitagesu, E.; Getachew, T. Evaluation and characterization of semen quality in rams of Menz, Dorper and Awassi crosses in different seasons in Ethiopia. Livest. Res. Rural Dev. 2020, 32, 11. [Google Scholar]
- González-Arto, M.; Hamilton, T.R.D.S.; Gallego, M.; Gaspar-Torrubia, E.; Aguilar, D.; Serrano-Blesa, E.; Abecia, J.A.; Pérez-Pé, R.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; et al. Evidence of melatonin synthesis in the ram reproductive tract. Andrology 2016, 4, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Casao, A.; Gallego, M.; Abecia, J.A.; Forcada, F.; Ṕrez-Ṕ, R.; Muio-Blanco, T.; Cebrin-Ṕrez, J.L. Identification and immunolocalisation of melatonin MT1 and MT2 receptors in Rasa Aragonesa ram spermatozoa. Reprod. Fertil. Dev. 2012, 24, 953–961. [Google Scholar] [CrossRef]
- Casao, A.; Mendoza, N.; Pérez-Pé, R.; Grasa, P.; Abecia, J.A.; Forcada, F.; Cebrián-Pérez, J.A.; Muino-Blanco, T. Melatonin prevents capacitation and apoptotic-like changes of ram spermatozoa and increases fertility rate. J. Pineal Res. 2010, 48, 39–46. [Google Scholar] [CrossRef]
- Casao, A.; Pérez-Pé, R.; Abecia, J.A.; Forcada, F.; Muiño-Blanco, T.; Cebrián-Pérez, J.A. The effect of exogenous melatonin during the non-reproductive season on the seminal plasma hormonal profile and the antioxidant defence system of Rasa Aragonesa rams. Anim. Reprod. Sci. 2013, 138, 168–174. [Google Scholar] [CrossRef]
- Gonzalez-Arto, M.; Luna, C.; Pérez-Pé, R.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; Casao, A. New evidence of melatonin receptor contribution to ram sperm functionality. Reprod. Fertil. Dev. 2016, 28, 924–935. [Google Scholar] [CrossRef]
- Gimeno-Martos, S.; Casao, A.; Yeste, M.; Cebrián-Pérez, J.A.; Muiño-Blanco, T.; Pérez-Pé, R. Melatonin reduces cAMP-stimulated capacitation of ram spermatozoa. Reprod. Fertil. Dev. 2019, 31, 420–431. [Google Scholar] [CrossRef]
- Moreno, D.C.; Grajales, H.A. Caracterization of ovine systems in Colombian high tropics: Management, productive, and reproductive performanceindicators. Rev. Med. Vet. Zoot 2017, 64, 36–51. [Google Scholar] [CrossRef]
- Ocampo, R.J.; Martinez, R.A.; Rocha, J.J.; Cardona, H. Genetic characterization of Colombian indigenous sheep. Rev. Colomb. Ciencias Pecu. 2017, 30, 116–125. [Google Scholar] [CrossRef]
- Bravo, S.; Larama, G.; Ortíz, M.; Sepúlveda, N. Genetic differentiation between ‘Araucana’ creole and ‘Hampshire Down’ sheeps in Chile. Chil. J. Agric. Res. 2015, 75, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Beaty, T.; Williams, H.L. The reproductive performance of British breeds of sheep in an equatorial environment. II. Lowland breeds. Br. Vet. J. 1971, 127, 10–19. [Google Scholar] [CrossRef]
- Carvajal-Serna, M.; Torres-Ruda, F.; Cardozo, J.A.; Grajales-Lombana, H.; Cebrián-Pérez, J.Á.; Muiño-Blanco, T.; Pérez-Pé, R.; Casao, A. Changes in melatonin concentrations in seminal plasma are not correlated with testosterone or antioxidant enzyme activity when rams are located in areas with an equatorial photoperiod. Anim. Reprod. Sci. 2019, 200. [Google Scholar] [CrossRef]
- Carvajal-Serna, M.; Neira-Rivera, E.; Cardozo, J.A.; Grajales-Lombana, H.; Cebrián-Pérez, J.Á.; Muiño-Blanco, T.; Pérez-Pé, R.; Casao, A. Melatonin membrane receptors MT1 and MT2 are expressed in ram spermatozoa from non-seasonal breeds. Trop. Anim. Health Prod. 2020, 52, 2549–2557. [Google Scholar] [CrossRef]
- Ollero, M.; Muiño-Blanco, T.; López-Pérez, M.J.; Cebrián-Pérez, J.A. Viability of ram spermatozoa in relation to the abstinence period and successive ejaculations. Int. J. Androl. 1996, 19, 287–292. [Google Scholar] [CrossRef]
- García-López, N.; Ollero, M.; Muiño-Blanco, T.; Cebrián-Pérez, J.A. A dextran swim-up procedure for separation of highly motile and viable ram spermatozoa from seminal plasma. Theriogenology 1996, 46, 141–151. [Google Scholar] [CrossRef]
- Grasa, P.; Pérez-Pé, R.; Báguena, O.; Forcada, F.; Abecia, A.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. Ram sperm selection by a dextran/swim-up procedure increases fertilization rates following intrauterine insemination in superovulated ewes. J. Androl. 2004, 25, 982–990. [Google Scholar] [CrossRef]
- Parrish, J.J.; Susko-Parrish, J.; Winer, M.A.; First, N.L. Capacitation of bovine sperm by heparin. Biol. Reprod. 1988, 38, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Serna, M.; Cardozo, J.A.; Grajales-Lombana, H.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. Sperm quality and seminal plasma proteins in three sheep breeds under high altitude and tropical conditions. Span. J. Agric. Res. 2018, 16. [Google Scholar] [CrossRef] [Green Version]
- Martí, E.; Pérez-Pé, R.; Colás, C.; Muiño-Blanco, T.; Cebrián-Pérez, J.A. Study of apoptosis-related markers in ram spermatozoa. Anim. Reprod. Sci. 2008, 106, 113–132. [Google Scholar] [CrossRef]
- Ward, C.R.; Storey, B.T. Determination of the time course of capacitation in mouse spermatozoa using a chlortetracycline fluorescence assay. Dev. Biol. 1984, 104, 287–296. [Google Scholar] [CrossRef]
- Gillan, L.; Evans, A.G.; Maxwell, W.M.C. Capacitation status and fertility of fresh and frozen-thawed ram spermatozoa. Reprod. Fertil. Dev. 1997, 9, 481–487. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, S.T.; Maxwell, W.M.C. Kinematic definition of ram sperm hyperactivation. Reprod. Fertil. Dev. 1999, 11, 25–30. [Google Scholar] [CrossRef]
- Koopman, G.; Reutelingsperger, C.P.M.; Kuijten, G.A.M.; Keehnen, R.M.J.; Pals, S.T.; Van Oers, M.H.J. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 1994, 84, 1415–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martí, E.; Pérez-Pé, R.; Muiño-Blanco, T.; Cebrián-Pérez, J.A. Comparative study of four different sperm washing methods using apoptotic markers in ram spermatozoa. J. Androl. 2006, 27, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Sabido, O.; Durand, P.; Levy, R. Phosphatidylserine externalization in human sperm induced by calcium ionophore A23187: Relationship with apoptosis, membrane scrambling and the acrosome reaction. Hum. Reprod. 2005, 20, 3459–3468. [Google Scholar] [CrossRef] [Green Version]
- Luna, C.; Mendoza, N.; Casao, A.; Pérez-Ṕ, R.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. c-Jun N-terminal kinase and p38 mitogen-activated protein kinase pathways link capacitation with apoptosis and seminal plasma proteins protect sperm by interfering with both routes. Biol. Reprod. 2017, 96, 800–815. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Pé, R.; Grasa, P.; Fernández-Juan, M.; Peleato, M.L.; Cebrián-Pérez, J.Á.; Muiño-Blanco, T. Seminal plasma proteins reduce protein tyrosine phosphorylation in the plasma membrane of cold-shocked ram spermatozoa. Mol. Reprod. Dev. 2002, 61, 226–233. [Google Scholar] [CrossRef]
- Gimeno-Martos, S.; González-Arto, M.; Casao, A.; Gallego, M.; Cebrián-Pérez, J.A.; Muiño-Blanco, T.; Pérez-Pé, R. Steroid hormone receptors and direct effects of steroid hormones on ram spermatozoa. Reproduction 2017, 154, 469–481. [Google Scholar] [CrossRef]
- Leahy, T.; Rickard, J.P.; Aitken, R.J.; De Graaf, S.P. Penicillamine prevents ram sperm agglutination in media that support capacitation. Reproduction 2016, 151, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Witt-Enderby, P.A.; Bennett, J.; Jarzynka, M.J.; Firestine, S.; Melan, M.A. Melatonin receptors and their regulation: Biochemical and structural mechanisms. Life Sci. 2003, 72, 2183–2198. [Google Scholar] [CrossRef]
- González-Arto, M.; Vicente-Carrillo, A.; Martínez-Pastor, F.; Fernández-Alegre, E.; Roca, J.; Miró, J.; Rigau, T.; Rodríguez-Gil, J.E.; Pérez-Pé, R.; Muiño-Blanco, T.; et al. Melatonin receptors MT1and MT2 are expressed in spermatozoa from several seasonal and nonseasonal breeder species. Theriogenology 2016, 86, 1958–1968. [Google Scholar] [CrossRef]
- Young, C.; Grasa, P.; Coward, K.; Davis, L.C.; Parrington, J. Phospholipase C zeta undergoes dynamic changes in its pattern of localization in sperm during capacitation and the acrosome reaction. Fertil. Steril. 2009, 91, 2230–2242. [Google Scholar] [CrossRef]
- Espino, J.; Sc, M.; Monllor, F.; Sc, B.; Garc, J.F.; Rodr, A.B. Melatonin protects human spermatozoa from apoptosis via melatonin receptor—And extracellular signal—Regulated kinase-mediated pathways. Male Factor 2011, 95, 2290–2296. [Google Scholar] [CrossRef]
- Fujinoki, M. Melatonin-enhanced hyperactivation of hamster sperm. Reproduction 2008, 136, 533–541. [Google Scholar] [CrossRef] [Green Version]



| Variables | Total Motility | Progressive Motility | ||||
|---|---|---|---|---|---|---|
| Breed | Creole | Romney Marsh | Hampshire | Creole | Romney Marsh | Hampshire |
| Rainy season | ||||||
| Swim-up | 77.9 ± 3.7 aA | 72.8 ± 3.0 bA | 65.7 ± 3.9 *cA | 64.7 ± 11.7 aA | 41.3 ± 9.0 *bA | 43.9 ± 5.1 *bA |
| Cap-TALP | 68.6 ± 3.0 *aB | 55.4 ± 6.2 *bB | 29.0 ± 5.2 *cB | 44.4 ± 4.9 *aB | 27.5 ± 5.9 *bB | 5.8 ± 3.0 *cB |
| Cap-CK | 76.7 ± 2.8 *aA | 47.2 ± 6.8 *bC | 22.0 ± 3.8 *cC | 32.8 ± 7.0 *aC | 17.0 ± 4.1 *bC | 1.2 ± 0.3 *cC |
| Dry season | ||||||
| Swim-up | 78.8 ± 3.4 aA | 69.2 ± 4.0 bA | 72.9 ± 3.3 bA | 59.8 ± 3.9 aA | 53.3 ± 5.0 bA | 54.2 ± 0.6 abA |
| Cap-TALP | 46.9 ± 13.6 aB | 39.8 ± 3.6 bB | 36.7 ± 9.6 bB | 23.0 ± 11.5 aB | 13.0 ± 4.6 bB | 13.2 ± 5.3 bB |
| Cap-CK | 33.8 ± 5.8 C | 33.8 ± 5.2 B | 28.1 ± 8.5 C | 5.7 ± 1.9 C | 3.9 ± 1.6 C | 5.9 ± 3.0 C |
| Variables | Viability (Plasma Membrane Integrity %) | Viable Sperm without PS Translocation (%) | ||||
|---|---|---|---|---|---|---|
| Breed | Creole | Romney Marsh | Hampshire | Creole | Romney Marsh | Hampshire |
| Rainy season | ||||||
| Swim-up | 88.6 ± 3.6 *aA | 80.4 ± 5.3 bA | 86.5 ± 4.2 *aA | 62.4 ± 5.2 aA | 47.0 ± 3.5 bA | 40.4 ± 9.2 bA |
| Cap-TALP | 80.1 ± 1.3 *aB | 68.2 ± 5.9 *bB | 49.1 ± 4.5 *cB | 53.6 ± 5.9 aB | 53.0 ± 5.0 aA | 35.4 ± 0.7 bA |
| Cap-CK | 86.0 ± 1.0 *aA | 63.0 ± 6.5 *bB | 40.6 ± 3.9 *cC | 56.3 ± 6.4 aAB | 55.5 ± 7.5 aA | 40.7 ± 1.3 *bA |
| Dry season | ||||||
| Swim-up | 85.7 ± 2.9 aA | 79.2 ± 3.3 bA | 80.8 ± 3.9 abA | 70.4 ± 11.9 aA | 50.0 ± 6.5 bA | 39.8 ± 0.2 cA |
| Cap-TALP | 68.9 ± 8.5 aB | 54.6 ± 3.9 bB | 55.6 ± 2.2 bB | 70.0 ± 2.6 aA | 42.0 ± 10.6 bB | 37.0 ± 3.0 bA |
| Cap-CK | 71.7 ± 4.8 aB | 56.7 ± 3.9 bB | 53.5 ± 8.3 bB | 51.3 ± 12.0 aB | 43.0 ± 7.2 aAB | 32.5 ± 4.5 bA |
| Variables | Total Motility | Progressive Motility | ||||
|---|---|---|---|---|---|---|
| Breed | Creole | Romney Marsh | Hampshire | Creole | Romney Marsh | Hampshire |
| Rainy season | ||||||
| Cap-CK | 76.7 ± 2.8 *aA | 47.2 ± 6.8 *bA | 22.0 ± 3.8 *cA | 32.8 ± 7.0 *aA | 17.0 ± 4.1 *bA | 1.2 ± 0.3 *cA |
| Cap-CK-100 pM MEL | 67.8 ± 5.1 *aB | 51.0 ± 5.4 *bA | 22.9 ± 3.9 cA | 30.0 ± 4.9 *aA | 17.7 ± 4.1 *bA | 2.3 ± 0.9 cA |
| Cap-CK-1 µM MEL | 77.5 ± 2.1 *aA | 57.1 ± 2.4 *bB | 18.6 ± 1.5 cA | 32.3 ± 2.5 *aA | 18.1 ± 1.8 *bA | 1.7 ± 0.6 cA |
| Dry season | ||||||
| Cap-CK | 33.8 ± 5.8 A | 33.8 ± 5.2 A | 28.1 ± 8.5 A | 5.7 ± 1.9 A | 3.9 ± 1.6 A | 5.9 ± 3.0 A |
| Cap-CK-100 pM MEL | 30.5 ± 5.6 aA | 33.9 ± 6.5 aA | 21.9 ± 5.0 bB | 5.1 ± 2.3 A | 3.8 ± 1.3 A | 2.4 ± 0.7 B |
| Cap-CK-1 µM MEL | 35.1 ± 6.6 aA | 30.0 ± 4.0 aA | 23.3 ± 6.1 bA | 5.9 ± 2.8 A | 3.6 ± 1.3 A | 3.0 ± 1.3 B |
| Variables | Viability (Plasma Membrane Integrity %) | Viable Sperm without PS Translocation (%) | ||||
|---|---|---|---|---|---|---|
| Breed | Creole | Romney Marsh | Hampshire | Creole | Romney Marsh | Hampshire |
| Rainy season | ||||||
| Cap-CK | 86.0 ± 1.0 *aA | 63.0 ± 6.5 *bA | 40.6 ± 3.9 *cA | 56.3 ± 6.4 aA | 55.5 ± 7.5 aA | 40.7 ± 1.3 *bA |
| Cap-CK-100 pM MEL | 80.3 ± 3.7 *aB | 65.7 ± 7.1 *bA | 44.0 ± 6.3 cA | 72.9 ± 6.4 *aB | 57.0 ± 6.2 bA | 49.7 ± 7.5 *bB |
| Cap-CK-1 µM MEL | 84.8 ± 1.6 *aAB | 73.7 ± 3.5 *bB | 36.1 ± 5.8 *cB | 64.7 ± 3.3 *aA | 62.7 ± 7.7 *aB | 44.2 ± 8.4 bA |
| Dry season | ||||||
| Cap-CK | 71.7 ± 4.8 aA | 56.7 ± 3.9 bA | 53.5 ± 8.3 bA | 51.3 ± 12.0 aA | 43.0 ± 7.2 aA | 32.5 ± 4.5 bA |
| Cap-CK-100 pM MEL | 72.2 ± 4.4 aA | 54.7 ± 4.5 bA | 50.0 ± 5.7 bA | 51.7 ± 5.4 aA | 50.0 ± 1.5 aAB | 41.0 ± 4.0 bAB |
| Cap-CK-1 µM MEL | 71.5 ± 4.2 aA | 54.0 ± 2.60 bA | 50.1 ± 3.8 bA | 46.0 ± 4.6 aA | 55.7 ± 0.7 bB | 47.5 ± 10.5 aB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvajal-Serna, M.; Cardozo-Cerquera, J.A.; Grajales-Lombana, H.A.; Casao, A.; Pérez-Pe, R. Sperm Behavior and Response to Melatonin under Capacitating Conditions in Three Sheep Breeds Subject to the Equatorial Photoperiod. Animals 2021, 11, 1828. https://doi.org/10.3390/ani11061828
Carvajal-Serna M, Cardozo-Cerquera JA, Grajales-Lombana HA, Casao A, Pérez-Pe R. Sperm Behavior and Response to Melatonin under Capacitating Conditions in Three Sheep Breeds Subject to the Equatorial Photoperiod. Animals. 2021; 11(6):1828. https://doi.org/10.3390/ani11061828
Chicago/Turabian StyleCarvajal-Serna, Melissa, Jaime Antonio Cardozo-Cerquera, Henry Alberto Grajales-Lombana, Adriana Casao, and Rosaura Pérez-Pe. 2021. "Sperm Behavior and Response to Melatonin under Capacitating Conditions in Three Sheep Breeds Subject to the Equatorial Photoperiod" Animals 11, no. 6: 1828. https://doi.org/10.3390/ani11061828







