Impact of Dietary Crude Protein Level on Hepatic Lipid Metabolism in Weaned Female Piglets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Piglets and Experimental Design
2.2. Serum and Liver Collection
2.3. Triglyceride, Cholesterol, HDL-C, and LDL-C Determination
2.4. Urea Determination
2.5. Quantitative Real-Time PCR
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
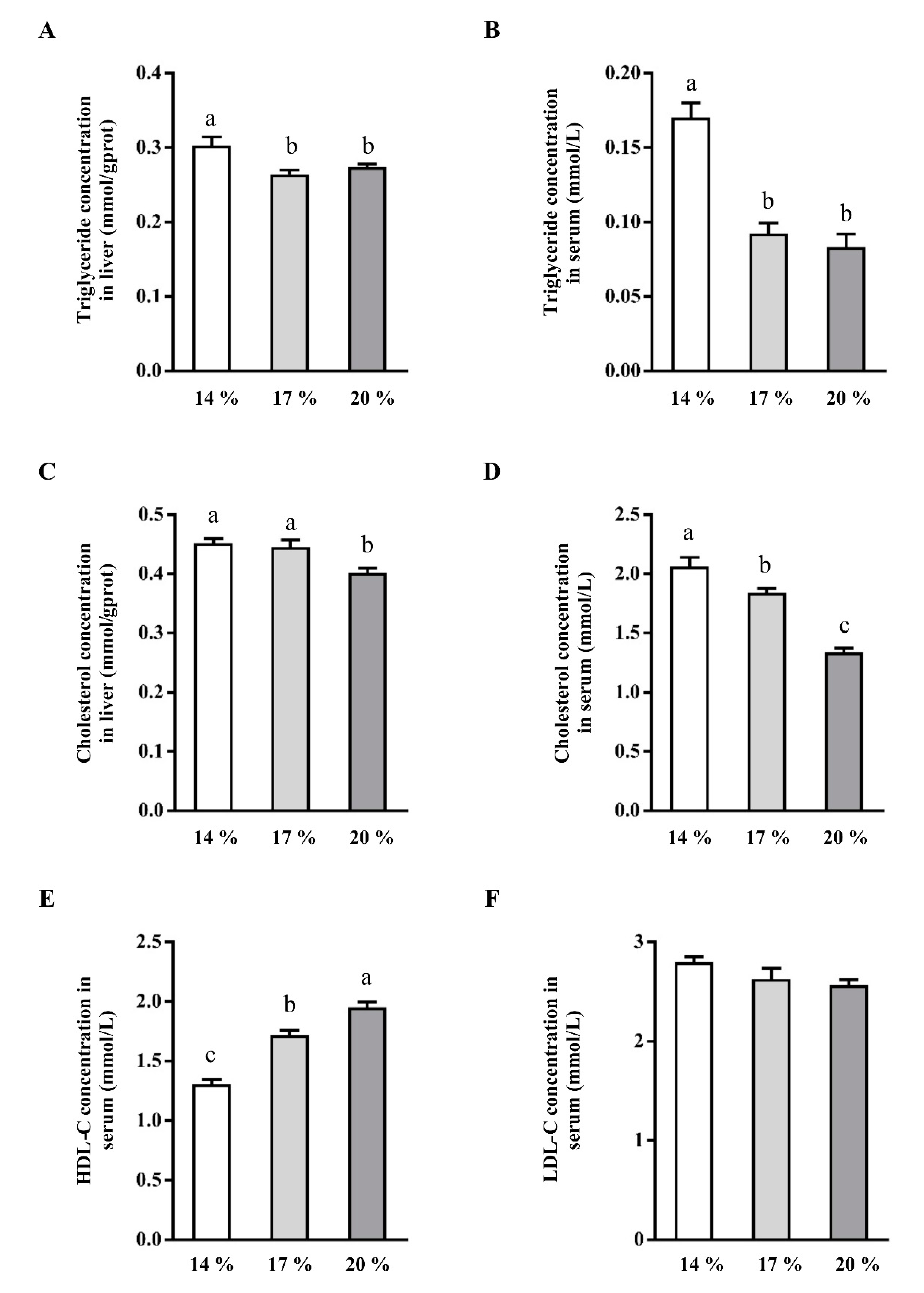
3.1. Effects of Dietary Crude Protein on Triglyceride and Cholesterol Concentration in Liver and Serum
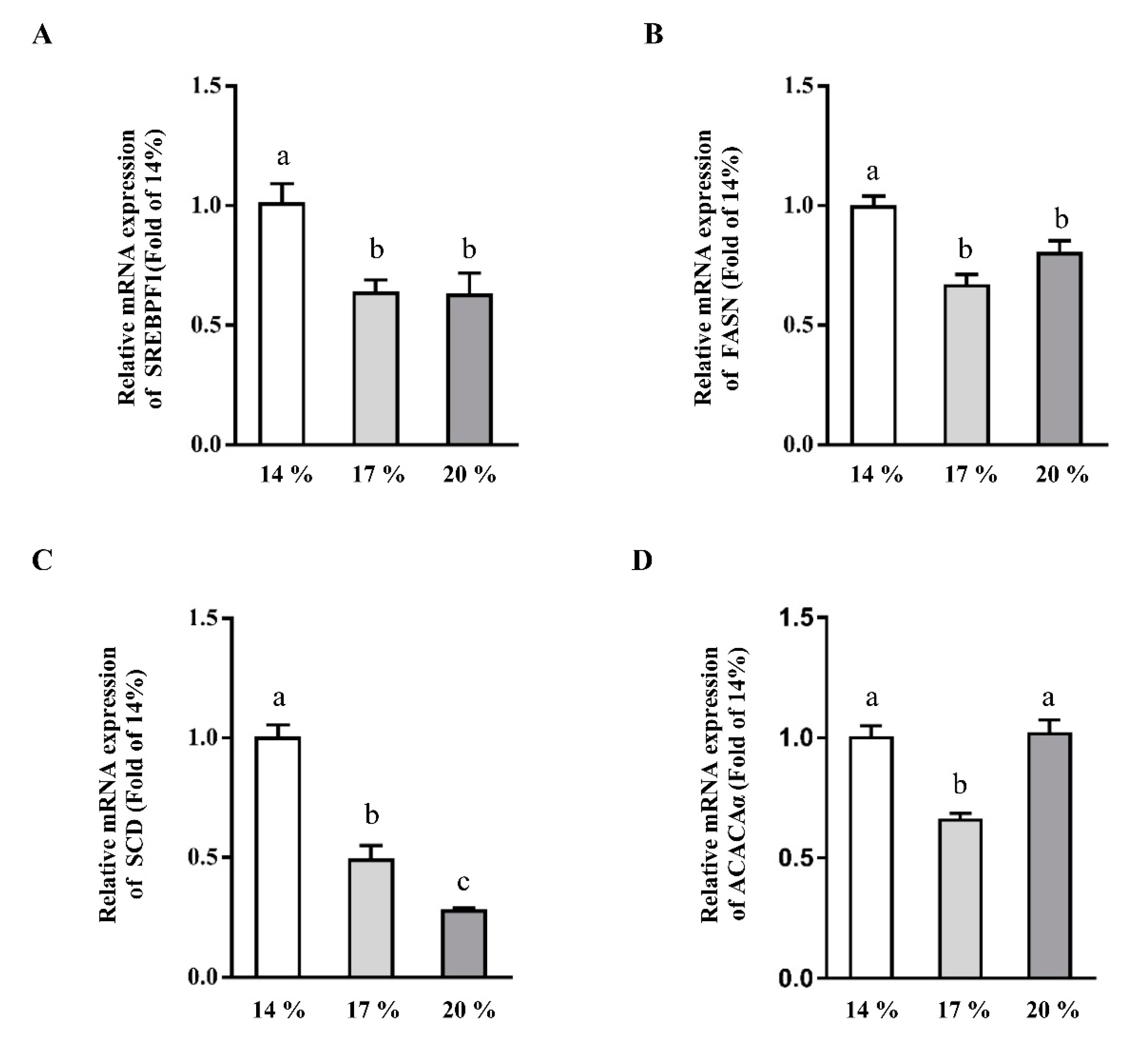
3.2. Effects of Dietary Crude Protein on Hepatic Lipogenesis Genes Expression
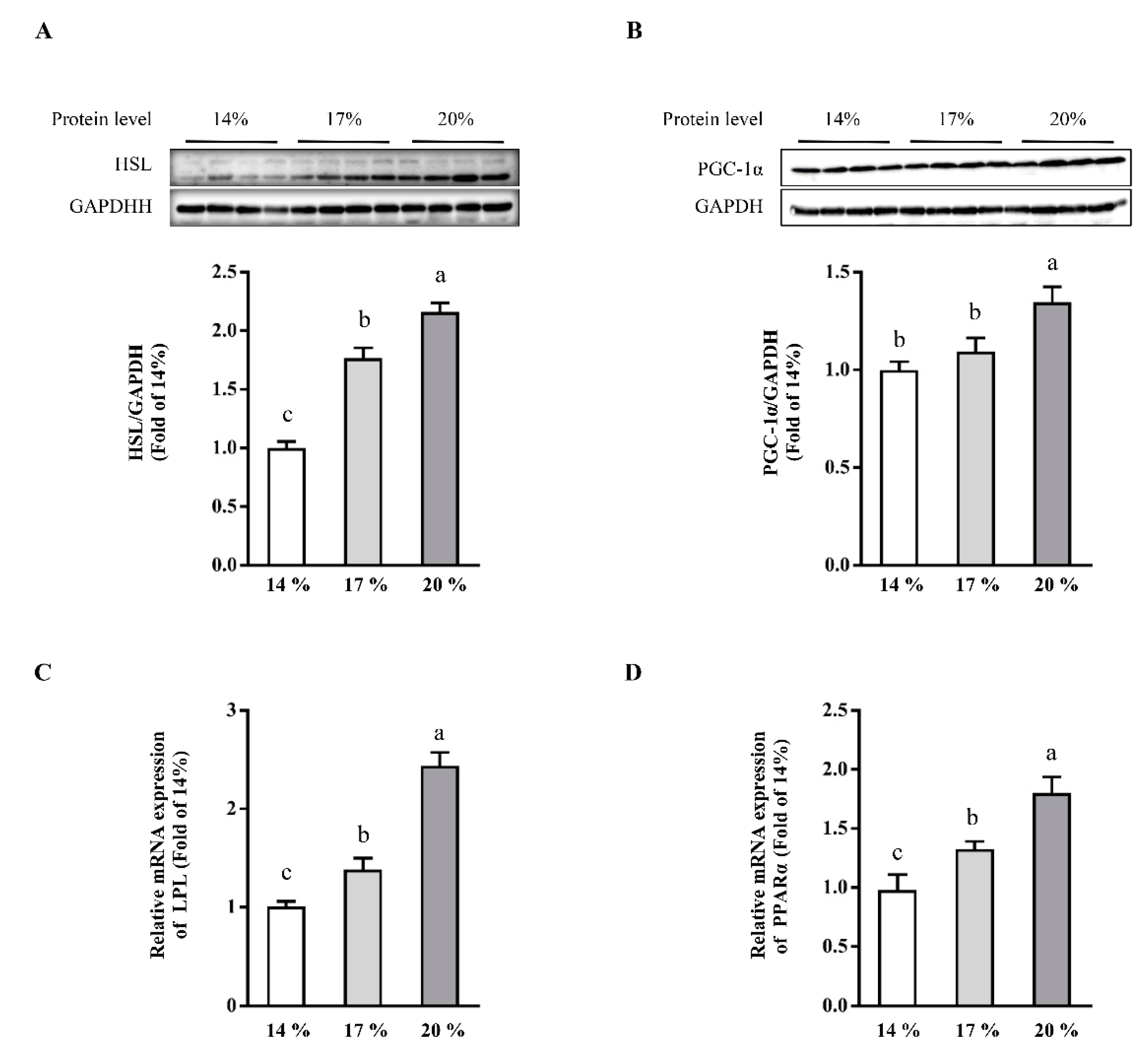
3.3. Effects of Dietary Crude Protein on Hepatic Lipolysis and Lipid Oxidation
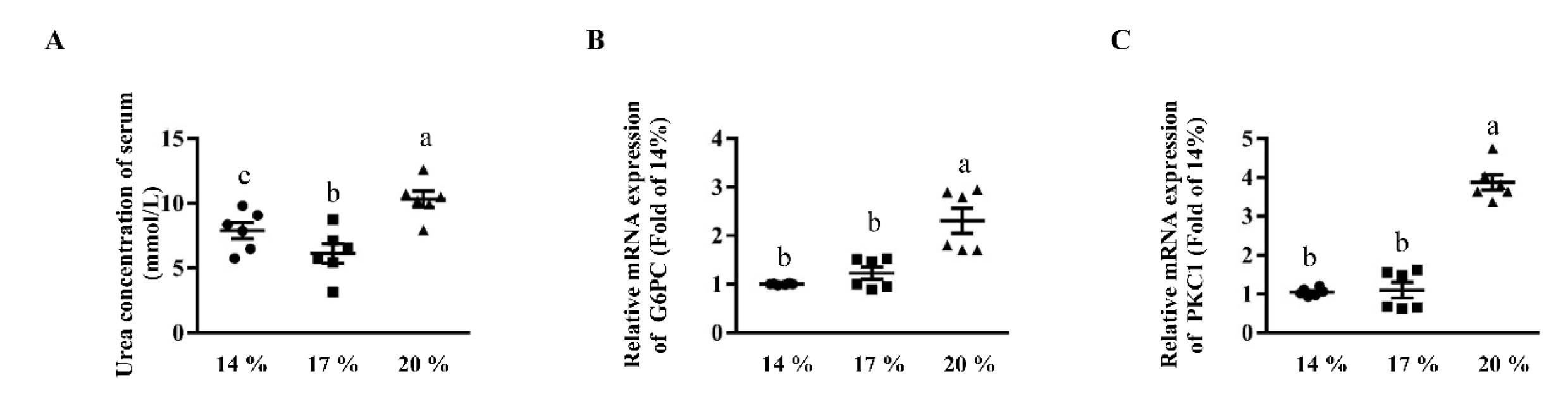
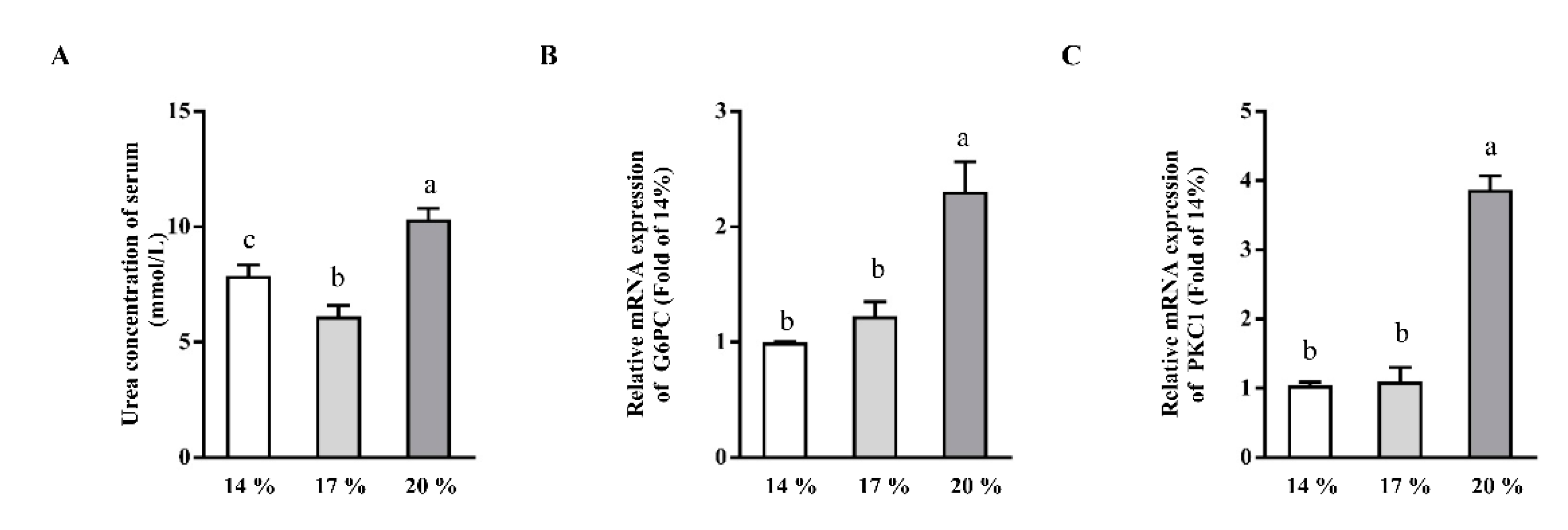
3.4. Effects of Dietary Crude Protein on Serum Urea Concentrations and Hepatic Gluconeogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACACα | Acetyl-CoA carboxylase alpha |
| AMPK | AMP-activated protein kinase |
| ApoB | Apolipoprotein B |
| BCAAs | Branched chain amino acids |
| ChREBP | Carbohydrate response element binding protein |
| CPT1B | Carnitine palmitoyltransferase 1B |
| EAA/NEAA | Essential Amino Acids/Nonessential amino acids |
| FA | Fatty acids |
| FASN | Fatty acid synthase |
| G6PC | Glucose-6-phosphatase catalytic subunit |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| HDL-C | High-density lipoprotein cholesterol |
| HMGCR | 3-Hydroxy-3-methylglutaryl coenzyme A reductase |
| HSL | Hormone-sensitive triglyceride lipase |
| LDL-C | Low-density lipoprotein cholesterol |
| LPL | Lipoprotein lipase |
| mmBCFAs | Monomethyl branched chain fatty acids |
| MTTP | Microsomal triglyceride transfer protein |
| NAFLD | Nonalcoholic fatty liver disease |
| NASH | Nonalcoholic steatohepatitis |
| NRC | National Research Council |
| PMSF | Phenylmethanesulfonyl fluoride |
| PKC1 | Phosphoenolpyruvate carboxykinase 1 |
| PGC-1α | Peroxisome proliferator-activated receptor-γ coactivator 1α |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| SCD | Stearoyl-CoA desaturase |
| SREBPF1 | Sterol regulatory element binding transcription factor 1 |
| TG | Triglyceride |
| UCP | Uncoupling protein |
Appendix A



References
- Lavoie, J.M.; Gauthier, M.S. Regulation of fat metabolism in the liver: Link to non-alcoholic hepatic steatosis and impact of physical exercise. Cell. Mol. Life Sci. 2006, 63, 1393–1409. [Google Scholar] [CrossRef] [PubMed]
- Yki-Järvinen, H. Liver fat in the pathogenesis of insulin resistance and type 2 diabetes. Dig. Dis. 2010, 28, 203–209. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postic, C.; Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J. Clin. Investig. 2008, 118, 829. [Google Scholar] [CrossRef] [Green Version]
- Fotschki, B.; Jurgonski, A.; Juskiewicz, J.; Zdunczyk, Z. Dietary Supplementation with Raspberry Seed Oil Modulates Liver Functions, Inflammatory State, and Lipid Metabolism in Rats. J. Nutr. 2015, 145, 1793–1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.M.; Brunt, E.M. Pathology of nonalcoholic fatty liver disease. Am. J. Clin. Pathol. 2007, 128, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Hooper, A.J.; Adams, L.A.; Burnett, J.R. Genetic determinants of hepatic steatosis in man. J. Lipid Res. 2011, 52, 593–617. [Google Scholar] [CrossRef] [Green Version]
- Dongiovanni, P.; Lanti, C.; Riso, P.; Valenti, L. Nutritional therapy for nonalcoholic fatty liver disease. J. Nutr. Biochem. 2016, 29, 1–11. [Google Scholar] [CrossRef]
- Garcia-Caraballo, S.C.; Comhair, T.M.; Verheyen, F.; Gaemers, I.; Schaap, F.G.; Houten, S.M.; Hakvoort, T.B.; Dejong, C.H.; Lamers, W.H.; Koehler, S.E. Prevention and reversal of hepatic steatosis with a high-protein diet in mice. Biochim. Biophys. Acta 2013, 1832, 685–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Wang, J.; Song, X.; Zhang, X.; Ge, C.; Gao, S. Impact of dietary protein on lipid metabolism-related gene expression in porcine adipose tissue. Nutr. Metab. 2010, 7, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Ruan, Z.; Gao, Y.; Yin, Y.; Zhou, X.; Wang, L.; Geng, M.; Hou, Y.; Wu, G. Dietary supplementation with L-arginine or N-carbamylglutamate enhances intestinal growth and heat shock protein-70 expression in weanling pigs fed a corn- and soybean meal-based diet. Amino Acids 2010, 39, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhu, W.; Hang, S. Effects of low-protein diet on the intestinal morphology, digestive enzyme activity, blood urea nitrogen, and gut microbiota and metabolites in weaned pigs. Arch. Anim. Nutr. 2019, 73, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.L.; Zhang, J.; Wu, G.; Zhu, W.Y. Utilization of amino acids by bacteria from the pig small intestine. Amino Acids 2010, 39, 1201–1215. [Google Scholar] [CrossRef]
- Zhang, S.; Qiao, S.; Ren, M.; Zeng, X.; Ma, X.; Wu, Z.; Thacker, P.; Wu, G. Supplementation with branched-chain amino acids to a low-protein diet regulates intestinal expression of amino acid and peptide transporters in weanling pigs. Amino Acids 2013, 45, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; He, L.Q.; Cui, Z.J.; Liu, G.; Yao, K.; Wu, F.; Li, J.; Li, T.J. Effects of reducing dietary protein on the expression of nutrition sensing genes (amino acid transporters) in weaned piglets. J. Zhejiang Univ. Sci. B 2015, 16, 496–502. [Google Scholar] [CrossRef] [Green Version]
- Haynes, T.E.; Li, P.; Li, X.; Shimotori, K.; Sato, H.; Flynn, N.E.; Wang, J.; Knabe, D.A.; Wu, G. L-Glutamine or L-alanyl-L-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids 2009, 37, 131–142. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Dai, Z.; Li, D.; Wang, J.; Wu, Z. Amino acid nutrition in animals: Protein synthesis and beyond. Annu. Rev. Anim. Biosci. 2014, 2, 387–417. [Google Scholar] [CrossRef]
- Borsheim, E.; Bui, Q.U.; Tissier, S.; Cree, M.G.; Ronsen, O.; Morio, B.; Ferrando, A.A.; Kobayashi, H.; Newcomer, B.R.; Wolfe, R.R. Amino acid supplementation decreases plasma and liver triacylglycerols in elderly. Nutrition 2009, 25, 281–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, J.; Masaki, T.; Arakawa, M.; Seike, M.; Yoshimatsu, H. Isoleucine prevents the accumulation of tissue triglycerides and upregulates the expression of PPARalpha and uncoupling protein in diet-induced obese mice. J. Nutr. 2010, 140, 496–500. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Satterfield, M.C.; Bazer, F.W.; Wu, G. Regulation of brown adipose tissue development and white fat reduction by L-arginine. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 529–538. [Google Scholar] [CrossRef]
- Wu, G.; Collins, J.K.; Perkins-Veazie, P.; Siddiq, M.; Dolan, K.D.; Kelly, K.A.; Heaps, C.L.; Meininger, C.J. Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J. Nutr. 2007, 137, 2680–2685. [Google Scholar] [CrossRef]
- Prada, P.; Hirabara, S.; De Souza, C.; Schenka, A.; Zecchin, H.; Vassallo, J.; Velloso, L.; Carneiro, E.; Carvalheira, J.; Curi, R. L-glutamine supplementation induces insulin resistance in adipose tissue and improves insulin signalling in liver and muscle of rats with diet-induced obesity. Diabetologia 2007, 50, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Kimball, S.R.; Jefferson, L.S. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J. Nutr. 2006, 136, 227S–231S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kniazeva, M.; Euler, T.; Han, M. A branched-chain fatty acid is involved in post-embryonic growth control in parallel to the insulin receptor pathway and its biosynthesis is feedback-regulated in C. elegans. Genes Dev. 2008, 22, 2102–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Dai, Z.; Wu, Z.; Lin, G.; Jia, S.; Hu, S.; Dahanayaka, S.; Wu, G. Glycine is a nutritionally essential amino acid for maximal growth of milk-fed young pigs. Amino Acids 2014, 46, 2037–2045. [Google Scholar] [CrossRef]
- Liu, N.; Dai, Z.; Zhang, Y.; Chen, J.; Yang, Y.; Wu, G.; Tso, P.; Wu, Z. Maternal L-proline supplementation enhances fetal survival, placental development, and nutrient transport in micedagger. Biol. Reprod. 2019, 100, 1073–1081. [Google Scholar] [CrossRef]
- Wang, H.; Ji, Y.; Wu, G.; Sun, K.; Sun, Y.; Li, W.; Wang, B.; He, B.; Zhang, Q.; Dai, Z.; et al. l-Tryptophan Activates Mammalian Target of Rapamycin and Enhances Expression of Tight Junction Proteins in Intestinal Porcine Epithelial Cells. J. Nutr. 2015, 145, 1156–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.; Ma, X.; Luo, X.; Zhang, Y.; He, Y.; Dai, Z.; Yang, Y.; Wu, G.; Wu, Z. L-Glutamine Attenuates Apoptosis in Porcine Enterocytes by Regulating Glutathione-Related Redox Homeostasis. J. Nutr. 2018, 148, 526–534. [Google Scholar] [CrossRef]
- Pierzchala, M.; Pareek, C.S.; Urbanski, P.; Goluch, D.; Kamyczek, M.; Rozycki, M.; Kuryl, J. Selection of reference genes for gene expression studies in porcine hepatic tissue using quantitative real-time polymerase chain reaction. Anim. Sci. Pap. Rep. 2011, 29, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.W.; Qiao, S.Y.; Li, D.F. Amino acids and gut function. Amino Acids 2009, 37, 105–110. [Google Scholar] [CrossRef]
- Reddy, J.K.; Rao, M.S. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G852–G858. [Google Scholar] [CrossRef] [Green Version]
- Stepien, M.; Gaudichon, C.; Fromentin, G.; Even, P.; Tome, D.; Azzout-Marniche, D. Increasing protein at the expense of carbohydrate in the diet down-regulates glucose utilization as glucose sparing effect in rats. PLoS ONE 2011, 6, e14664. [Google Scholar] [CrossRef]
- Nakao, H.; Yoneda, M. The intertwisted correlations among non-alcoholic fatty liver disease, atherosclerosis, and metabolic syndrome. J. Gastroenterol. 2009, 44, 1162–1164. [Google Scholar] [CrossRef]
- Bortolotti, M.; Kreis, R.; Debard, C.; Cariou, B.; Faeh, D.; Chetiveaux, M.; Ith, M.; Vermathen, P.; Stefanoni, N.; Lê, K.-A. High protein intake reduces intrahepatocellular lipid deposition in humans. Am. J. Clin. Nutr. 2009, 90, 1002–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia Caraballo, S.C.; Comhair, T.M.; Houten, S.M.; Dejong, C.H.; Lamers, W.H.; Koehler, S.E. High-protein diets prevent steatosis and induce hepatic accumulation of monomethyl branched-chain fatty acids. J. Nutr. Biochem. 2014, 25, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.S.; Tjonn, S.L.; Swan, P.D. High-protein, low-fat diets are effective for weight loss and favorably alter biomarkers in healthy adults. J. Nutr. 2004, 134, 586–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belobrajdic, D.P.; McIntosh, G.H.; Owens, J.A. A high-whey-protein diet reduces body weight gain and alters insulin sensitivity relative to red meat in wistar rats. J. Nutr. 2004, 134, 1454–1458. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, E.; Ishihara, A.; Tamai, S.; Nemoto, A.; Iwase, K.; Hiwasa, T.; Shibata, S.; Takiguchi, M. Time of day and nutrients in feeding govern daily expression rhythms of the gene for sterol regulatory element-binding protein (SREBP)-1 in the mouse liver. J. Biol. Chem. 2010, 285, 33028–33036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, S.M.; Dobrzyn, A.; Dobrzyn, P.; Lee, S.H.; Miyazaki, M.; Ntambi, J.M. Stearoyl-CoA desaturase 1 deficiency elevates insulin-signaling components and down-regulates protein-tyrosine phosphatase 1B in muscle. Proc. Natl. Acad. Sci. USA 2003, 100, 11110–11115. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, M.; Dobrzyn, A.; Sampath, H.; Lee, S.H.; Man, W.C.; Chu, K.; Peters, J.M.; Gonzalez, F.J.; Ntambi, J.M. Reduced adiposity and liver steatosis by stearoyl-CoA desaturase deficiency are independent of peroxisome proliferator-activated receptor-alpha. J. Biol. Chem. 2004, 279, 35017–35024. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Ma, X.; Yang, Y.; Dai, Z.; Wu, Z.; Wu, G. Glycine enhances expression of adiponectin and IL-10 in 3T3-L1 adipocytes without affecting adipogenesis and lipolysis. Amino Acids 2018, 50, 629–640. [Google Scholar] [CrossRef]
- Miles, J.M.; Park, Y.S.; Walewicz, D.; Russell-Lopez, C.; Windsor, S.; Isley, W.L.; Coppack, S.W.; Harris, W.S. Systemic and Forearm Triglyceride Metabolism Fate of Lipoprotein Lipase-Generated Glycerol and Free Fatty Acids. Diabetes 2004, 53, 521–527. [Google Scholar] [CrossRef] [Green Version]
- Egan, J.J.; Greenberg, A.S.; Chang, M.-K.; Wek, S.A.; Moos, M.C.; Londos, C. Mechanism of hormone-stimulated lipolysis in adipocytes: Translocation of hormone-sensitive lipase to the lipid storage droplet. Proc. Natl. Acad. Sci. USA 1992, 89, 8537–8541. [Google Scholar] [CrossRef] [Green Version]
- Bender, D.A. The metabolism of “surplus” amino acids. Br. J. Nutr. 2012, 108 (Suppl. S2), S113–S121. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; He, J.; Ji, X.; Zheng, W.; Yao, W. A Moderate Reduction of Dietary Crude Protein Provide Comparable Growth Performance and Improve Metabolism via Changing Intestinal Microbiota in Sushan Nursery Pigs. Animals 2021, 11, 1166. [Google Scholar] [CrossRef] [PubMed]
- Marin-Garcia, P.J.; Lopez-Lujan, M.C.; Rodenas, L.; Martinez-Paredes, E.M.; Blas, E.; Pascual, J.J. Plasmatic Urea Nitrogen in Growing Rabbits with Different Combinations of Dietary Levels of Lysine, Sulphur Amino Acids and Threonine. Animals 2020, 10, 946. [Google Scholar] [CrossRef]
- Attia, Y.A.; Bovera, F.; Wang, J.; Al-Harthi, M.A.; Kim, W.K. Multiple Amino Acid Supplementations to Low-Protein Diets: Effect on Performance, Carcass Yield, Meat Quality and Nitrogen Excretion of Finishing Broilers under Hot Climate Conditions. Animals 2020, 10, 973. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; De Jesús, R.; Freire, T.; Oliveira, C.; Castro Lyra, A.; Lyra, L. Possible molecular mechanisms soy-mediated in preventing and treating nonalcoholic fatty liver disease. Nutr. Hosp. 2012, 27, 991–998. [Google Scholar]
- Doisaki, M.; Katano, Y.; Nakano, I.; Hirooka, Y.; Itoh, A.; Ishigami, M.; Hayashi, K.; Goto, H.; Fujita, Y.; Kadota, Y. Regulation of hepatic branched-chain α-keto acid dehydrogenase kinase in a rat model for type 2 diabetes mellitus at different stages of the disease. Biochem. Biophys. Res. Commun. 2010, 393, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Chevrier, G.; Mitchell, P.L.; Rioux, L.E.; Hasan, F.; Jin, T.; Roblet, C.R.; Doyen, A.; Pilon, G.; St-Pierre, P.; Lavigne, C.; et al. Low-Molecular-Weight Peptides from Salmon Protein Prevent Obesity-Linked Glucose Intolerance, Inflammation, and Dyslipidemia in LDLR-/-/ApoB100/100 Mice. J. Nutr. 2015, 145, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Item | Content, % | ||
|---|---|---|---|
| 14% CP | 17% CP | 20% CP | |
| Crude protein | 14.14 | 17.32 | 20.17 |
| EAA 2 | |||
| Arginine | 0.71 | 0.93 | 1.09 |
| Histidine | 0.30 | 0.37 | 0.44 |
| Isoleucine | 0.46 | 0.60 | 0.71 |
| Leucine | 1.11 | 1.32 | 1.52 |
| Lysine | 1.26 | 1.25 | 1.26 |
| Methionine | 0.41 | 0.42 | 0.40 |
| Methionine + Cystine | 0.63 | 0.65 | 0.62 |
| Phenylalanine | 0.56 | 0.70 | 0.81 |
| Threonine | 0.76 | 0.75 | 0.76 |
| Tryptophan | 0.20 | 0.20 | 0.20 |
| Tyrosine | 0.41 | 0.50 | 0.59 |
| Valine | 0.54 | 0.64 | 0.72 |
| NEAA 3 | |||
| Alanine | 0.75 | 0.90 | 1.07 |
| Asparagine | 1.15 | 1.49 | 1.76 |
| Cystine | 0.22 | 0.23 | 0.22 |
| Glutamate | 2.28 | 2.78 | 3.15 |
| Glycine | 0.53 | 0.71 | 0.92 |
| Proline | 0.90 | 1.04 | 1.17 |
| Serine | 0.60 | 0.74 | 0.85 |
| EAA | 6.29 | 7.18 | 7.91 |
| NEAA | 6.84 | 8.40 | 9.74 |
| EAA/NEAA | 0.92 | 0.85 | 0.81 |
| Calculated nutritional value | |||
| DE 4 (MJ/kg) | 14.60 | 14.60 | 14.60 |
| Total calcium | 0.70 | 0.71 | 0.69 |
| Total phosphorus | 0.53 | 0.55 | 0.57 |
| Genes | Accession No. | Primers |
|---|---|---|
| SREBPF1 | NM_214157.1 | F: 5′-GACCCCACCAGTCCTGATG-3′ |
| R: 5′-ACGGGTACATCTTCAGCGG-3′ | ||
| FASN | NM_001099930.1 | F: 5′-GTTCCAAGGAGCAAGGTGTG-3′ |
| R: 5′-GCTTCGATGTACTCCAGGGA-3′ | ||
| ACACα | NM_001114269.1 | F: 5′-ATGTCTGGCTTGCACCTAGT-3′ |
| R: 5′-ATAAGACCACCGGCGGATAG-3′ | ||
| SCD | NM_213781.1 | F: 5′-AGAAGACATCCGCCCTGAAA-3′ |
| R: 5′-TCTTGCAGGTGGGGATCAAT-3′ | ||
| LPL | NM_214286.1 | F: 5′-CGCGGACAGAATTTCAGGAG-3′ |
| R: 5′-GGCAAGTGTCCTCAACTGTG-3′ | ||
| PPARα | NM_001044526.1 | F: 5′-GCAAGCTTGGACTTGAACGA-3′ |
| R: 5′-GCATCCCGTCCTTGTTCATC-3′ | ||
| G6PC | NM_001113445.1 | F: 5′-TGTGGGCATCAAACTCCTCT-3′ |
| R: 5′-GCTTTATCAGTGGCACCGAG-3′ | ||
| PKC1 | FJ668384.1 | F: 5′-CGTTTACTGGGAAGGCATCG-3′ |
| R: 5′-TTCCCCTACAACAGCCAGAG-3′ | ||
| GAPDH | NM_001206359.1 | F: 5′-GTCGGAGTGAACGGATTTGG-3′ |
| R: 5′-AGTGGAGGTCAATGAAGGGG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Ji, Y.; Yang, Y.; Jia, H.; Si, X.; Jiang, D.; Zhang, Y.; Dai, Z.; Wu, Z. Impact of Dietary Crude Protein Level on Hepatic Lipid Metabolism in Weaned Female Piglets. Animals 2021, 11, 1829. https://doi.org/10.3390/ani11061829
Liu N, Ji Y, Yang Y, Jia H, Si X, Jiang D, Zhang Y, Dai Z, Wu Z. Impact of Dietary Crude Protein Level on Hepatic Lipid Metabolism in Weaned Female Piglets. Animals. 2021; 11(6):1829. https://doi.org/10.3390/ani11061829
Chicago/Turabian StyleLiu, Ning, Yun Ji, Ying Yang, Hai Jia, Xuemeng Si, Da Jiang, Yunchang Zhang, Zhaolai Dai, and Zhenlong Wu. 2021. "Impact of Dietary Crude Protein Level on Hepatic Lipid Metabolism in Weaned Female Piglets" Animals 11, no. 6: 1829. https://doi.org/10.3390/ani11061829
APA StyleLiu, N., Ji, Y., Yang, Y., Jia, H., Si, X., Jiang, D., Zhang, Y., Dai, Z., & Wu, Z. (2021). Impact of Dietary Crude Protein Level on Hepatic Lipid Metabolism in Weaned Female Piglets. Animals, 11(6), 1829. https://doi.org/10.3390/ani11061829







