Reproductive Anatomy of Chondrichthyans: Notes on Specimen Handling and Sperm Extraction. I. Rays and Skates
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Origins of the Specimens
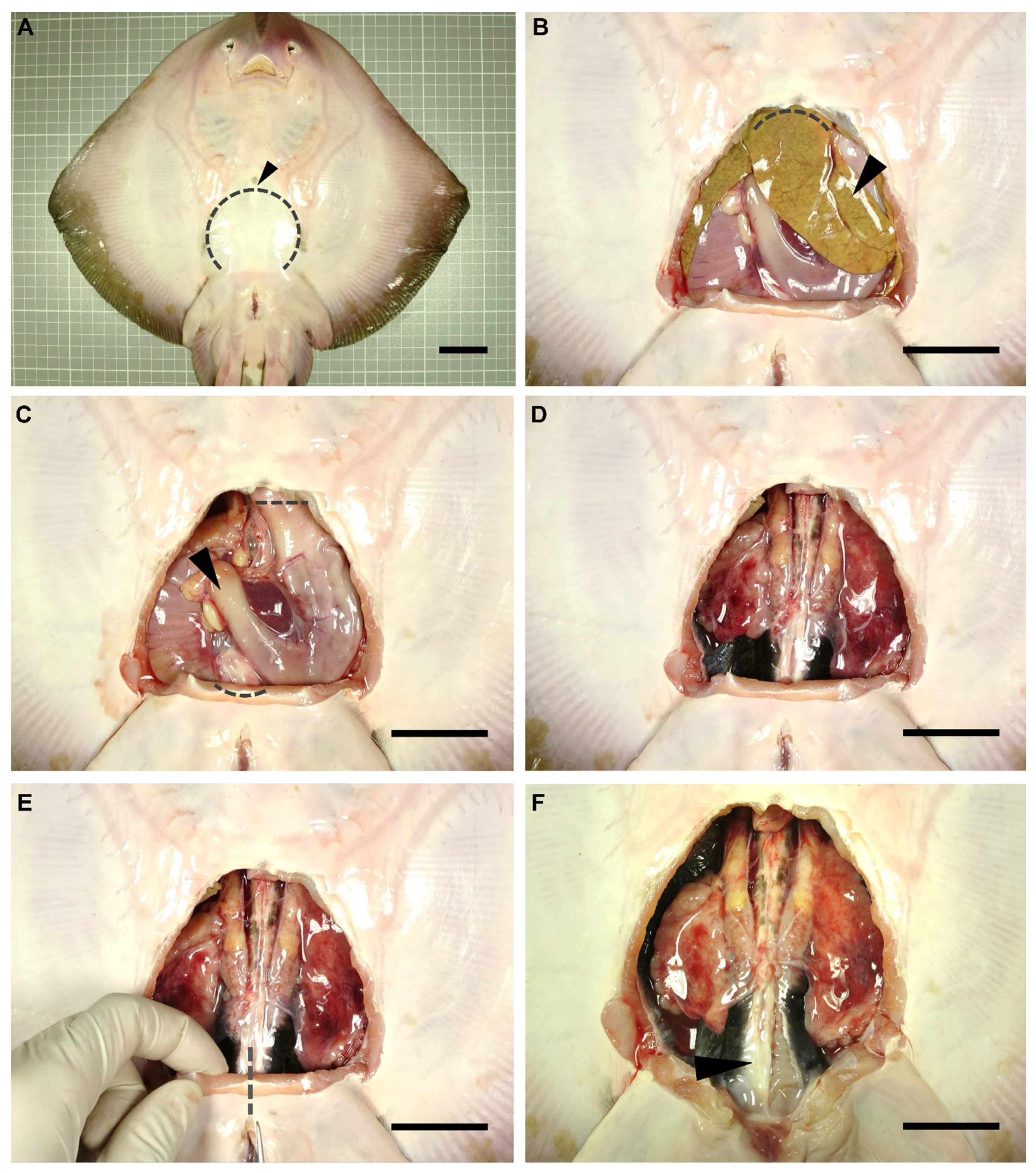
2.2. Dissection Procedure
2.3. Description of Reproductive Structures
2.4. Sperm Collection
2.4.1. In Vivo Sperm Extraction
2.4.2. Post-Mortem Sperm Extraction
3. Results and Disscusions
3.1. Female General Anatomy
3.2. Female Comparative Anatomy
3.3. Anatomic Notes for Artificial Insemination
3.4. Male General Anatomy
3.5. Male Comparative Anatomy
3.6. Anatomic Notes for Sperm Extraction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Compagno, L.J.V. Alternative life-history styles of cartilaginous fishes in time and space. Environ. Biol. Fishes 1990, 28, 33–75. [Google Scholar] [CrossRef]
- Stevens, J. The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 2000, 57, 476–494. [Google Scholar] [CrossRef]
- Fricke, R.; Eschmeyer, W.N.; Van der Laan, R. CAS—Eschmeyer’s Catalog of Fishes. Available online: https://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 7 April 2021).
- Weigmann, S. Annotated checklist of the living sharks, batoids and chimaeras (Chondrichthyes) of the world, with a focus on biogeographical diversity. J. Fish Biol. 2016, 88, 837–1037. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.L.; Cavanagh, R.D. Sharks, Rays and Chimaeras: The Status of the Chondrichthyan Fishes: Status Survey; IUCN: Gland, Switzerland, 2005; Volume 63, ISBN 2831707005. [Google Scholar]
- Last, P.; Naylor, G.; Séret, B.; White, W.; de Carvalho, M.; Stehmann, M. Rays of the World; CSIRO Publishing: Clayton, Australia, 2016; ISBN 0643109145. [Google Scholar]
- García, V.B.; Lucifora, L.O.; Myers, R.A. The importance of habitat and life history to extinction risk in sharks, skates, rays and chimaeras. Proc. R. Soc. B Biol. Sci. 2008, 275, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulvy, N.K.; Forrest, R.E. Life histories, population dynamics and extinction risks in chondrichthyans. In Biology of Sharks and their Relatives, 2nd ed.; Carrier, J.D., Musick, J.A., Heithaus, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2010; Volume 2, pp. 639–679. [Google Scholar]
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.; Fordham, S.V.; Francis, M.P.; et al. Extinction risk and conservation of the world’s sharks and rays. Elife 2014, 3, 1–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacoureau, N.; Rigby, C.L.; Kyne, P.M.; Sherley, R.B.; Winker, H.; Carlson, J.K.; Fordham, S.V.; Barreto, R.; Fernando, D.; Francis, M.P. Half a century of global decline in oceanic sharks and rays. Nature 2021, 589, 567–571. [Google Scholar] [CrossRef]
- Cortés, E. Life History Patterns and Correlations in Sharks. Rev. Fish. Sci. 2000, 8, 299–344. [Google Scholar] [CrossRef]
- Walker, T.I. Reproduction of Chondrichthyans. In Reproduction in Aquatic Animals; Yoshida., M., Asturiano, J.F., Eds.; Springer: Singapore, 2020; pp. 193–223. [Google Scholar]
- Musick, J.A.; Ellis, J.K.; Hamlett, W. Reproductive evolution of chondrichthyans. In Reproductive Biology and Phylogeny of Chondrichthyes, Sharks, Batoids and Chimaeras; Hamlett, W.C., Ed.; Science Publishers: Enfield, NH, USA, 2005; pp. 45–71. [Google Scholar]
- Conrath, C.L.; Musick, J.A. Reproductive biology of elasmobranchs. In Biology of Sharks and their Relatives, 2nd ed.; Carrier, J.D., Musick, J.A., Heithaus, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2010; Volume 2, pp. 291–311. [Google Scholar]
- Wourms, J.P. Reproduction and development in chondrichthyan fishes. Am. Zool. 1977, 17, 379–410. [Google Scholar] [CrossRef]
- Janse, M.; Zimmerman, B.; Geerlings, L.; Brown, C.; Nagelkerke, L.A.J. Sustainable species management of the elasmobranch populations within European aquariums: A conservation challenge. J. Zoo Aquar. Res. 2017, 5, 172–181. [Google Scholar] [CrossRef]
- Henningsen, A.D.; Smale, M.J.; Gordon, I.; Garner, R.; Marin-Osorno, R.; Kinnunen, N. Captive breeding and sexual conflict in elasmobranchs. In The Elasmobranch Husbandry Manual: Captive Care of Sharks, Rays and their Relatives; Smith, M., Warmolts, D., Thoney, D., Hueter, R., Eds.; Special Publication of the Ohio Biological Survey: Columbus, OH, USA, 2004; pp. 237–248. [Google Scholar]
- Daly, J.; Jones, R. The use of reproductive technologies in breeding programs for elasmobranchs in aquaria. In The Elasmobranch Husbandry Manual II: Recent Advances in the Care of Sharks, Rays and their Relatives; Smith, M., Warmolts, D., Thoney, D., Hueter, R., Murray, M., Ezcurra, J., Eds.; Special Publication of the Ohio Biological Survey: Columbus, OH, USA, 2017; pp. 363–374. [Google Scholar]
- Luer, C.A.; Walsh, C.J.; Bodine, A.B.; Wyffels, J.T. Normal embryonic development in the clearnose skate, Raja eglanteria, with experimental observations on artificial insemination. In Biology of Skates; Ebert, D.A., Sulikowski, J.A., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 133–149. [Google Scholar]
- Daochai, C.; Keschumras, N.; Chansue, N.; Haetrakul, T. Preliminary of intra-vagina artificial insemination using fresh semen in Ocellate river stingray (Potamotrygon motoro). Thai J. Vet. Med. 2020, 50, 383–385. [Google Scholar]
- Penfold, L.M.; Wyffels, J.T. Reproductive science in sharks and rays. Adv. Exp. Med. Biol. 2019, 1200, 465–488. [Google Scholar] [CrossRef]
- Daly, J.; Holland, M.; Galloway, D. Preliminary investigations on sperm cryopreservation of a stingray, the sparsely spotted stingaree. In Cryopreservation in Aquatic Species, 2nd ed.; Tiersch, T.R., Green, C.C., Eds.; World Aquaculture Society: Baton Rouge, LA, USA, 2011; pp. 337–344. [Google Scholar]
- Dzyuba, V.; Ninhaus-Silveira, A.; Kahanec, M.; Veríssimo-Silveira, R.; Rodina, M.; Holt, W.V.; Dzyuba, B. Sperm motility in ocellate river stingrays: Evidence for post-testicular sperm maturation and capacitation in Chondrichthyes. J. Zool. 2019, 307, 9–16. [Google Scholar] [CrossRef]
- Morales-Gamba, R.D.; Caldas, J.S.; Godoy, L.; Marcon, J.L. Sperm characterization of the Amazonian freshwater cururu stingray Potamotrygon wallacei (Potamotryogonidae): Basic knowledge for reproduction and conservation plans. Zygote 2019, 27, 259–261. [Google Scholar] [CrossRef]
- Ebert, D.A.; Dando, M. Field Guide to Sharks, Rays & Chimaeras of Europe and the Mediterranean; Princeton University Press: Princeton, NJ, USA, 2020; ISBN 0691205981. [Google Scholar]
- Gillian, M.; King, D.R.N. Colour Atlas of Vetebrate Anatomy; Blackwell Scientific Publications: Oxford, UK, 1982. [Google Scholar]
- Crow, G.L.; Brock, J.A. Necropsy Methods and Procedures for Elasmobranchs. In The Elasmobranch Husbandry Manual: Captive Care of Sharks, Rays and their Relatives; Smith, M., Warmolts, D., Thoney, D., Hueter, R., Eds.; Special Publication of the Ohio Biological Survey: Columbus, OH, USA, 2004; pp. 467–471. [Google Scholar]
- De Iuliis, G.; Pulerà, D. The Dissection of Vertebrates, 2nd ed.; Academic Press: Oxford, UK, 2019; ISBN 0124105009. [Google Scholar]
- Henningsen, A.D. Tonic immobility in 12 elasmobranchs: Use as an aid in captive husbandry. Zoo Biol. 1994, 13, 325–332. [Google Scholar] [CrossRef]
- Smith, M.; Marshall, A.; Correia, J.P.; Rupp, P. Elasmobranch Transport Techniques and Equipment. In Elasmobranch Husbandry Manual: Captive Care of Sharks, Rays, and their Relatives; Smith, M., Warmolts, D., Thoney, D., Hueter, R., Eds.; Special Publication of the Ohio Biological Survey: Columbus, OH, USA, 2004; pp. 105–131. [Google Scholar]
- Kessel, S.T.; Hussey, N.E. Tonic immobility as an anaesthetic for elasmobranchs during surgical implantation procedures. Can. J. Fish. Aquat. Sci. 2015, 72, 1287–1291. [Google Scholar] [CrossRef]
- García-Salinas, P.; Gallego, V.; Asturiano, J.F. Development of sperm cryopreservation protocols for sharks and rays: New tools for elasmobranch conservation. Front. Mar. Sci. 2021. under review. [Google Scholar]
- Hamlett, W.C.; Knight, D.P.; Koob, T.J.; Jezior, M.; Luong, T.; Rozycki, T.; Brunette, N.; Hysell, M.K. Survey of oviducal gland structure and function in elasmobranchs. J. Exp. Zool. 1998, 282, 399–420. [Google Scholar] [CrossRef]
- Villalobos-Segura, E.; Underwood, C.J. Radiation and Divergence Times of Batoidea. J. Vertebr. Paleontol. 2020, 40, 3. [Google Scholar] [CrossRef]
- Saadaoui, A.; Saidi, B.; Enajjar, S.; Bradai, M.N. Reproductive biology of the common stingray Dasyatis pastinaca (Linnaeus, 1758) off the Gulf of Gabès (Central Mediterranean Sea). Cah. Biol. Mar. 2015, 56, 389–396. [Google Scholar]
- Capapé, C.; Guélorget, O.; Vergne, Y.; Quignard, J.P. Reproductive biology of the common eagle ray Myliobatis aquila (Chondrichthyes: Myliobatidae) from the coast of Languedoc (southern France, northern Mediterranean). Vie Milieu 2007, 57, 125–130. [Google Scholar]
- Araújo, P.R.V.; Oddone, M.C.; Velasco, G. Reproductive biology of the stingrays, Myliobatis goodei and Myliobatis ridens (Chondrichthyes: Myliobatidae), in southern Brazil. J. Fish Biol. 2016, 89, 1043–1067. [Google Scholar] [CrossRef]
- Swider, D.A.; Corwin, A.L.; Kamerman, T.Y.; Zimmerman, S.L.; Violetta, G.C.; Davis, J.; Janse, M. Reproduction of spotted eagle rays, Aetobatus narinari, in aquaria. In The Elasmobranch Husbandry Manual II: Recent Advances in the Care of Sharks, Rays and their Relatives; Smith, M., Warmolts, D., Thoney, D., Hueter, R., Murray, M., Ezcurra, J., Eds.; Special Publication of the Ohio Biological Survey: Columbus, OH, USA, 2017; pp. 422–442. [Google Scholar]
- Snelson Jr, F.F.; Williams-Hooper, S.E.; Schmid, T.H. Reproduction and ecology of the Atlantic stingray, Dasyatis sabina, in Florida coastal lagoons. Copeia 1988, 729–739. [Google Scholar] [CrossRef]
- Henningsen, A.D.; Smale, M.; Garner, R.; Kinnunen, N. Reproduction, embryonic development, and reproductive physiology of elasmobranchs. In The Elasmobranch Husbandry Manual: Captive Care of Sharks, Rays and Their Relatives; Smith, M., Warmolts, D., Thoney, D., Hueter, R., Eds.; Special Publication of the Ohio Biological Survey: Columbus, OH, USA, 2004; pp. 227–236. [Google Scholar]
- Lacy, E.R. Alkaline glands and clasper glands of batoids. In Reproductive Biology and Phylogeny of Chondrichthyes: Sharks, Batoids and Chimaeras; Hamlett, W.C., Ed.; Science Publishers: Enfield, NH, USA, 2005; pp. 336–360. [Google Scholar]
- Piercy, A.; Gelsleichter, J.; Snelson Jr, F.F. Morphological changes in the clasper gland of the Atlantic stingray, Dasyatis sabina, associated with the seasonal reproductive cycle. J. Morphol. 2006, 267, 109–114. [Google Scholar] [CrossRef]
- Jamieson, B.G.M.; Hamlett, W.C. Chondrichthyan spermatozoa and phylogeny. In Reproductive Biology and Phylogeny of Chondrichthyes: Sharks, Batoids and Chimaeras; Hamlett, W.C., Ed.; Science Publishers: Enfield, NH, USA, 2005; pp. 201–236. [Google Scholar]
- Marongiu, M.F.; Porcu, C.; Bellodi, A.; Cuccu, D.; Mulas, A.; Follesa, M.C. Oviducal gland microstructure of Raja miraletus and Dipturus oxyrinchus (Elasmobranchii, Rajidae). J. Morphol. 2015, 276, 1392–1403. [Google Scholar] [CrossRef]
- Pratt, H.L. The storage of spermatozoa in the oviducal glands of western North Atlantic sharks. In The Reproduction and Development of Sharks, Skates, Rays and Ratfishes; Demski, L.S., Wourms, J.P., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 139–149. [Google Scholar]
- IUCN/SSC. IUCN Species Survival Commission Guidelines on the Use of Ex Situ Management for Species Conservation; Version 2; IUCN: Gland, Switzerland, 2014; pp. 1–7. [Google Scholar]
- Grassmann, M.; McNeil, B.; Wharton, J. Sharks in captivity: The role of husbandry, breeding, education, and citizen science in shark conservation. Adv. Mar. Biol. 2017, 78, 89–119. [Google Scholar] [CrossRef]
- Buckley, K.A.; Crook, D.A.; Pillans, R.D.; Smith, L.; Kyne, P.M. Sustainability of threatened species displayed in public aquaria, with a case study of Australian sharks and rays. Rev. Fish Biol. Fish. 2018, 28, 137–151. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Davidson, L.N.K.; Kyne, P.M.; Simpfendorfer, C.A.; Harrison, L.R.; Carlson, J.K.; Fordham, S.V. Ghosts of the coast: Global extinction risk and conservation of sawfishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 134–153. [Google Scholar] [CrossRef]
- Kyne, P.M.; Jabado, R.W.; Rigby, C.L.; Gore, M.A.; Pollock, C.M.; Herman, K.B.; Cheok, J.; Ebert, D.A.; Simpfendorfer, C.A.; Dulvy, N.K. The thin edge of the wedge: Extremely high extinction risk in wedgefishes and giant guitarfishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1337–1361. [Google Scholar] [CrossRef]
- Moore, A.B.M. Are guitarfishes the next sawfishes? Extinction risk and an urgent call for conservation action. Endanger. Species Res. 2017, 34, 75–88. [Google Scholar] [CrossRef] [Green Version]
- Harrison, L.R.; Dulvy, N.K. Sawfish: A Global Strategy for Conservation; IUCN Species Survival Commission’s Shark Specialist Group: Vancouver, QC, Canada, 2014; ISBN 9780956106339. [Google Scholar]





| Common Name | Scientific Name | NM | NF | IUCN | Source | Range (cm) |
|---|---|---|---|---|---|---|
| Rough skate | Raja radula | 3 | 3 | EN | FM | 47–63 |
| Spotted skate | Raja montagui | 2 | 1 | LC | FM | 55–67 |
| Mediterranean starry skate | Raja asterias | 2 | 4 | NT | AQ/FM | 61–68 |
| Thornback skate | Raja clavata | 1 | 1 | NT | FM | 68–72 |
| Undulate skate | Raja undulata | 1 | NT | AQ | 86 | |
| Longnosed skate | Dipturus oxyrinchus | 1 | 1 | NT | BC | 104–112 |
| Spiny butterfly ray | Gymnura altavela | 1 | CR | BC | 104 | |
| Common stingray | Dasyatis pastinaca | 2 | 3 | VU | BC | 52–56 |
| Common eagle ray | Myliobatis aquila | 2 | 2 | VU | BC | 54–63 |
| Bull ray | Aetomylaeus bovinus | 2 | 2 | CR | BC | 89–103 |
| Marbled electric ray | Torpedo marmorata | 2 | 2 | LC | BC | 22–51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Salinas, P.; Gallego, V.; Asturiano, J.F. Reproductive Anatomy of Chondrichthyans: Notes on Specimen Handling and Sperm Extraction. I. Rays and Skates. Animals 2021, 11, 1888. https://doi.org/10.3390/ani11071888
García-Salinas P, Gallego V, Asturiano JF. Reproductive Anatomy of Chondrichthyans: Notes on Specimen Handling and Sperm Extraction. I. Rays and Skates. Animals. 2021; 11(7):1888. https://doi.org/10.3390/ani11071888
Chicago/Turabian StyleGarcía-Salinas, Pablo, Victor Gallego, and Juan F. Asturiano. 2021. "Reproductive Anatomy of Chondrichthyans: Notes on Specimen Handling and Sperm Extraction. I. Rays and Skates" Animals 11, no. 7: 1888. https://doi.org/10.3390/ani11071888






