Post-Prandial Amino Acid Changes in Gilthead Sea Bream
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Setup and Operation
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Houlihan, D.F.; Mathers, E.; Foster, A.R. Biochemical correlates of growth rate in fish. In Fish Ecophysiology; Rankin, J.C., Jensen, F.B., Eds.; Chapman and Hall: London, UK, 1993; pp. 45–71. [Google Scholar]
- Carter, C.G.; Houlihan, D.F. Protein synthesis. In Nitrogen Excretion, Fish Physiology; Wright, P.A., Anderson, P.M., Eds.; Academic Press: San Diego, CA, USA, 2001; Volume 20, pp. 31–75. [Google Scholar]
- Wilson, R.P. Amino acid requirements of finfish. In Amino Acids in Farm Animal Nutrition; D’Mello, J.P.F., Ed.; CAB International: Wallingford, UK, 1994; pp. 377–399. [Google Scholar]
- Bequette, B.J. Amino acid metabolism in animals: An overview. In Amino Acids in Animal Nutrition; D’Mello, J.P.F., Ed.; CABI Publishing: Wallingford, UK, 2003; pp. 87–101. [Google Scholar]
- Langer, H.; Guillaume, J. Effect of feeding pattern and dietary protein source on protein synthesis in European sea bass (Dicentrarchus labrax). Comp. Biochem. Physiol. 1994, 108A, 461–466. [Google Scholar] [CrossRef]
- Houlihan, D.F. Protein turnover in ectotherms and its relationships to energetics. In Advances in Comparative and Environmental Physiology; Gilles, R., Ed.; Springer: Berlin, Germany, 1991; Volume 7, pp. 1–47. [Google Scholar]
- Kimball, S.R.; Jefferson, L.S. Regulation of protein synthesis by branched chain amino acids. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K. The role of leucine in weight loss diets and glucose homeostasis. J. Nutr. 2003, 133, 261S–267S. [Google Scholar] [CrossRef] [PubMed]
- Mente, E.; Coutteau, P.; Houlihan, D.; Davidson, I.; Sorgeloos, P. Protein turnover, amino acid profile and amino acid flux in juvenile shrimp Litopenaeus vannamei: Effects of dietary protein source. J. Exp. Biol. 2002, 205, 3107–3122. [Google Scholar] [CrossRef]
- Panserat, S.; Kaushik, S.J. Regulation of gene expression by nutritional factors in fish. Aquac. Res. 2010, 41, 751–762. [Google Scholar] [CrossRef]
- Langer, H.; Guillaume, J.; Metailler, R.; Fauconneau, B. Augmentation of protein synthesis and degradation by poor dietary amino acid balance on European sea bass (Dicentrarchus labrax). J. Nutr. 1993, 123, 1754–1761. [Google Scholar] [CrossRef]
- De la Higuera, M.; Akharbach, H.; Hidalgo, M.C.; Peragon, J.; Lupianez, J.A.; Garcia-Gallego, M. Liver and white muscle protein turnover rates in the European eel (Anguilla anguilla): Effects of dietary protein quality. Aquaculture 1999, 179, 203–216. [Google Scholar] [CrossRef]
- Katersky, R.S.; Carter, C.G. The effect of temperature on post-prandial protein synthesis in juvenile barramundi, Lates calcarifer. Comp. Biochem. Physiol. A Mol. Int. Physiol. 2010, 156, 529–536. [Google Scholar] [CrossRef]
- Carter, C.G.; Mente, E.; Barnes, R.; Nengas, I. Protein synthesis in gilthead sea bream: Response to partial fishmeal replacement. Br. J. Nutr. 2012, 108, 2190–2197. [Google Scholar] [CrossRef]
- Larsen, B.K.; Dalsgaard, J.; Pedersen, P.B. Effects of plant proteins on postprandial, free plasma amino acid concentrations in rainbow trout (Oncorhynchus mykiss). Aquaculture 2012, 326–329, 90–98. [Google Scholar] [CrossRef]
- Nengas, I.; Alexis, M.N.; Davis, S.J. Partial substitution of fishmeal with soybean meal products and derivatives in diets for the gilthead sea bream Sparus aurata (L.). Aquac. Res. 1996, 27, 147–156. [Google Scholar] [CrossRef]
- Robaina, L.; Izquirdo, M.S.; Moyano, F.J.; Socorro, J.; Vergara, J.M.; Montero, D.; Fernandez-Palacios, H. Soybean and lupin seed meals as protein sources in diets for gilthead bream (Sparus aurata)-nutritional and histological implications. Aquaculture 1995, 130, 219–233. [Google Scholar] [CrossRef]
- Kissil, G.W.; Lupatsch, I.; Higgs, D.A.; Hary, R.W. Dietary substitution of soy and rapeseed protein concentrates for fish meal, and their effects on growth and nutrient utilization in gilthead seabream Sparus aurata L. Aquac. Res. 2000, 31, 595–601. [Google Scholar] [CrossRef]
- Gatlin, D.M.; Barrows, F.T.; Brown, P.; Dabrowski, K.; Gaylord, T.G.; Hardy, R.W.; Herman, E.; Hu, G.S.; Krogdahl, A.; Nelson, R.; et al. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquac. Res. 2007, 38, 551–579. [Google Scholar] [CrossRef]
- Carter, C.; Houlihan, D.F.H.; Kiessling, A.; Medale, F.; Jobling, M. Physiological effects of feeding. In Food Intake in Fish; Houlihan, D.F.H., Boujard, T., Jobling, M., Eds.; Blackwell Science: Oxford, UK, 2001; pp. 297–332. [Google Scholar]
- Kaushik, S.J.; Luquet, P. Influence of dietary amino acid patterns on the free amino acid contents of blood and muscle of rainbow trout (Salmo gairdnerii R.). Comp. Biochem. Physiol. B 1979, 64, 175–180. [Google Scholar] [CrossRef]
- Lyndon, A.R.; Davidson, I.; Houlihan, D.F. Changes in tissue and plasma free amino acid concentrations after feeding in Atlantic cod. Fish Physiol. Biochem. 1993, 10, 365–375. [Google Scholar] [CrossRef]
- Carter, C.G.; He, Z.Y.; Houlihan, D.F.; McCarthy, I.D.; Davidson, I. Effect of feeding on tissue free amino acid concentrations in rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 1995, 14, 153–164. [Google Scholar] [CrossRef]
- Carter, C.G.; Houlihan, D.F.; He, Z.-Y. Changes in tissue free amino acid concentrations in Atlantic salmon, Salmo salar L., after consumption of a low ration. Fish Physiol. Biochem. 2000, 23, 295–306. [Google Scholar] [CrossRef]
- Mente, E.; Deguara, S.; Santos, M.B.; Houlihan, D. White muscle free amino acid concentrations following feeding a maize gluten dietary protein in Atlantic salmon (Salmo salar L.). Aquaculture 2003, 225, 133–147. [Google Scholar] [CrossRef]
- McCarthy, I.D.; Fuiman, L.A. Post-prandial changes in protein synthesis in red drum (Sciaenops ocellatus) larvae. J. Exp. Biol. 2011, 214, 1821–1828. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, D.; He, G.; Mai, K.; Zhou, H.; Xu, W.; Song, F. Postprandial nutrient-sensing and metabolic responses after partial dietary fishmeal replacement by soyabean meal in turbot (Scophthalmus maximus L.). Br. J. Nutr. 2016, 115, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Requeni, P.; Mingarro, M.; Kirchner, S.; Calduch-Giner, J.A.; Medale, F.; Corraze, G.; Panserat, S.; Martin, S.A.M.; Houlihan, D.F.; Kaushik, S.J.; et al. Protein growth performance, amino acid utilisation and somatotropic axis responsiveness to fish meal replacement by plant protein sources in gilthead sea bream (Sparus aurata). Aquaculture 2004, 232, 493–510. [Google Scholar] [CrossRef]
- Santigosa, E.; Sanchez, J.; Medale, F.; Kaushil, S.J.; Perez-Sanchez, J.; Gallardo, M.A. Modifications of digestives enzymes in trout (Onchorynchus mykiss) and sea bream (Sparus aurata) in response to dietary fish meal replacement by plant protein sources. Aquaculture 2008, 317, 68–74. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Prentice-Hall: Bergen, NJ, USA, 1996; p. 663. [Google Scholar]
- Mente, E.; Stratakos, A.; Boziaris, I.S.; Kormas, K.A.; Karalazos, V.; Karapanagiotidis, I.T.; Catsiki, A.V.; Leondiadis, L. The effect of organic and conventional production methods on sea bream growth, health and body composition; a field experiment. Sci. Mar. 2012, 76, 549–560. [Google Scholar] [CrossRef]
- Rønnestad, I.; Morais, S. Digestion. In Fish Larval Physiology; Finn, R., Kapoor, B., Eds.; Science Publishers: Enfield, NH, USA, 2008; pp. 201–262. [Google Scholar]
- Houlihan, D.F.; Carter, C.G.; McCarthy, I.D. Protein turnover in animals. In Nitrogen and Excretion; Wright, P.J., Walsh, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 1995; pp. 1–29. [Google Scholar]
- Borey, M.; Pancerat, S.; Surget, A.; Cluzeaud, M.; Plagnes-Juan, E.; Herman, A.; Lazzaarotto, V.; Corraze, G.; Medale, F.; Lauga, B.; et al. Postprandial kinetics of gene expression of proteins involved in the digestive process in rainbow trout (O. mykiss) and impact of diet composition. Fish Physiol. Biochem. 2016, 42, 1187–1202. [Google Scholar] [CrossRef]
- Figueiredo-Silva, C.A.; Saravanan, S.; Schrama, J.W.; Pancerat, S.; Kaushik, S.; Geurden, I. A comparative study of the metabolic response in rainbow trout and nile tilapia to changes in dietary macronutrient composition. Br. J. Nutr. 2013, 109, 816–826. [Google Scholar] [CrossRef][Green Version]
- Wade, N.M.; Skiba-Casst, S.; Dias, K.; Glencross, B.D. Postprandial molecular responses in the liver of the barramundi, Lates calcarifer. Fish Physiol. Biochem. 2014, 40, 427–443. [Google Scholar] [CrossRef]
- Walton, M.J.; Wilson, R.P. Postprandial changes in plasma and liver free amino acids of rainbow trout fed complete diets containing casein. Aquaculture 1986, 51, 105–115. [Google Scholar] [CrossRef]
- Kaushik, S.J.; Luquet, P. Influence of nutritional status on the daily pattern of nitrogen excretion in the carp (Cyprinus carpio) and the rainbow trout (Salmo gairdneri R.). Reprod. Nutr. Dev. 1980, 20, 1751–1765. [Google Scholar] [CrossRef]
- Espe, M.; Lied, E.; Torrissen, K.R. Changes in plasma and muscle free amino acids in Atlantic salmon (Salmo salar) during absorption of dies containing different amounts of hydrolysed cod muscle protein. Comp. Biochem. Physiol. 1993, 105A, 555–562. [Google Scholar] [CrossRef]
- Tibaldi, E.; Kaushik, S.J. Amino acid requirements of Mediterranean fish species. In Mediterranean Fish Nutrition; Montero, D., Basurco, B., Nengas, I., Alexis, M., Izquierdo, M., Eds.; CIHEAM (Cahiers Options Méditerranéennes): Zaragoza, Spain, 2005; Volume 63, pp. 59–65. [Google Scholar]
- Ogata, H. Correlation of essential amino acid patterns between the dietary protein and the blood, hepatopancreas, or skeletal muscle in carp. Bull. Jpn. Soc. Sci. Fish 1986, 52, 307–312. [Google Scholar] [CrossRef]
- Jurss, K.; Bastrop, R. Amino acid metabolism in fish. In Biochemistry and Molecular Biology of Fishes; Hochachka, P., Mommsen, T., Eds.; Elsevier Press: Amsterdam, The Netherlands, 1992; Volume 4, pp. 159–189. [Google Scholar]
- Dabrowski, K.; Guderley, H. Intermediary metabolism. In Fish Nutrition, 3rd ed.; Halver, J.E., Ed.; Academic Press: San Diego, CA, USA, 2002; pp. 309–365. [Google Scholar]
- Wu, G.; Morris, M. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.A.; Campbell, A.; Morgan, R.L.; Rosenberger, A.G.; Murray, B.W. Dogmas and controversies in the handling of nitrogenous wastes expression of arginase Type I and II genes in rainbow trout: Influence of fasting on liver enzyme activity and mRNA levels in juveniles. J. Exp. Biol. 2004, 207, 2033–2042. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.J. Whole body amino acid composition of European seabass (Dicentrarchus labrax), gilthead seabream (Sparus aurata) and turbot (Psetta maxima) with an estimation of their IAA requirement profiles. Aquat. Liv. Res. 1998, 11, 355–358. [Google Scholar] [CrossRef]
- Ballantyne, J.S. Amino acid metabolism. In Nitrogen Excretion Fish Physiology; Wright, P.A., Anderson, A.J., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. 77–107. [Google Scholar]
- Aragão, C.; Conceição, L.E.C.; Martins, D.; Rønnestad, I.; Gomes, E.; Dinis, M.T. A balanced dietary amino acid profile improves amino acid retention in post-larval Senegalese sole (Solea senegalensis). Aquaculture 2004, 233, 293–304. [Google Scholar] [CrossRef]
- Gomez-Requeni, P.; Mingarro, M.; Kirchner, S.; Calduch-Giner, J.A.; Medale, F.; Corraze, G.; Panserat, S.; Martin, S.A.M.; Houlihan, D.F.; Kaushik, S.J.; et al. Effects of dietary amino acid profile on growth performance, key metabolic enzymes and somatotropic axis responsiveness of gilthead sea bream (Sparus aurata). Aquaculture 2003, 220, 749–767. [Google Scholar] [CrossRef]
- Kormas, K.A.; Meziti, A.; Mente, E.; Fretzos, A. Gut microorganisms in organic and conventional sea bream. Microbiol. Open 2014, 3, 718–728. [Google Scholar] [CrossRef]
- Mente, E.; Jokumsen, A.; Carter, C.; Tacon, A.; Antonopoulou, E. Nutrition in relation to organic aquaculture: Sources and strategies. In Organic Aquaculture. Impacts and Future Developments; Lembo, P., Mente, E., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 141–188. ISBN 978-3-030-05603-2. [Google Scholar]
- Takagi, S.; Shimeno, S.; Hosokawa, H.; Ukawa, M. Effect of lysine and methionine supplementation to soy protein concentrate diet for red sea bream Pagrus major. Fish. Sci. 2001, 67, 1088–1096. [Google Scholar] [CrossRef]
- Pereira, T.G.; Oliva-Teles, A. Evaluation of corn gluten meal as a protein source in diets for gilthead sea bream (Sparus aurata L.) juveniles. Aquac. Res. 2003, 34, 1111–1117. [Google Scholar] [CrossRef]
- Oliva-Teles, A.; Lupatsch, I.; Nengas, I. Nutrition and feeding of Sparidae. In Sparidae: Biology and Aquaculture of Gilthead Sea Bream and Other Species, 1st ed.; Pavlidis, M., Mylonas, C.C., Eds.; Wiley-Blackwell Ltd: West Sussex, UK, 2011; pp. 199–233. [Google Scholar]
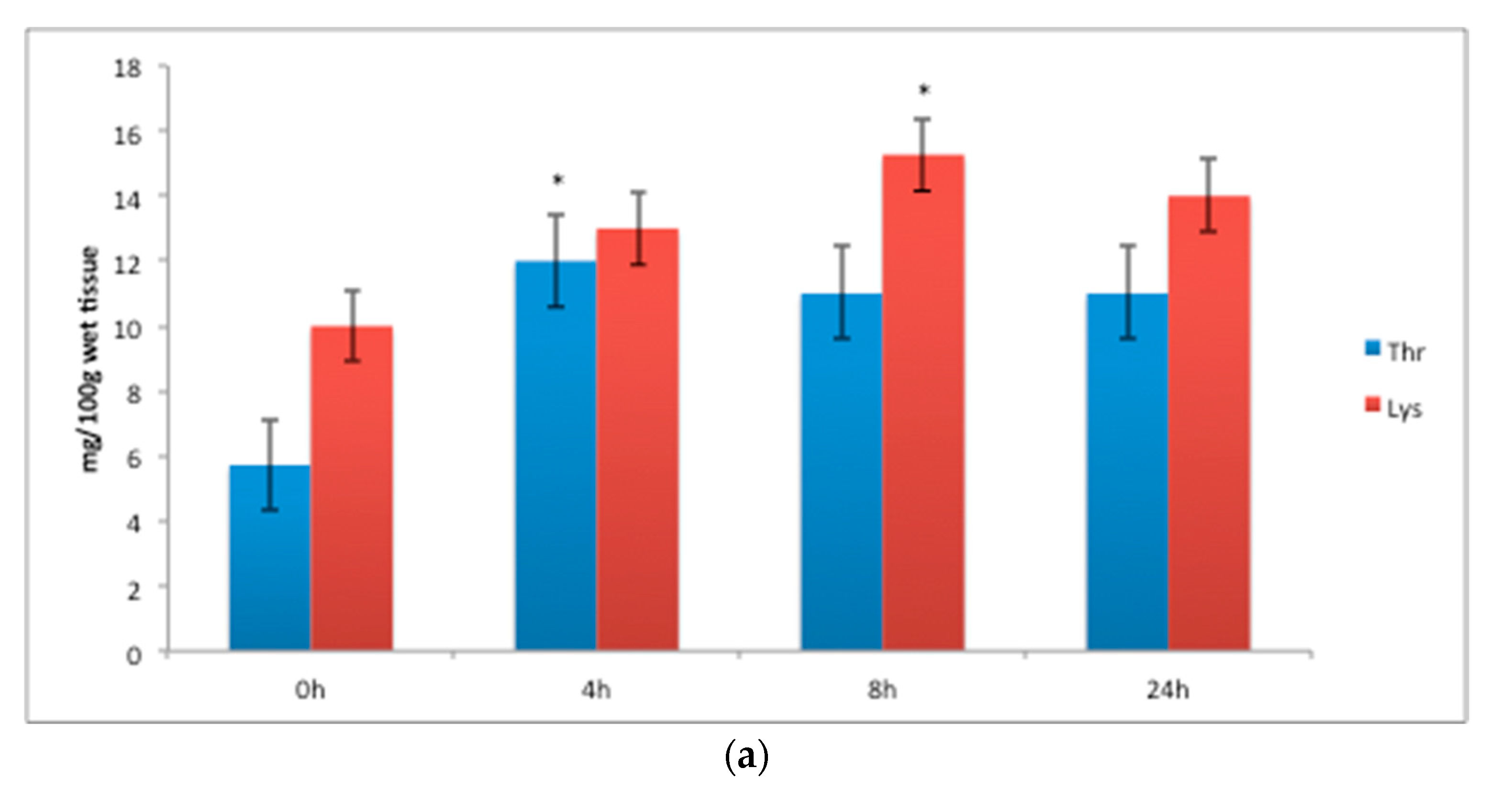

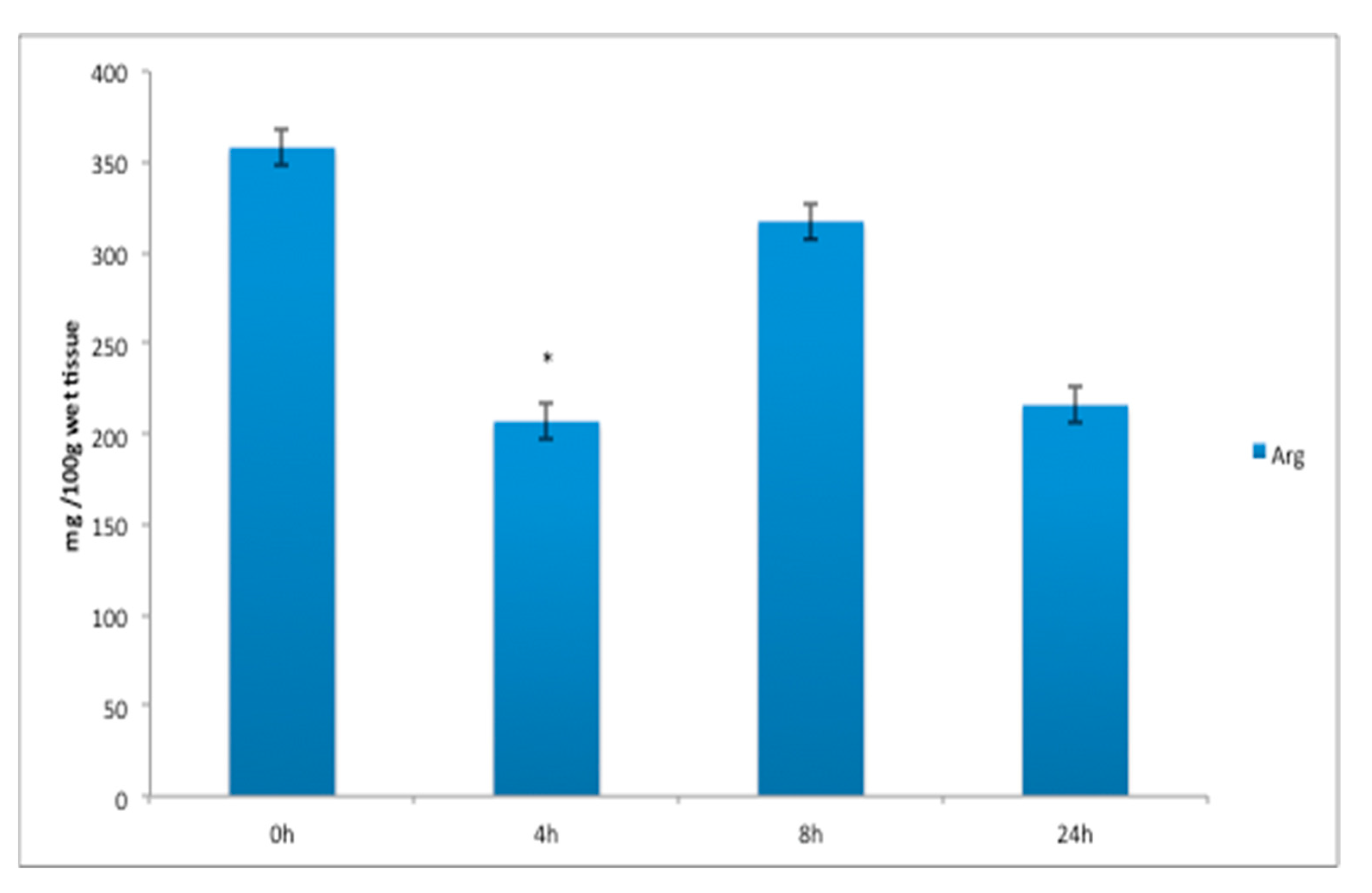

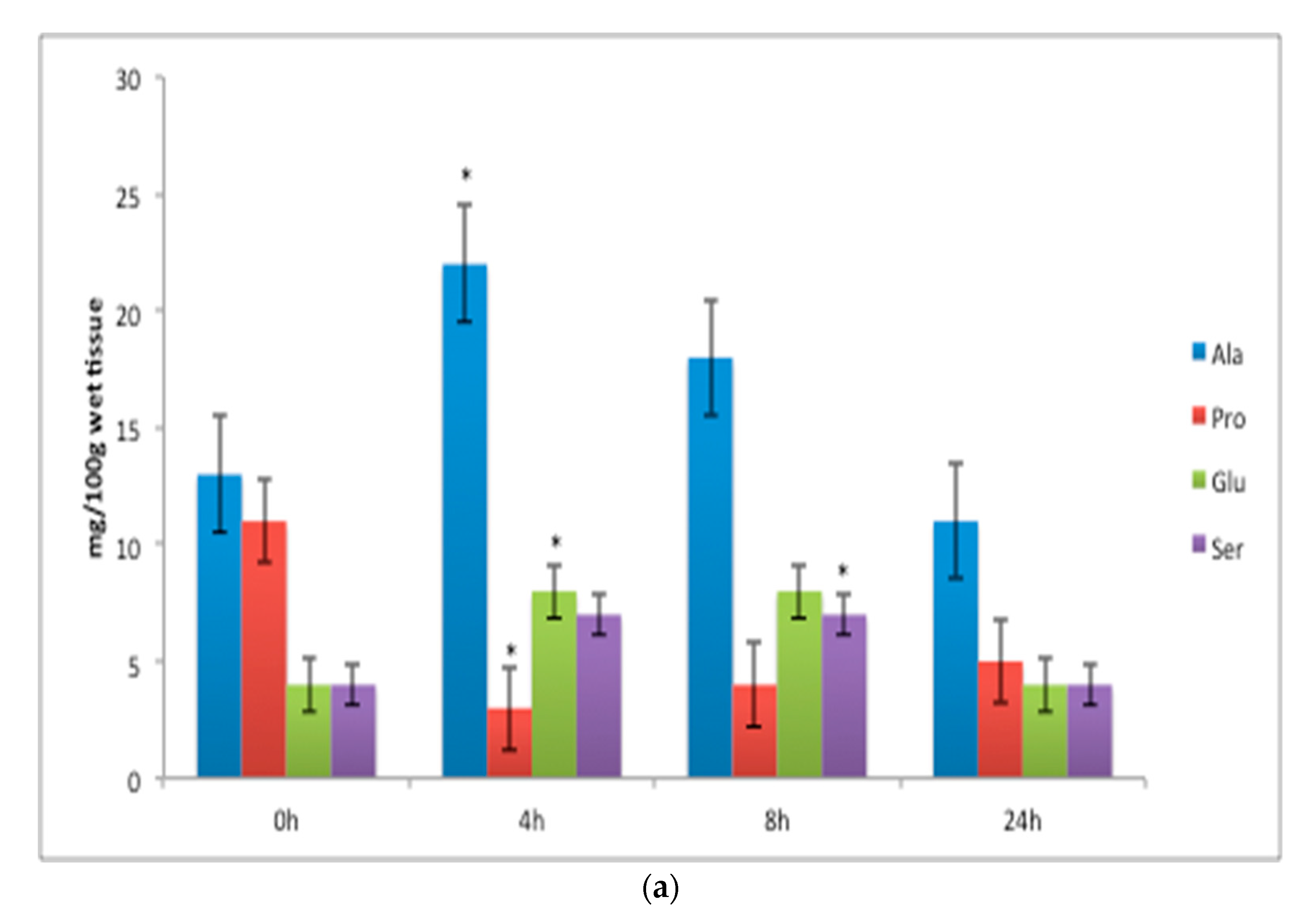

| Amino Acid (% of Total) | Amino Acid Code | Diet (COM) 1 | Diet (LAB) | Diet (ORG) 1 | p-Value | SE | |
| Aspartic Acid | Asp | 8.10 | 11.97 * | 7.90 | 0.001 | 0.150 | |
| Glutamic Acid | Glu | 12.74 | 14.79 * | 11.84 | 0.007 | 0.298 | |
| Serine | Ser | 5.47 | 3.52 * | 5.02 | 0.025 | 0.077 | |
| Glycine | Gly | 8.63 | 9.86 | 10.03 * | 0.003 | 0.106 | |
| Histidine | His | 1.44 | 2.11 * | 1.63 | 0.007 | 0.049 | |
| Arginine | Arg | 3.90 | 4.93 * | 3.99 | 0.002 | 0.110 | |
| Threonine | Thr | 4.69 | 4.93 | 5.20 | 0.186 | 0.313 | |
| Alanine | Ala | 9.62 | 7.75 | 10.36 | 0.104 | 0.233 | |
| Proline | Pro | 6.86 | 4.70 | 5.81 | 0.938 | 0.289 | |
| Tyrosine | Tyr | 2.59 | 2.11 * | 2.37 | 0.001 | 0.034 | |
| Valine | Val | 6.17 | 7.04 * | 6.55 | 0.003 | 0.105 | |
| Methionine | Met | 2.26 | 2.82 | 2.83 | 0.176 | 0.235 | |
| Isoleucine | Ile | 5.06 | 6.34 | 5.06 | 0.185 | 0.355 | |
| Leucine | Leu | 10.52 | 8.45 * | 8.87 | 0.045 | 0.254 | |
| Phenylalanine | Phe | 4.89 | 4.23 * | 4.13 | 0.004 | 0.068 | |
| Lysine | Lys | 7.07 | 7.75 | 8.36 * | 0.001 | 0.131 | |
| Amino Acid (% of Total) | Amino Acid Code | A/E Wild WM | A/E Wild L | A/E Wild G | Free A/E Gut Fed the COM Diet | Free A/E Gut Fed the LAB Diet | Free A/E Gut Fed the ORG Diet |
| Arginine | Arg | 10.71 | 11.80 | 72.41 | 78.67 | 71.43 | 75.71 |
| Histidine | His | 3.28 | 5.90 | 4.07 | 5.03 | 6.03 | 4.79 |
| Isoleucine | Ile | 11.95 | 15.61 | 1.62 | 0.86 | 1.22 | 0.94 |
| Leucine | Leu | 14.04 | 17.70 | 8.32 | 1.75 | 2.68 | 2.25 |
| Lysine | Lys | 15.23 | 11.80 | 2.41 | 1.32 | 2.15 | 1.71 |
| Methionine | Met | 3.28 | 5.90 | 1.22 | 0.93 | 1.20 | 0.61 |
| Phenylalanine | Phe | 5.38 | 7.68 | 1.63 | 0.96 | 1.54 | 1.27 |
| Threonine | Thr | 8.66 | 5.90 | 2.78 | 1.52 | 2.10 | 2.25 |
| Tyrosine | Tyr | 4.47 | 5.9 | 1.41 | 0.83 | 1.39 | 1.10 |
| Valine | Val | 11.95 | 11.8 | 2.70 | 1.26 | 1.66 | 1.62 |
| Time after Feeding (h) | 0 | 4 | 8 | 24 |
|---|---|---|---|---|
| Total AA liver | 30.77 ± 0.16 a | 24.57 ± 7.17 b | 26.11 ± 1.84 a | 19.30 ± 3.95 a |
| Total EAA liver | 35.21 ± 1.07 a | 31.56 ± 7.91 b | 32.51 ± 0.86 b | 23.65 ± 2.84 a |
| Total EAA white muscle | 26.56 ± 1.13 a | 28.01 ± 2.12 b | 25.18 ± 3.50 a | 21.76 ± 2.65 a |
| Total AA white muscle | 18.26 ± 5.56 a | 19.06 ± 6.52 a | 17.04 ± 4.65 a | 16.07 ± 6.36 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mente, E.; Carter, C.G.; Barnes, R.S.; Vlahos, N.; Nengas, I. Post-Prandial Amino Acid Changes in Gilthead Sea Bream. Animals 2021, 11, 1889. https://doi.org/10.3390/ani11071889
Mente E, Carter CG, Barnes RS, Vlahos N, Nengas I. Post-Prandial Amino Acid Changes in Gilthead Sea Bream. Animals. 2021; 11(7):1889. https://doi.org/10.3390/ani11071889
Chicago/Turabian StyleMente, Eleni, Chris G. Carter, Robin S. (Katersky) Barnes, Nikolaos Vlahos, and Ioannis Nengas. 2021. "Post-Prandial Amino Acid Changes in Gilthead Sea Bream" Animals 11, no. 7: 1889. https://doi.org/10.3390/ani11071889
APA StyleMente, E., Carter, C. G., Barnes, R. S., Vlahos, N., & Nengas, I. (2021). Post-Prandial Amino Acid Changes in Gilthead Sea Bream. Animals, 11(7), 1889. https://doi.org/10.3390/ani11071889







