The Crossroads between Zinc and Steroidal Implant-Induced Growth of Beef Cattle
Abstract
:Simple Summary
Abstract
1. Introduction
2. Steroidal Implants
2.1. Estrogen and TBA in Steroidal Implants
2.2. Genomic Mode of Action
2.3. Non-Genomic Mode of Action
3. Biological Importance of Zinc in Mammals
3.1. Classical Zinc Literature
3.2. Zinc Requirement
3.3. Zinc Absorption and Status
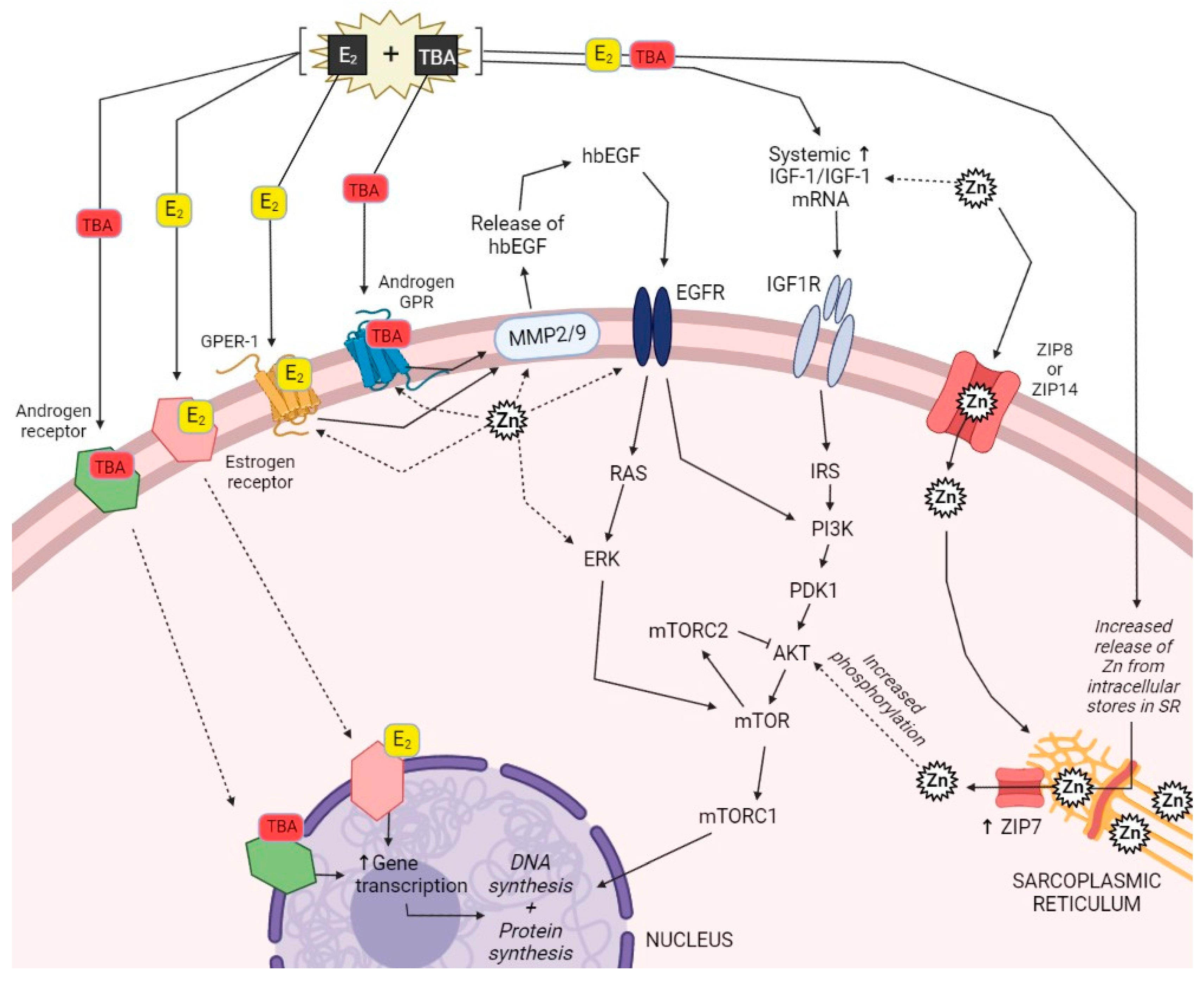
4. Interconnections between Steroidal Implants and Zinc
4.1. Hormone Receptors and Downstream Signaling
4.2. Insulin-Like Growth Factor-1
4.3. Glucose Metabolism in Muscle
4.4. Protein Synthesis Pathways
4.5. Satellite Cell Proliferation and Differentiation
4.6. Cattle Growth
4.7. Zinc Metabolism
| Ref. | Sex | Steroidal Implant 1 | Potency 2 | Supplemental Zn 3 | Day 4 | ∆ 5 ADG, % | ∆ 5 Plasma Zn, % | ∆ 5 Liver Zn, % |
|---|---|---|---|---|---|---|---|---|
| [1] | Steer | 200 mg TBA + 20 mg E2 | High | 0 | 69 | -- | -- | +10.6 |
| 30 a | 69 | -- | -- | +11.0 | ||||
| 100 a | 69 | -- | -- | +22.9 | ||||
| [14] | Steer | 200 mg TBA + 20 mg E2 | High | 30 a | 13/14 | +29.0 | −11.2 | −6.6 |
| [118] | Steer | 200 mg P + 20 mg EB | Moderate | 0 | 59 | +7.6 | +1.8 | −26.1 |
| 200 a | 59 | +17.4 | +3.2 | +30.7 | ||||
| 200 b | 59 | -- | −15.0 | +8.5 | ||||
| [118] | Heifer | 200 mg TP + 20 mg EB | Moderate | 0 | 50 | +11.0 | +7.0 | −38.2 |
| 200 a | 50 | +17.0 | −2.5 | +25.4 | ||||
| 200 b | 50 | −26.0 | +24.7 | −40.8 | ||||
| [119] | Steer | 80 mg TBA +16 mg E2 (Initial) | Moderate | 100 c | 14/15 | -- | −3.1 | −1.0 |
| 200 mg TBA + 20 mg E2 | High | 100 c | 14/15 | -- | −12.8 | +4.8 | ||
| [120] | Steer | 200 mg TBA + 20 mg E2 | High | 0 | 18 | +8.0 | −4.6 | -- |
| 30 a | 18 | +3.1 | −9.7 | -- | ||||
| 100 a | 18 | +4.5 | −7.0 | -- | ||||
| 150 a | 18 | +21.6 | −3.5 | -- | ||||
| [124] | Steer | 200 mg TBA | High | 55 c | 2 | -- | −4.3 | −16.2 |
| 120 mg TBA +24 mg E2 | Moderate | 55 c | 2 | -- | −8.5 | −16.2 | ||
| [125] | Steer | 200 mg P + 20 mg EB (Initial) | Moderate | 360 d mg·steer−1·d−1 | 28 | -- | −4.8 | +12.2 |
| 80 mg TBA +16 mg E2 | Moderate | 360 d mg·steer−1·d−1 | 56 | -- | −7.7 | +4.7 |
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niedermayer, E.K.; Genther-Schroeder, O.N.; Loy, D.D.; Hansen, S.L. Effect of varying trace mineral supplementation of steers with or without hormone implants on growth and carcass characteristics. J. Anim. Sci. 2018, 96, 1159–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engle, T.E.; Nockels, C.F.; Kimberling, C.V.; Weaber, D.L.; Johnson, A.B. Zinc repletion with organic or inorganic forms of zinc and protein turnover in marginally zinc-deficient calves. J. Anim. Sci. 1997, 75, 3074–3081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spears, J.W.; Kegley, E.B. Effect of zinc source (zinc oxide vs zinc proteinate) and level on performance, carcass characteristics, and immune response of growing and finishing steers1,2. J. Anim. Sci. 2002, 80, 2747–2752. [Google Scholar] [CrossRef] [Green Version]
- Genther-Schroeder, O.N.; Branine, M.E.; Hansen, S.L. The effects of increasing supplementation of zinc-amino acid complex on growth performance, carcass characteristics, and inflammatory response of beef cattle fed ractopamine hydrochloride. J. Anim. Sci. 2016, 94, 3389–3398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cousins, R.J.; Liuzzi, J.P.; Lichten, L.A. Mammalian Zinc Transport, Trafficking, and Signals. J. Biol. Chem. 2006, 281, 24085–24089. [Google Scholar] [CrossRef] [Green Version]
- Beyersmann, D.; Haase, H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. BioMetals 2001, 14, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Ninh, N.X.; Thissen, J.P.; Collette, L.; Gérard, G.; Khói, H.H.; Ketelslegers, J.M. Zinc supplementation increases growth and circulating insulin-like growth factor I (IGF-I) in growth-retarded Vietnamese children. Am. J. Clin. Nutr. 1996, 63, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.T.; Hamed, A.I.; Sallam, M.T. Effect of zinc supplementation on growth Hormone Insulin growth factor axis in short Egyptian children with zinc deficiency. Ital. J. Pediatr. 2012, 38, 21. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.K.; Miller, W.J. Experimental Zinc Deficiency and Recovery of Calves. J. Nutr. 1962, 76, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.F.; Dalgarno, A.C.; Williams, R.B.; Quarterman, J. Zinc deficiency and the zinc requirements of calves and lambs. Br. J. Nutr. 1967, 21, 751–768. [Google Scholar] [CrossRef] [Green Version]
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef]
- Chesters, J.K.; Petrie, L.; Vint, H. Specificity and timing of the Zn2+ requirement for DNA synthesis by 3T3 cells. Exp. Cell Res. 1989, 184, 499–508. [Google Scholar] [CrossRef]
- Duckett, S.K.; Pratt, S.L. MEAT SCIENCE AND MUSCLE BIOLOGY SYMPOSIUM—Anabolic implants and meat quality1. J. Anim. Sci. 2014, 92, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messersmith, E.M. The Effect of Copper Supplementation on Performance and Carcass Characteristics of Cattle Utilizing Growth Promoting Technologies. Master’s Thesis, Iowa State University, Ames, IA, USA, 2018. [Google Scholar]
- Johnson, B.; Beckett, J. Application of Growth Enhancing Compounds in Modern Beef Production Executive Summary; Reference Paper; American Meat Science Association: Champaign, IL, USA, 2014; pp. 1–15. [Google Scholar]
- Smith, Z.K.; Johnson, B.J. Mechanisms of steroidal implants to improve beef cattle growth: A review. J. Appl. Anim. Res. 2020, 48, 133–141. [Google Scholar] [CrossRef]
- FDA. Revalor XH Freedom of Information Summary; NADA 141-269; Food Drug Admin.: Silver Spring, MD, USA, 2017. [Google Scholar]
- Bouffault, J.; Willemart, J. Anabolic activity of trenbolone acetate alone or in association with estrogens testosterone analog, anabolisants, beef cattle, veal calves, young bulls, steers carcasse quality. In Anabolics in Animal Production; Public Health Aspects, Analytical Methods and Regulation: Paris, France, 1983. [Google Scholar]
- Johnson, B.J.; Anderson, P.T.; Meiske, J.C.; Dayton, W.R. Effect of a combined trenbolone acetate and estradiol implant on feedlot performance, carcass characteristics, and carcass composition of feedlot steers. J. Anim. Sci. 1996, 74, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Cleale, R.M.; Amodie, D.; Bechtol, D.T.; Drouillard, J.S.; Edmonds, J.D.; Edmonds, M.; Hunsaker, B.D.; Kraft, L.A.; Lawrence, T.; Rulli, R.D.; et al. Effects of estradiol benzoate and trenbolone acetate, alone or in combination at dose levels present in Synovex Choice, on performance by feedlot heifers1. J. Anim. Sci. 2013, 91, 970–977. [Google Scholar] [CrossRef]
- Filardo, E.J.; Thomas, P. Minireview: G Protein-Coupled Estrogen Receptor-1, GPER-1: Its Mechanism of Action and Role in Female Reproductive Cancer, Renal and Vascular Physiology. Endocrinology 2012, 153, 2953–2962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Zhao, W.; Zhao, J.; Pan, J.; Wu, Q.; Zhang, Y.; Bauman, W.A.; Cardozo, C.P. Identification of Androgen Response Elements in the Insulin-Like Growth Factor I Upstream Promoter. Endocrinology 2007, 148, 2984–2993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prossnitz, E.R.; Maggiolini, M. Mechanisms of estrogen signaling and gene expression via GPR30. Mol. Cell. Endocrinol. 2009, 308, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P. Rapid steroid hormone actions initiated at the cell surface and the receptors that mediate them with an emphasis on recent progress in fish models. Gen. Comp. Endocrinol. 2012, 175, 367–383. [Google Scholar] [CrossRef] [Green Version]
- Thornton, K.J.; Kamange-Sollo, E.; White, M.E.; Dayton, W.R. Role of G protein–coupled receptors (GPCR), matrix metalloproteinases 2 and 9 (MMP2 and MMP9), heparin-binding epidermal growth factor–like growth factor (hbEGF), epidermal growth factor receptor (EGFR), erbB2, and insulin-like growth factor 1 receptor (IGF-1R) in trenbolone acetate–stimulated bovine satellite cell proliferation1. J. Anim. Sci. 2015, 93, 4291–4301. [Google Scholar] [CrossRef] [Green Version]
- Thornton, K.J.; Kamanga-Sollo, E.; White, M.E.; Dayton, W.R. Active G protein–coupled receptors (GPCR), matrix metalloproteinases 2/9 (MMP2/9), heparin-binding epidermal growth factor (hbEGF), epidermal growth factor receptor (EGFR), erbB2, and insulin-like growth factor 1 receptor (IGF-1R) are necessary for trenbolone acetate–induced alterations in protein turnover rate of fused bovine satellite cell cultures1. J. Anim. Sci. 2016, 94, 2332–2343. [Google Scholar] [CrossRef] [Green Version]
- Dehm, S.M.; Tindall, D.J. Androgen Receptor Structural and Functional Elements: Role and Regulation in Prostate Cancer. Mol. Endocrinol. 2007, 21, 2855–2863. [Google Scholar] [CrossRef]
- Heinlein, C.A.; Chang, C. Androgen Receptor (AR) Coregulators: An Overview. Endocr. Rev. 2002, 23, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Cheskis, B.J.; Greger, J.G.; Nagpal, S.; Freedman, L.P. Signaling by estrogens. J. Cell. Physiol. 2007, 213, 610–617. [Google Scholar] [CrossRef]
- Dayton, W.R.; White, M.E. Mechanisms of Anabolic Steroid Action in Bovine Skeletal Muscle. In Evaluating Veterinary Pharmaceutical Behavior in the Environment; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2013; Volume 1126, pp. 1–12. [Google Scholar]
- Preston, R.L. Biological Responses to Estrogen Additives in Meat Producing Cattle and Lambs. J. Anim. Sci. 1975, 41, 1414–1430. [Google Scholar] [CrossRef]
- Trenkle, A. The anabolic effect of estrogens on nitrogen metabolism of growing and finishing cattle and sheep. Environ. Qual. safety. Suppl. 1976, 5, 79–88. [Google Scholar]
- Heinlein, C.A.; Chang, C. The Roles of Androgen Receptors and Androgen-Binding Proteins in Nongenomic Androgen Actions. Mol. Endocrinol. 2002, 16, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Estrada, M.; Espinosa, A.; Müller, M.; Jaimovich, E. Testosterone Stimulates Intracellular Calcium Release and Mitogen-Activated Protein Kinases Via a G Protein-Coupled Receptor in Skeletal Muscle Cells. Endocrinology 2003, 144, 3586–3597. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bosonea, A.-M.; Fernandez-Patron, C. Metalloproteinases: Key and common mediators of multiple GPCRs and candidate therapeutic targets in models of hypertensive cardiac disease. Drug Discov. Today Dis. Model. 2012, 9, e103–e108. [Google Scholar] [CrossRef] [Green Version]
- Kamanga-Sollo, E.; White, M.; Chung, K.; Johnson, B.; Dayton, W. Potential role of G-protein-coupled receptor 30 (GPR30) in estradiol-17β-stimulated IGF-I mRNA expression in bovine satellite cell cultures. Domest. Anim. Endocrinol. 2008, 35, 254–262. [Google Scholar] [CrossRef]
- Bologa, C.; Revankar, C.M.; Young, S.M.; Edwards, B.S.; Arterburn, J.B.; Kiselyov, A.S.; Parker, M.A.; Tkachenko, S.E.; Savchuck, N.P.; Sklar, L.A.; et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat. Chem. Biol. 2006, 2, 207–212. [Google Scholar] [CrossRef]
- Boonyaratanakornkit, V.; Edwards, D.P. Receptor mechanisms mediating non-genomic actions of sex steroids. In Seminars in Reproductive Medicine; Thieme Medical Publishers, Inc.: New York, NY, USA, 2007; Volume 25, pp. 139–153. [Google Scholar]
- Kamanga-Sollo, E.; Thornton, K.; White, M.; Dayton, W. Role of G protein-coupled estrogen receptor-1, matrix metalloproteinases 2 and 9, and heparin binding epidermal growth factor-like growth factor in estradiol-17β-stimulated bovine satellite cell proliferation. Domest. Anim. Endocrinol. 2014, 49, 20–26. [Google Scholar] [CrossRef]
- Levin, E.R. Bidirectional Signaling between the Estrogen Receptor and the Epidermal Growth Factor Receptor. Mol. Endocrinol. 2003, 17, 309–317. [Google Scholar] [CrossRef]
- Von Bülow, V.; Rink, L.; Haase, H. Zinc-Mediated Inhibition of Cyclic Nucleotide Phosphodiesterase Activity and Expression Suppresses TNF-α and IL-1β Production in Monocytes by Elevation of Guanosine 3′,5′-Cyclic Monophosphate. J. Immunol. 2005, 175, 4697–4705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnamoorthy, L.; Cotruvo, J.A.; Chan, J.; Kaluarachchi, H.; Muchenditsi, A.; Pendyala, V.S.; Jia, S.; Aron, A.T.; Ackerman, C.M.; Wal, M.N.V.; et al. Copper regulates cyclic-AMP-dependent lipolysis. Nat. Chem. Biol. 2016, 12, 586–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, A.S.; Oberleas, D.; Wolf, P.; Horwitz, J.P.; Miller, E.R.; Luecke, R.W. Changes in Trace Elements and Enzyme Activities in Tissues of Zinc-Deficient Pigs. Am. J. Clin. Nutr. 1969, 22, 628–637. [Google Scholar] [CrossRef]
- Oberleas, D.; Prasad, A.S. Growth as Affected by Zinc and Protein Nutrition. Am. J. Clin. Nutr. 1969, 22, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.B.; Chesters, J.K. The effects of early zinc deficiency on DNA and protein synthesis in the rat. Br. J. Nutr. 1970, 24, 1053–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NASEM. Nutrient Requirements of Beef Cattle, 8th ed.; The National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- NRC. Nutrient Requirements of Beef Cattle, 6th ed.; The National Academies Press: Washington, DC, USA, 1984. [Google Scholar]
- Samuelson, K.L.; Hubbert, M.E.; Galyean, M.L.; Löest, C.A. Nutritional recommendations of feedlot consulting nutritionists: The 2015 New Mexico State and Texas Tech University survey1. J. Anim. Sci. 2016, 94, 2648–2663. [Google Scholar] [CrossRef]
- Suttle, N.F. Mineral Nutrition of Livestock; Cabi: Wallingford, UK, 2010. [Google Scholar]
- Capper, J.L. The environmental impact of beef production in the United States: 1977 compared with 2007. J. Anim. Sci. 2011, 89, 4249–4261. [Google Scholar] [CrossRef] [Green Version]
- Dufner-Beattie, J.; Wang, F.; Kuo, Y.-M.; Gitschier, J.; Eide, D.; Andrews, G.K. The Acrodermatitis Enteropathica Gene ZIP4 Encodes a Tissue-specific, Zinc-regulated Zinc Transporter in Mice. J. Biol. Chem. 2003, 278, 33474–33481. [Google Scholar] [CrossRef] [Green Version]
- McMahon, R.J.; Cousins, R.J. Regulation of the zinc transporter ZnT-1 by dietary zinc. Proc. Natl. Acad. Sci. USA 1998, 95, 4841–4846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cousins, R.J. Absorption, transport, and hepatic metabolism of copper and zinc: Special reference to metallothionein and ceruloplasmin. Physiol. Rev. 1985, 65, 238–309. [Google Scholar] [CrossRef]
- Lönnerdal, B. Dietary Factors Influencing Zinc Absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef] [PubMed]
- Sandström, B.; Arvidsson, B.; Cederblad, A.; Björn-Rasmussen, E. Zinc absorption from composite meals I. The significance of wheat extraction rate, zinc, calcium, and protein content in meals based on bread. Am. J. Clin. Nutr. 1980, 33, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, R.N.; Genther-Schroeder, O.N.; Deters, E.L.; Jackson, T.D.; Messersmith, E.M.; VanValin, K.R.; Hansen, S.L. The influence of supplemental zinc and dietary fiber concentration on mineral retention of beef steers1. Transl. Anim. Sci. 2019, 3, 784–795. [Google Scholar] [CrossRef] [Green Version]
- Carmichael, R.N.; Genther-Schroeder, O.N.; Blank, C.P.; Deters, E.L.; Hartman, S.J.; Niedermayer, E.K.; Hansen, S.L. The influence of supplemental zinc and ractopamine hydrochloride on trace mineral and nitrogen retention of beef steers. J. Anim. Sci. 2018, 96, 2939–2948. [Google Scholar] [CrossRef] [PubMed]
- Fukada, T.; Kambe, T. Zinc Signaling; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Miller, W. Zinc Nutrition of Cattle: A Review. J. Dairy Sci. 1970, 53, 1123–1135. [Google Scholar] [CrossRef]
- Kincaid, R.L. Assessment of trace mineral status of ruminants: A review. J. Anim. Sci. 2000, 77, 1–10. [Google Scholar] [CrossRef]
- Pisano, A.; Santolla, M.F.; De Francesco, E.M.; De Marco, P.; Rigiracciolo, D.C.; Perri, M.G.; Vivacqua, A.; Abonante, S.; Cappello, A.R.; Dolce, V.; et al. GPER, IGF-IR, and EGFR transduction signaling are involved in stimulatory effects of zinc in breast cancer cells and cancer-associated fibroblasts. Mol. Carcinog. 2017, 56, 580–593. [Google Scholar] [CrossRef]
- Wu, W.; Graves, L.M.; Jaspers, I.; Devlin, R.B.; Reed, W.; Samet, J.M. Activation of the EGF receptor signaling pathway in human airway epithelial cells exposed to metals. Am. J. Physiol. Cell. Mol. Physiol. 1999, 277, L924–L931. [Google Scholar] [CrossRef]
- Wu, W.; Graves, L.M.; Gill, G.N.; Parsons, S.J.; Samet, J.M. Src-dependent Phosphorylation of the Epidermal Growth Factor Receptor on Tyrosine 845 Is Required for Zinc-induced Ras Activation. J. Biol. Chem. 2002, 277, 24252–24257. [Google Scholar] [CrossRef] [Green Version]
- Samet, J.M.; Dewar, B.J.; Wu, W.; Graves, L.M. Mechanisms of Zn2+-induced signal initiation through the epidermal growth factor receptor. Toxicol. Appl. Pharmacol. 2003, 191, 86–93. [Google Scholar] [CrossRef]
- Wu, W.; Samet, J.M.; Silbajoris, R.; Dailey, L.A.; Sheppard, D.; Bromberg, P.A.; Graves, L.M. Heparin-Binding Epidermal Growth Factor Cleavage Mediates Zinc-Induced Epidermal Growth Factor Receptor Phosphorylation. Am. J. Respir. Cell Mol. Biol. 2004, 30, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Wells, A. EGF receptor. Int. J. Biochem. Cell Biol. 1999, 31, 637–643. [Google Scholar] [CrossRef]
- Shi, F.; Sheng, Q.; Xu, X.; Huang, W.; Kang, Y.J. Zinc supplementation suppresses the progression of bile duct ligation-induced liver fibrosis in mice. Exp. Biol. Med. 2014, 240, 1197–1204. [Google Scholar] [CrossRef] [Green Version]
- Zong, L.; Wei, X.; Gou, W.; Huang, P.; Lv, Y. Zinc improves learning and memory abilities of fetal growth restriction rats and promotes trophoblast cell invasion and migration via enhancing STAT3-MMP-2/9 axis activity. Oncotarget 2017, 8, 115190–115201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardo, A.; Selman, M. MMP-1: The elder of the family. Int. J. Biochem. Cell Biol. 2005, 37, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Krane, S.M.; Inada, M. Matrix metalloproteinases and bone. Bone 2008, 43, 7–18. [Google Scholar] [CrossRef]
- Dempsey, P.J.; Meise, K.S.; Yoshitake, Y.; Nishikawa, K.; Coffey, R.J. Apical Enrichment of Human EGF Precursor in Madin-Darby Canine Kidney Cells Involves Preferential Basolateral Ectodomain Cleavage Sensitive to a Metalloprotease Inhibitor. J. Cell Biol. 1997, 138, 747–758. [Google Scholar] [CrossRef] [Green Version]
- Dethlefsen, S.M.; Raab, G.; Moses, M.A.; Adam, R.M.; Klagsbrun, M.; Freeman, M.R. Extracellular calcium influx stimu-lates metalloproteinase cleavage and secretion of heparin-binding EGF-like growth factor independently of protein kinase C. J. Cell. Biochem. 1998, 69, 143–153. [Google Scholar] [CrossRef]
- Berg, H.; Rice, C.D.; Rahman, S.; Dong, J.; Thomas, P. Identification and Characterization of Membrane Androgen Receptors in the ZIP9 Zinc Transporter Subfamily: I. Discovery in Female Atlantic Croaker and Evidence ZIP9 Mediates Testosterone-Induced Apoptosis of Ovarian Follicle Cells. Endocrinology 2014, 155, 4237–4249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, P.; Pang, Y.; Dong, J.; Berg, H. Identification and Characterization of Membrane Androgen Receptors in the ZIP9 Zinc Transporter Subfamily: II. Role of Human ZIP9 in Testosterone-Induced Prostate and Breast Cancer Cell Apoptosis. Endocrinology 2014, 155, 4250–4265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Converse, A.; Thomas, P. Androgens regulate follicle stage-dependent pro- and anti-apoptosis in teleost ovaries through ZIP9 activation of different G proteins. Biol. Reprod. 2019, 101, 377–391. [Google Scholar] [CrossRef]
- Johnson, B.J.; White, M.E.; Hathaway, M.R.; Christians, C.J.; Dayton, W.R. Effect of a combined trenbolone acetate and estradiol implant on steady-state IGF-I mRNA concentrations in the liver of wethers and the longissimus muscle of steers. J. Anim. Sci. 1998, 76, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Parr, S.L.; Brown, T.R.; Ribeiro, F.R.B.; Chung, K.Y.; Hutcheson, J.P.; Blackwell, B.; Smith, P.; Johnson, B.J. Biological responses of beef steers to steroidal implants and zilpaterol hydrochloride1. J. Anim. Sci. 2014, 92, 3348–3363. [Google Scholar] [CrossRef]
- Johnson, B.J.; Hathaway, M.R.; Anderson, P.T.; Meiske, J.C.; Dayton, W.R. Stimulation of circulating insulin-like growth factor I (IGF-I) and insulin-like growth factor binding proteins (IGFBP) due to administration of a combined trenbolone acetate and estradiol implant in feedlot cattle. J. Anim. Sci. 1996, 74, 372–379. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Liu, J.-L.; Yakar, S.; LeRoith, D. Conditional knockout of mouse insulin-like growth factor-1 gene using the Cre/loxP system (44500). Proc. Soc. Exp. Biol. Med. 2000, 223, 344–351. [Google Scholar] [CrossRef]
- Underwood, L.E.; Thissen, J.-P.; Lemozy, S.; Ketelslegers, J.-M.; Clemmons, D.R. Hormonal and Nutritional Regulation of IGF-I and Its Binding Proteins. Horm. Res. 1994, 42, 145–151. [Google Scholar] [CrossRef]
- Dørup, I.; Flyvbjerg, A.; Everts, M.E.; Clausen, T. Role of insulin-like growth factor-1 and growth hormone in growth inhibition induced by magnesium and zinc deficiencies. Br. J. Nutr. 1991, 66, 505–521. [Google Scholar] [CrossRef]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, C.; Clemmons, D.R. Differential Expression and Biological Effects of Insulin-like Growth Factor-binding Protein-4 and -5 in Vascular Smooth Muscle Cells. J. Biol. Chem. 1998, 273, 16836–16842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesur, Y.; Yordaman, N.; Doğan, M. Serum Insulin-like Growth Factor-I and Insulin-like Growth Factor Binding Protein-3 Levels in Children with Zinc Deficiency and the Effect of Zinc Supplementation on these Parameters. J. Pediatr. Endocrinol. Metab. 2009, 22, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.G.; Kelleher, S.L.; Lönnerdal, B.; Philipps, A.F. A Graded Model of Dietary Zinc Deficiency: Effects on Growth, Insulin-Like Growth Factor-I, and the Glucose/Insulin Axis in Weanling Rats. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 72–80. [Google Scholar] [CrossRef]
- Nishi, Y.; Hatano, S.; Aihara, K.; Fujie, A.; Kihara, M. Transient partial growth hormone deficiency due to zinc deficiency. J. Am. Coll. Nutr. 1989, 8, 93–97. [Google Scholar] [CrossRef]
- Nakamura, T.; Nishiyama, S.; Futagoishi-Suginohara, Y.; Matsuda, I.; Higashi, A. Mild to moderate zinc deficiency in short children: Effect of zinc supplementation on linear growth velocity. J. Pediatr. 1993, 123, 65–69. [Google Scholar] [CrossRef]
- Sasaki, S.-I. Mechanism of insulin action on glucose metabolism in ruminants. Anim. Sci. J. 2002, 73, 423–433. [Google Scholar] [CrossRef]
- Yang, J. Enhanced Skeletal Muscle for Effective Glucose Homeostasis. In Progress in Molecular Biology and Translational Science; Tao, Y.-X., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 121, pp. 133–163. [Google Scholar]
- McDowell, G.H. Hormonal control of glucose homoeostasis in ruminants. Proc. Nutr. Soc. 1983, 42, 149–167. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Pigera, S.; Galappatthy, P.; Katulanda, P.; Constantine, G.R. Zinc and diabetes mellitus: Understanding molecular mechanisms and clinical implications. DARU J. Pharm. Sci. 2015, 23, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelishadi, R.; Hashemipour, M.; Adeli, K.; Tavakoli, N.; Movahedian-Attar, A.; Shapouri, J.; Poursafa, P.; Rouzbahani, A. Effect of Zinc Supplementation on Markers of Insulin Resistance, Oxidative Stress, and Inflammation among Prepubescent Children with Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2010, 8, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Le-Tien, H.; Goldstein, B.J.; Shin, P.; Lai, R.; Fantus, I.G. Decreased in situ insulin receptor dephosphorylation in hyperglycemia-induced insulin resistance in rat adipocytes. Diabetes 2001, 50, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Miranda, E.R.; Dey, C.S. Effect of Chromium and Zinc on Insulin Signaling in Skeletal Muscle Cells. Biol. Trace Elem. Res. 2004, 101, 19–36. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, H.; Yang, H.; Li, C.; Sang, Q.; Liu, X.; Liu, Y.; Wang, Y.; Sun, Z. Zinc stimulates glucose consumption by modulating the insulin signaling pathway in L6 myotubes: Essential roles of Akt–GLUT4, GSK3β and mTOR–S6K1. J. Nutr. Biochem. 2016, 34, 126–135. [Google Scholar] [CrossRef]
- Myers, S.A.; Nield, A.; Chew, G.-S.; Myers, M.A. The Zinc Transporter, Slc39a7 (Zip7) Is Implicated in Glycaemic Control in Skeletal Muscle Cells. PLoS ONE 2013, 8, e79316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peppa, M.; Koliaki, C.; Nikolopoulos, P.; Raptis, S.A. Skeletal Muscle Insulin Resistance in Endocrine Disease. J. Biomed. Biotechnol. 2010, 2010, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, G.E.; McGilchrist, P.; Pethick, D.W. Ruminant glycogen metabolism. Anim. Prod. Sci. 2014, 54, 1575–1583. [Google Scholar] [CrossRef]
- Ohashi, K.; Nagata, Y.; Wada, E.; Zammit, P.S.; Shiozuka, M.; Matsuda, R. Zinc promotes proliferation and activation of myogenic cells via the PI3K/Akt and ERK signaling cascade. Exp. Cell Res. 2015, 333, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Vernon, B.G.; Buttery, P.J. The effect of trenbolone acetate with time on the various responses of protein synthesis of the rat. Br. J. Nutr. 1978, 40, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Lobley, G.E.; Connell, A.; Mollison, G.S.; Brewer, A.; Harris, C.I.; Buchan, V.; Galbraith, H. The effects of a combined implant of trenbolone acetate and oestradiol-17β on protein and energy metabolism in growing beef steers. Br. J. Nutr. 1985, 54, 681–694. [Google Scholar] [CrossRef]
- Preston, R. Hormone containing growth promoting implants in farmed livestock. Adv. Drug Deliv. Rev. 1999, 38, 123–138. [Google Scholar] [CrossRef]
- Schmelzle, T.; Hall, M.N. TOR, a Central Controller of Cell Growth. Cell 2000, 103, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Manning, B.D.; Cantley, L.C. AKT/PKB Signaling: Navigating Downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamanga-Sollo, E.; White, M.; Hathaway, M.; Weber, W.; Dayton, W. Effect of Estradiol-17β on protein synthesis and degradation rates in fused bovine satellite cell cultures. Domest. Anim. Endocrinol. 2010, 39, 54–62. [Google Scholar] [CrossRef]
- Nimmanon, T.; Ziliotto, S.; Morris, S.; Flanagan, L.; Taylor, K.M. Phosphorylation of zinc channel ZIP7 drives MAPK, PI3K and mTOR growth and proliferation signalling. Metallomics 2017, 9, 471–481. [Google Scholar] [CrossRef] [Green Version]
- Mnatsakanyan, H.; I Serra, R.S.; Rico, P.; Salmerón-Sánchez, M. Zinc uptake promotes myoblast differentiation via Zip7 transporter and activation of Akt signalling transduction pathway. Sci. Rep. 2018, 8, 13642. [Google Scholar] [CrossRef]
- Morgan, J.E.; Partridge, T.A. Muscle satellite cells. Int. J. Biochem. Cell Biol. 2003, 35, 1151–1156. [Google Scholar] [CrossRef]
- Kamanga-Sollo, E.; White, M.; Hathaway, M.; Chung, K.; Johnson, B.; Dayton, W. Roles of IGF-I and the estrogen, androgen and IGF-I receptors in estradiol-17β- and trenbolone acetate-stimulated proliferation of cultured bovine satellite cells. Domest. Anim. Endocrinol. 2008, 35, 88–97. [Google Scholar] [CrossRef]
- Kamanga-Sollo, E.; White, M.; Hathaway, M.; Weber, W.; Dayton, W. Effect of trenbolone acetate on protein synthesis and degradation rates in fused bovine satellite cell cultures. Domest. Anim. Endocrinol. 2011, 40, 60–66. [Google Scholar] [CrossRef]
- Fu, R.; Liu, J.; Li, R.; Li, D.; Cui, S.; Fan, J.; Yin, J. Novel evidence that testosterone promotes cell proliferation and differentiation via G protein-coupled receptors in the rat L6 skeletal muscle myoblast cell line. J. Cell. Physiol. 2011, 227, 98–107. [Google Scholar] [CrossRef]
- Ogawa, M.; Yamaji, R.; Higashimura, Y.; Harada, N.; Ashida, H.; Nakano, Y.; Inui, H. 17β-Estradiol Represses Myogenic Differentiation by Increasing Ubiquitin-specific Peptidase 19 through Estrogen Receptor α. J. Biol. Chem. 2011, 286, 41455–41465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paskavitz, A.L.; Quintana, J.; Cangussu, D.; Tavera-Montañez, C.; Xiao, Y.; Ortiz-Miranda, S.; Navea, J.G.; Padilla-Benavides, T. Differential expression of zinc transporters accompanies the differentiation of C2C12 myoblasts. J. Trace Elem. Med. Biol. 2018, 49, 27–34. [Google Scholar] [CrossRef]
- Petrie, L.; Chesters, J.K.; Franklin, M. Inhibition of myoblast differentiation by lack of zinc. Biochem. J. 1991, 276, 109–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrie, L.; Buskin, J.N.; Chesters, J.K. Zinc and the initiation of myoblast differentiation. J. Nutr. Biochem. 1996, 7, 670–676. [Google Scholar] [CrossRef]
- Hergenreder, J.E.; Legako, J.F.; Dinh, T.T.N.; Spivey, K.S.; Baggerman, J.O.; Broadway, P.R.; Beckett, J.L.; Branine, M.E.; Johnson, B.J. Zinc Methionine Supplementation Impacts Gene and Protein Expression in Calf-Fed Holstein Steers with Minimal Impact on Feedlot Performance. Biol. Trace Elem. Res. 2015, 171, 315–327. [Google Scholar] [CrossRef] [Green Version]
- Huerta, M.; Kincaid, R.; Cronrath, J.; Busboom, J.; Johnson, A.; Swenson, C. Interaction of dietary zinc and growth implants on weight gain, carcass traits and zinc in tissues of growing beef steers and heifers. Anim. Feed. Sci. Technol. 2002, 95, 15–32. [Google Scholar] [CrossRef]
- Messersmith, E.M.; Boyer, A.; Nuzback, D.; Hansen, S.L. 185 Effect of zinc source and implant strategy on performance, carcass characteristics, and tissue mineral concentrations in finishing beef steers. J. Anim. Sci. 2020, 98, 159. [Google Scholar] [CrossRef]
- Messersmith, E.M.; Hansen, S.L. 162 Increasing Concentrations of Supplemental Zinc Influence Performance, Carcass Characteristics, and Trace Mineral Status of Non-implanted and Implanted Steers. J. Anim. Sci. 2021, 99, 123. [Google Scholar] [CrossRef]
- Messersmith, E.M.; Niedermayer, E.; Crawford, G.; Hansen, S.L. 103 Effect of a single Revalor-XH or Revalor-200/Revalor-200 re-implant program and zinc supplementation on performance, carcass characteristics, and liver mineral of feedlot heifers. J. Anim. Sci. 2020, 98, 133. [Google Scholar] [CrossRef]
- Hufstedler, G.D.; Greene, L.W. Mineral and nitrogen balance in lambs implanted with zeranol. J. Anim. Sci. 1995, 73, 3785–3788. [Google Scholar] [CrossRef] [PubMed]
- Messersmith, E.; (Iowa State University, Ames, IA, USA); Hansen, S.L.; (Iowa State University, Ames, IA, USA). Personal communication, 2019.
- Messersmith, E.M.; Reichhardt, C.C.; Thornton, K.J.; Hansen, S.L. 186 Hormone content of anabolic implants differentially affects plasma and liver trace mineral concentrations. J. Anim. Sci. 2020, 98, 154–155. [Google Scholar] [CrossRef]
- Dorton, K.L.; Wagner, J.J.; Larson, C.K.; Enns, R.M.; Engle, T.E. Effects of Trace Mineral Source and Growth Implants on Trace Mineral Status of Growing and Finishing Feedlot Steers. Asian Australas. J. Anim. Sci. 2010, 23, 907–915. [Google Scholar] [CrossRef]
- Golden, B.E.; Golden, M.H.N. Plasma zinc, rate of weight gain, and the energy cost of tissue deposition in children recovering from severe malnutrition on a cow’s milk or soya protein based diet. Am. J. Clin. Nutr. 1981, 34, 892–899. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messersmith, E.M.; Smerchek, D.T.; Hansen, S.L. The Crossroads between Zinc and Steroidal Implant-Induced Growth of Beef Cattle. Animals 2021, 11, 1914. https://doi.org/10.3390/ani11071914
Messersmith EM, Smerchek DT, Hansen SL. The Crossroads between Zinc and Steroidal Implant-Induced Growth of Beef Cattle. Animals. 2021; 11(7):1914. https://doi.org/10.3390/ani11071914
Chicago/Turabian StyleMessersmith, Elizabeth M., Dathan T. Smerchek, and Stephanie L. Hansen. 2021. "The Crossroads between Zinc and Steroidal Implant-Induced Growth of Beef Cattle" Animals 11, no. 7: 1914. https://doi.org/10.3390/ani11071914
APA StyleMessersmith, E. M., Smerchek, D. T., & Hansen, S. L. (2021). The Crossroads between Zinc and Steroidal Implant-Induced Growth of Beef Cattle. Animals, 11(7), 1914. https://doi.org/10.3390/ani11071914






