Aglepristone Administration in Mid-Proestrus Reduces the LH Peak but Does Not Prevent Ovulation in the Bitch
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Clinical Observations and Vaginal Cytology
2.3. Hormone Assays
2.4. Estimation of Ovulation
2.5. Statistical Analysis
3. Results
3.1. Progesterone Plasma Levels
3.2. LH Plasma Levels
3.3. AUC for LH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Groppetti, D.; Aralla, M.; Bronzo, V.; Bosi, G.; Pecile, A.; Arrighi, S. Periovulatory time in the bitch: What’s new to know? Comparison between ovarian histology and clinical features. Anim. Reprod. Sci. 2015, 152, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Concannon, P.W. Endocrinologic control of normal canine ovarian function. Reprod. Domest. Anim. 2009, 44, 3–15. [Google Scholar] [CrossRef]
- Concannon, P.W. Reproductive cycles of the domestic bitch. Anim. Reprod. Sci. 2011, 124, 200–210. [Google Scholar] [CrossRef] [PubMed]
- de Gier, J.; Kooistra, H.S.; Djajadiningrat-Laanen, S.C.; Dieleman, S.J.; Okkens, A.C. Temporal relations between plasma concentrations of luteinizing hormone, follicle-stimulating hormone, estradiol-17beta, progesterone, prolactin, and alpha-melanocyte-stimulating hormone during the follicular, ovulatory, and early luteal phase in the bitch. Theriogenology 2006, 65, 1346–1359. [Google Scholar] [CrossRef]
- Concannon, P.W.; Hansel, W.; McEntee, K. Changes in LH, progesterone and sexual behavior associated with preovulatory luteinization in the bitch. Biol. Reprod. 1977, 17, 604–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, B.; Schneider, S. Secretion and release of luteinizing hormone during the luteal phase of the oestrous cycle in the dog. J. Reprod. Fertil. 1993, 47, 85–91. [Google Scholar]
- Hollinshead, F.; Hanlon, D. Normal progesterone profiles during estrus in the bitch: A prospective analysis of 1420 estrous cycles. Theriogenology 2019, 125, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Badinand, F.; Fontbonne, A.; Maurel, M.C.; Siliart, B. Fertilisation time in the bitch in relation to plasma concentration of oestradiol, progesterone and luteinizing hormone and vaginal smears. J. Reprod. Fertil. 1993, 47, 63–67. [Google Scholar]
- Ververidis, H.N.; Boscos, C.M.; Stefanakis, A.; Krambovitis, E. Use of enzyme-immunoassay for oestradiol-17beta and progesterone quantification in canine serum. Anim. Reprod. Sci. 2002, 69, 53–64. [Google Scholar] [CrossRef]
- Levy, X.; Fontbonne, A. Determining the optimal time of mating in bitches: Particularities. Rev. Bras. Reprod. Anim. 2007, 31, 128–134. [Google Scholar]
- Liu, J.H.; Yen, S.S. Induction of midcycle gonadotropin surge by ovarian steroids in women: A critical evaluation. J. Clin. Endocrinol. Metab. 1983, 57, 797–802. [Google Scholar]
- Spirtos, N.J.; Foote, C.; Downing, J.; Askew, M.J.; Subramanian, M.G. Evaluation of the preovulatory rise of follicle stimulating hormone and progesterone in normally ovulating women of reproductive age. Int. J. Fertil. 1989, 34, 62–66. [Google Scholar]
- Hashimoto, I.; Isomoto, N.; Eto, M.; Kawaminami, M.; Sunazuka, C.; Ueki, N. Preovulatory secretion of progesterone, luteinizing hormone, and prolactin in 4-day and 5-day cycling rats. Biol. Reprod. 1987, 36, 599–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dozortsev, D.I.; Diamond, M.P. Luteinizing hormone–independent rise of progesterone as the physiological trigger of the ovulatory gonadotropins surge in the human. Fertil. Steril. 2020, 114, 191–199. [Google Scholar] [CrossRef]
- Rao, I.M.; Mahesh, V.B. Role of progesterone in the modulation of the preovulatory surge of gonadotropins and ovulation in the pregnant mare’s serum gonadotropin-primed immature rat and the adult rat. Biol. Reprod. 1986, 35, 1154–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uilenbroek, J.T. Hormone concentrations and ovulatory response in rats treated with antiprogestagens. J. Endocrinol. 1991, 129, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.C.; Cartledge, T.P.; Zellmer, A.W.; Nieman, L.K.; Merriam, G.R.; Loriaux, D.L. Evidence for a critical role of progesterone in the regulation of the midcycle gonadotropin surge and ovulation. J. Clin. Endocrinol. Metab. 1992, 74, 565–570. [Google Scholar]
- Mahesh, V.B.; Brann, D.W. Interaction between ovarian and adrenal steroids in the regulation of gonadotropin secretion. J. Steroid. Biochem. Molec. Biol. 1992, 41, 495–513. [Google Scholar] [CrossRef]
- Troisi, A.; Polisca, A.; Cardinali, L.; Orlandi, R.; Brecchia, G.; Menchetti, L.; Zerani, M.; Maranesi, M.; Di Mari, W.; Verstegen, J.P. Effect of aglepristone (RU 534) administration during follicular phase on progesterone, estradiol-17β, and LH serum concentrations in bitches. Reprod. Domest. Anim. 2020, 55, 1794–1802. [Google Scholar] [CrossRef]
- Reynaud, K.; Saint-Dizier, M.; Tahir, M.Z.; Havard, T.; Harichaux, G.; Labas, V.; Thoumire, S.; Fontbonne, A.; Grimard, B.; Chastant-Maillard, S. Progesterone plays a critical role in canine oocyte maturation and fertilization. Biol. Reprod. 2015, 93, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bladowska, K.; Barański, W.; Janowski, T. Preovulatory progesterone secretion terminates the duration of reproductive behaviour during heat in the bitch. Polish J. Vet. Sci. 2018, 21, 615–622. [Google Scholar]
- Günzel-Apel, A.R.; Höftmann, T.; Nottorf, S.; Politt, E.; Meyer-Lindenberg, A.; Hoppen, H.O.; Einspanier, A.; Knijn, H.M.; Mischke, R. Influence of progesterone withdrawal on pregnancy-related parameters during post-implantation early pregnancy loss. Reprod. Dom. Anim. 2009, 44, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Schuler, G. Receptor blockers-general aspects with respect to their use in domestic animal reproduction. Anim. Reprod. Sci. 2000, 60–61, 295–312. [Google Scholar] [CrossRef]
- England, G.C.W. Dog Breeding, Whelping and Puppy Care, 1st ed.; Wiley-Blackwell: Oxford, UK, 2013; pp. 42–49. [Google Scholar]
- Hoffmann, B.; Höveler, R.; Hasan, S.H.; Failing, K. Ovarian and pituitary function in the dogs after hysterectomy. J. Rep. Fert. 1992, 96, 837–845. [Google Scholar] [CrossRef] [Green Version]
- Guerin, C.; Maurel, M.C.; Launais, M.; Deletang, F.; Badinand, F. Use of an immunoenzymatic assay to detect the luteinizing hormone peak in bitches. J. Reprod. Fertil. 1997, 51, 277–281. [Google Scholar]
- Holst, P.A.; Phemister, R.D. Onset of diestrus in the Beagle bitch: Definition and significance. Am. J. Vet. Res. 1974, 35, 401–406. [Google Scholar]
- Mahesh, V.B.; Brann, D.W. Regulation of preovulatory gonadotropin surge by endogenous steroids. Steroids 1998, 63, 616–629. [Google Scholar] [CrossRef]
- Gonçalves, S.C.; Marques, C.C.; Stöckemann, K.; Wang, W.; Horta, A.E. Influence of an antiprogestin (onapristone) on in vivo and in vitro fertilization. Anim. Reprod. Sci. 1997, 46, 55–67. [Google Scholar] [CrossRef]
- Ledger, W.L.; Sweeting, V.M.; Hillier, H.; Baird, D.T. Inhibition of ovulation by low-dose mifepristone (RU 486). Hum. Reprod. 1992, 7, 945–950. [Google Scholar] [CrossRef]
- Galac, S.; Kooistra, H.S.; Dieleman, S.J.; Cestnik, V.; Okkens, A.C. Effects of aglépristone, a progesterone receptor antagonist, administered during the early luteal phase in non-pregnant bitches. Theriogenology 2004, 62, 494–500. [Google Scholar] [CrossRef]
- Polisca, A.; Scotti, L.; Orlandi, R.; Brecchia, G.; Maranesi, M.; Zerani, M.; Boiti, C. Aglepristone (RU534) administration to non-pregnant bitches in the midluteal phase induces early luteal regression. Theriogenology 2010, 74, 672–681. [Google Scholar] [CrossRef]
- Blendinger, K.; Bostedt, H.; Hoffmann, B. Hormonal state and effects of the use of an antiprogestin in bitches with pyometra. J. Reprod. Fertil. Suppl. 1997, 51, 317–325. [Google Scholar] [PubMed]
- Fieni, F.; Martal, J.; Marnet, P.G.; Siliart, B.; Bernhard, F.; Riou, M.; Bruyas, J.F.; Tainturier, D. Hormonal variation in bitches after early or mid-pregnancy termination with aglepristone (RU 534). J. Reprod. Fertil. Suppl. 2001, 57, 243–248. [Google Scholar] [PubMed]
- Kowalewski, M.P.; Beceriklisoy, H.B.; Pfarrer, C.; Aslan, S.; Kindahl, H.; Kücükaslan, I.; Hoffmann, B. Canine placenta: A source of prepartal prostaglandins during normal and antiprogestin-induced parturition. Reproduction 2010, 139, 655–664. [Google Scholar] [CrossRef]
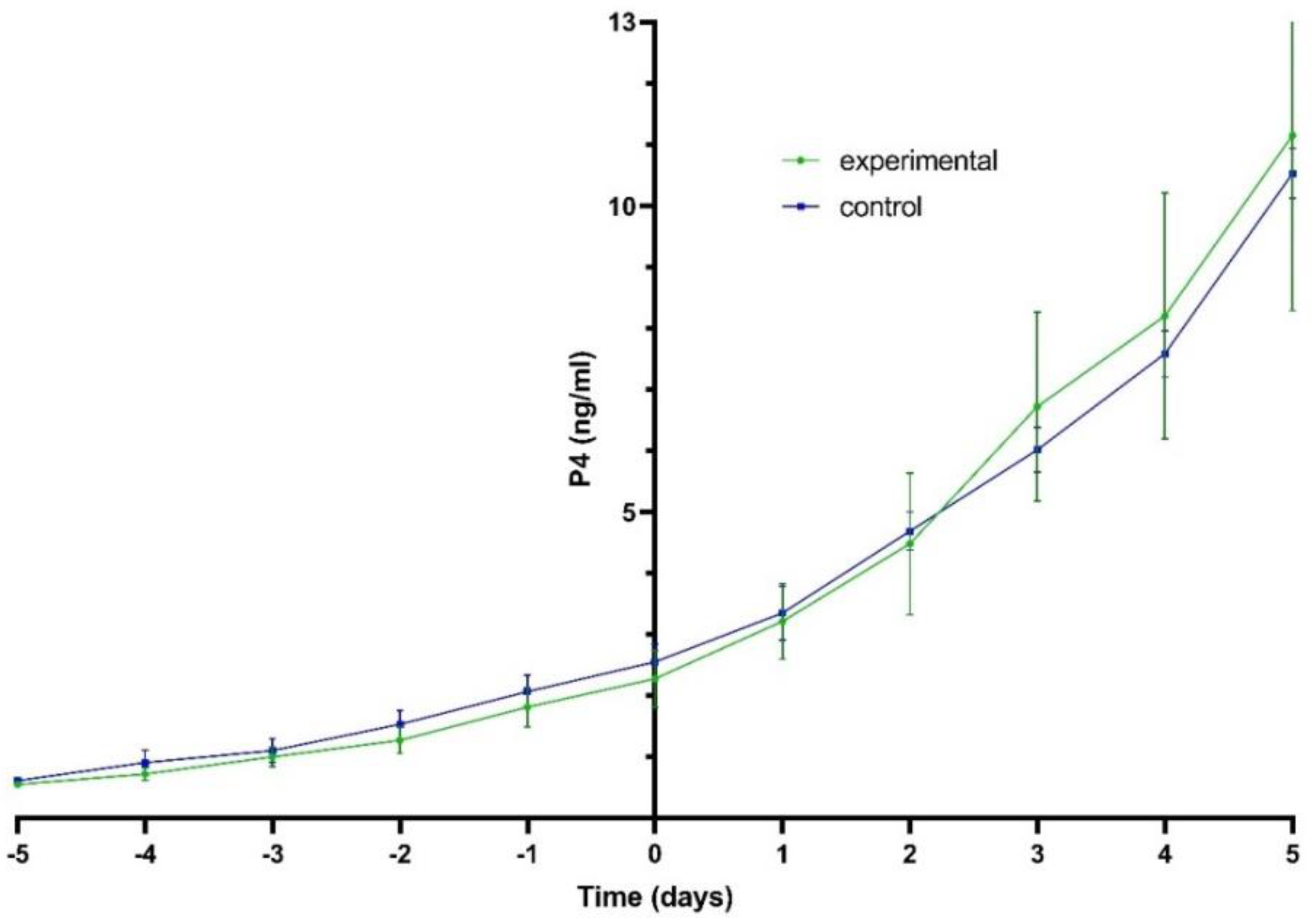
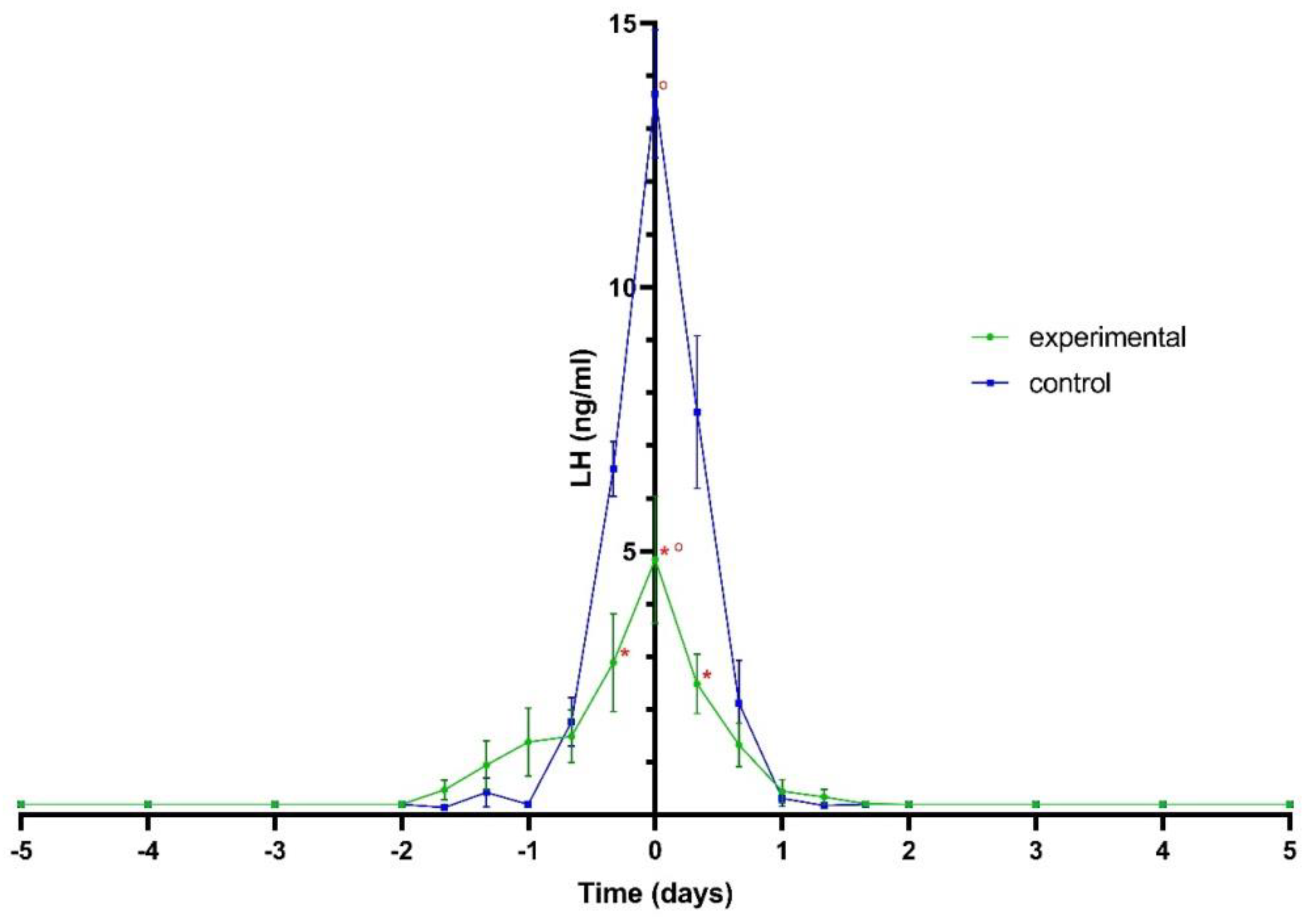
| Variables | Experimental Group (n = −7) | Control Group (n = 7) |
|---|---|---|
| The AUC for LH (ng/mL/d, mean ± SE) | 6.85 ± 1.21 a | 12.25 ± 1.35 b |
| Interval from first administration of aglepristone to the LH peak (days, mean ± SD) | 4.25 ± 1.26 | 5.36 ± 1.45 |
| Ovulation rate (%) | 100.00 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Socha, P.; Bladowska, K.; Zduńczyk, S.; Janowski, T. Aglepristone Administration in Mid-Proestrus Reduces the LH Peak but Does Not Prevent Ovulation in the Bitch. Animals 2021, 11, 1922. https://doi.org/10.3390/ani11071922
Socha P, Bladowska K, Zduńczyk S, Janowski T. Aglepristone Administration in Mid-Proestrus Reduces the LH Peak but Does Not Prevent Ovulation in the Bitch. Animals. 2021; 11(7):1922. https://doi.org/10.3390/ani11071922
Chicago/Turabian StyleSocha, Piotr, Katarzyna Bladowska, Sławomir Zduńczyk, and Tomasz Janowski. 2021. "Aglepristone Administration in Mid-Proestrus Reduces the LH Peak but Does Not Prevent Ovulation in the Bitch" Animals 11, no. 7: 1922. https://doi.org/10.3390/ani11071922
APA StyleSocha, P., Bladowska, K., Zduńczyk, S., & Janowski, T. (2021). Aglepristone Administration in Mid-Proestrus Reduces the LH Peak but Does Not Prevent Ovulation in the Bitch. Animals, 11(7), 1922. https://doi.org/10.3390/ani11071922






