A Review of the Effects and Production of Spore-Forming Probiotics for Poultry
Abstract
:Simple Summary
Abstract
1. Introduction
2. Where to Start? Brief Diving into the Chicken’s Gastrointestinal Tract and Its Commensal Microbiota
2.1. Oral Cavity and Goiter
2.2. Glandular Stomach and Gizzard
2.3. The Small Intestine
2.4. Cecum
2.5. Colon
2.6. Differences in the Microbiota of Chickens and Factors Affecting Them
3. Chicken Probiotics: Why Spore-Formers?
4. Spore-Forming Probiotics and Improving Poultry Health
4.1. Immuno-Modulation by Spore-Forming Probiotics
4.2. Improvement of Metabolic Activities by Spore-Forming Probiotics
4.3. Spore Formers in Health Promotion
5. Control of Microbial Pathogens
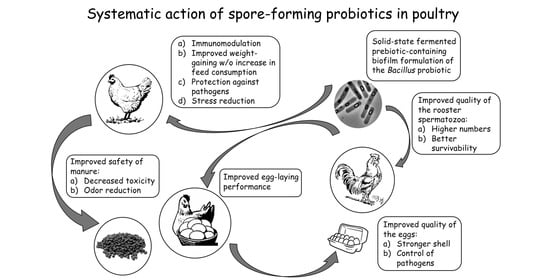
6. Spore-Forming Probiotics: Benefits for the Poultry Industry
- Biochemical blood parameters showing the intensity of carbohydrate and protein metabolism (protein, glucose, urea) [174];
- Dynamics of live weight (weight gain) [177];
- Feed conversion rate (this appears to be increased by improved digestion and absorption of nutrients, leading to increased productivity) [178];
- Quantitative and qualitative composition of the microbiota [179];
- The level of oxidative stress (mRNA expression of antioxidant genes, oxidative damage index, etc.) [180];
- Meat quality (pH, cooking loss, shear, color, short-chain fatty acids, taste) [181];
- Egg production [182].
- Egg quality (yolk cholesterol, improved shell thickness, egg weight) [183];
- Sperm quality (volume of ejaculate, total number and concentration of spermatozoa in the ejaculate, number of morphologically abnormal cells in the ejaculate) [184];
7. Spore-Forming Probiotics Manufacturing, Exploiting Their Biosynthetic Potential
7.1. Cultivation Conditions for Bacillus spp. Growth and Spore Production
7.2. Fermentation Methods for the Production of Probiotics
7.2.1. Solid-State Fermentation
7.2.2. Perspectives on Scaling up Fermentation Processes
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Animal | Spore-Forming Probiotic Strain, Supplemental Level | Way of Probiotic Administration | Performance Parameters | Results of the Experimental Group Compared to the Control Group | References |
|---|---|---|---|---|---|
| Eggs of broiler Cobb 500 (Gallus gallus domesticus) | B. subtilis fermentation extract (each egg received 1 × 107 CFU of the bacterium/200 mL saline diluent) | In ovo | Hatch performance (pipped eggs, late dead eggs, hatchability, average chick weight, chick body weight/initial egg weight, %) | In ovo injection of saline and probiotics resulted in a significant decrease of pipped eggs compare to intact control eggs. | Oladokun et al., 2021 [238] |
| Average daily feed intake (g/bird) | No significant difference. | ||||
| Average daily gain (g/bird) | No significant difference. | ||||
| Feed conversion ratio | No significant difference. | ||||
| Broiler Cobb 500 (Gallus gallus domesticus) | In-water probiotic formulation containing 2.5 × 108 CFU of B. subtilis/L of drinking water | Supplementing drinking water | Average daily feed intake (g/bird) | No significant difference. | |
| Average daily gain (g/bird) | No significant difference. | ||||
| Feed conversion ratio | No significant difference. | ||||
| In-feed probiotic formulation containing 5 × 108 CFU of B. subtilis/kg of feed | Supplementing standard diet | Average daily feed intake (g/bird) | No significant difference. | ||
| Average daily gain (g/bird) | No significant difference. | ||||
| Feed conversion ratio | No significant difference. | ||||
| Hy-Line Brown laying hens (Gallus gallus domesticus) | B. subtilis C-3102 spores at 9.3 × 109 CFU/kg | Supplementing standard diet | Egg production, % | No significant difference. | Wang et al., 2021 [239] |
| Egg weight, g | No significant difference. | ||||
| Average daily feed intake, g | No significant difference. | ||||
| Food conversion ratio (feed:egg, g:g) | A significant decrease from 2.15 in the control group to 2.08 in the treatment group. | ||||
| Hy-Line Brown laying hens (Gallus gallus domesticus) | B. velezensis (Group I, 1 × 1010 CFU/kg; Group II, 2 × 1010 CFU/kg) | Supplementing standard diet | Average egg production rate, % | A significant increase from 78.889 ± 0.007 in the control group to 80.827 ± 0.005 (Group I) and 81.905 ± 0.006 (Group II). | Ye et al., 2020 [240] |
| Average egg weight, g | A significant increase from 61.972 ± 0.150 in the control group to 60.362 ± 0.140 (Group I) and 61.192 ± 0.111 (Group II). | ||||
| Average daily feed intake, g | A significant increase from 110.608 ± 0.368 in the control group to 112.546 ± 0.281 (Group I) and 111.435 ± 0.229 (Group II). | ||||
| Feed conversion ratio | No significant difference. | ||||
| Hy-Line Brown laying hens (Gallus gallus domesticus) | B. amyloliquefaciens BLCC1-0238 (2 × 1010 CFU/g) at 0.01%, 0.03%, or 0.06% levels | Supplementing standard diet | Egg production, % | A significant increase from 93.8 ± 0.3 in the control group to 97.9 ± 0.5 (0.01%) and 97.7 ± 0.2 (0.06%) in groups treated with spore-forming probiotics. | Zhou et al., 2020 [241] |
| Egg weight, g | No significant difference. | ||||
| Egg mass, g/hen per day | Significant increase from 54.0 ± 0.5 in control group to 57.1 ± 0.7 (0.01%) and 57.0 ± 0.4 (0.06%) in groups treated with spore-forming probiotics. | ||||
| Feed intake, g/hen per day | No significant difference. | ||||
| Feed conversion, g/g | No significant difference. | ||||
| Lohmann pink hens (Gallus gallus domesticus) | B. subtilis C-3102 at 5 × 108 CFU/kg (and mixed with montmorillonite, which was not covered by this review) | Supplementing basal diet | Egg production, % | A significant increase from 94.33 in the control group to 95.94 in the group treated with spore-forming probiotics. | Chen et al., 2020 [242] |
| Egg mass, g/hen per d | No significant difference. | ||||
| Feed conversion ratio, g of feed/g of egg | No significant difference. | ||||
| Shaver White laying hens (Gallus gallus domesticus) | B. subtilis DSM29784, 1.1 × 108 CFU/kg (low); B. subtilis DSM29784, 2.2 × 108 CFU/kg (medium), B. subtilis DSM29784; 1.1 × 109 CFU/kg (high) | Supplementing corn–soybean meal | Body weight (kg) | Significant improvement from 1549 to 1568 (low), 1601 (medium), 1581 (high) observed in week 32. No significant difference in other dates/periods. | Neijat et al., 2019 [10] |
| Feed intake (g/day/bird) | Significant improvement from 81.3 to 86.3 (low), 83.5 (high) observed in week 20. No significant difference in other dates/periods. | ||||
| Egg production (% hen-day) | Significant improvement from 31.8 to 38.2 (low), 41.6 (medium) observed in week 19. No significant difference in other dates/periods. | ||||
| Egg weight (g/egg) | A significant decrease from 44.1 for medium dose compared to other doses (44.5 for control, 45.6 for low, 44.6 for high) observed in week 20. No significant difference in other dates/periods. | ||||
| Egg mass (g/egg) | Significant improvement in week 20, resulting in an overall improvement across the layer I phase (tabular values are not presented). No significant difference in the layer II phase. | ||||
| Feed conversion ratio (feed intake, g:egg mass, g) | Significant improvement from 1.59 to 1.62 (high) was observed in week 40. No significant difference in other dates/periods. | ||||
| Hisex Brown hens (Gallus gallus domesticus) | B. subtilis KATMIRA1933 (Group I; 107–109 CFU viable spores per gram of the probiotic supplement), B. amyloliquefaciens B-1895 (Group II; 107–109 CFU viable spores per gram of the probiotic supplement), both strains (Group III; equal amounts, 107–109 CFU viable spores per gram of the probiotic supplement) | Supplementing the standard diet via solid phase fermentation | Egg laying, % | Egg laying in the experimental groups exceeded the control by 1.8% (p < 0.05) in Group I, 0.62% (p < 0.05) in Group II, and 0.36% (p < 0.05) in Group III. | Prazdnova et al., 2019 [243] |
| Egg weight, g | The weight of eggs in the experimental groups exceeded the control by 3.00% in Group I (p < 0.05), 1.99% in Group II (p < 0.05), and 2.38% in Group III (p < 0.05). | ||||
| Yolk mass, g | The weight of yolk in the experimental groups exceeded the control by 3.49% in Group I (p < 0.05), 1.96% in Group II, and 2.28% in Group III. | ||||
| Albumen mass/yolk weight | In the experimental groups, the ratios decreased to 1.90 (p < 0.05), 1.92 (p < 0.05), and 1.91 (p < 0.05) in groups I, II, III, respectively, compared to 1.93 (p < 0.05) in the control. | ||||
| Protein index | In the experimental groups, it was significantly higher than the control at 8.77 (p < 0.01) in Group I, 6.14 (p < 0.05) in Group II, and 7.89 (p < 0.01) in Group III. | ||||
| Egg hatching, % | The hatching rate in Groups I and II was 86.76% (p < 0.05), which was 2.94% (p < 0.05) higher than the control. The hatching rate of Group III was 84.55% (p < 0.05), which exceeded the control by 0.73% (p < 0.05). | ||||
| Egg fertilization, % | High rates of chickens’ output in experimental Groups I and II were obtained due to the increase in egg fertilization up to 97.06% (p < 0.05), and the hatchability of eggs reached a maximum value of 89.39% (p < 0.05). | ||||
| Hy-LineBrown pullets (Gallus gallus domesticus) | GalliProMax/B. subtilis, 500 g/ton (GPM) 8 × 105 CFU/g; GalliPro Tect/B. licheniformis, 500 g/ton (GMT) 8 × 105 CFU/g | Supplementing the standard diet | Body weight, g | No significant difference. | Upadhaya et al., 2019 [244] |
| Body weight gain, g | No significant difference. | ||||
| Feed intake, g | A significant decrease from 3241 in the control group to 3123 (GPM) and an increase to 3312 (GMT). | ||||
| Feed conversion ratio | No significant difference. | ||||
| Egg production, % | A significant increase from 92.07 in the control group to 94.91 (GPM). | ||||
| Lohmann pink laying hens (Gallus gallus domesticus) | Clostridium butyricum (the dose of the dietary probiotic supplement was added with reference to the company’s commercial recommendations) | Supplementing a standard diet (0.5 g/kg of probiotic) | Average daily feed intake | A significant decrease from 105.5 ± 1.80 in the control group to 104.1 ± 1.14 in the treatment group. | Xiang et al., 2019 [245] |
| Average egg weight, g | No significant difference. | ||||
| Feed conversion (g of feed/g of egg) | A significant decrease from 1.97 ± 0.04 in the control group to 1.92 ± 0.03 in the treatment group. | ||||
| Laying rate, % | No significant difference. | ||||
| Mortality. % | No significant difference. | ||||
| Average cracked eggs, % | No significant difference. | ||||
| Hy-Line Brown laying hens (Gallus gallus domesticus) | B. licheniformis yb-214245 (BL; 1.0 × 106 CFU/kg), B. subtilis yb-114246 (BS; 1.0 × 106 CFU/kg), both strains (CB; (6.6 × 105:3.3 × 105 BL:BS) | Supplementing the standard diet via blending with 99.01 kg basic mass feed. | Laying rate, % | A significant increase from 75.9 in the control group to 79.1 (BL), 82.3 (BS), and 82.2 (CB) in the 60–72 weeks period. | Yang et al., 2019 [246] |
| Egg weight, g | No significant difference. | ||||
| Egg mass, g/hen per day | A significant increase from 46.9 in the control group to 49.5 (BL), 50.8 (BS), and 51.0 (CB) in the 60–72 weeks period. | ||||
| Feed consumption, g/hen per day | No significant difference. | ||||
| Feed conversion | A significant decrease from 2.49 in the control group to 2.36 (BL), 2.3 (BS), and 2.28 (CB) in the 60–72 weeks period. | ||||
| Soft broken egg rate, % | A significant decrease from 1.61 in the control group to 0.43 (BL), 0.65 (BS), and 0.33 (CB) in the 60–72 weeks period. | ||||
| Malformed egg rate, % | A significant decrease from 0.17 in control group to 0.12 (BL), 0.10 (BS), and 0.09 (CB) in the 60–72 weeks period. | ||||
| Arbor Acres broilerchickens (Gallus gallus domesticus) | B. subtilis CGMCCN 0943 at 1.0 × 1011 CFU/g | Supplementing standard diet at 0.2 g/kg of probiotic (BS-1), at 0.3 g/kg of probiotic (BS-2), at 0.4 g/kg of probiotic (BS-3), at 0.5 g/kg of probiotic (BS-4) | Body weight, g/bird | No significant difference. | Bai et al., 2018 [247] |
| Feed intake, g/bird | No significant difference. | ||||
| Feed conversion ratio, g/g | No significant difference. | ||||
| ISA brown laying hens (Gallus gallus domesticus) | Complex strain of spray-dried spores forming B. amyloliquefaciens | Supplementing a standard diet of 1.0 × 107 CFU/kg probiotic (P1) and 2.0 × 107 CFU/kg probiotic (P2) | Egg production, % | Significant improvement from 89.4 in the control group to 91.8 (P1) and 92.0 (P2) at 4–6 weeks. | Tang et al., 2018 [248] |
| Egg weight, g | No significant difference. | ||||
| Jinghong-1 strain laying hens (Gallus gallus domesticus) | C. butyricum at 2.5 × 104 (CB1), 5 × 104 (CB2), 1 × 105 (CB3), and 2 × 105 (CB4) CFU/g | Supplementing standard corn–soybean basal diet | Egg production, % | A significant increase from 85.4 in the control group to 91.4 (CB2). | Zhan et al., 2018 [249] |
| Egg weight, g/hen per day | No significant difference. | ||||
| Egg mass, g/hen per day | A significant increase from 52.5 in control group to 57.1 (CB2). | ||||
| Feed intake, g/hen per day | No significant difference. | ||||
| Feed conversion ratio | No significant difference. | ||||
| Japanese quails (Coturnix coturnix japonica) | B. subtilis at 109 CFU/g | Supplementing the basal diet | Feed intake, g/bird/day | A significant decrease from 25.924 in the control group to 24.694 in the group treated with spore-forming probiotics in the 9 to 23 week period. A significant decrease from 25.552 in the control group to 23.922 in the group treated with spore-forming probiotics in the 24 to 39 weeks period. | Lemos et al., 2018 [250] |
| Egg production, % | A significant increase from 90.102 in the control group to 95.981 in the group treated by spore-forming probiotics in the 9 to 23 weeks period. A significant increase from 89.223 in the control group to 92.961 in the group treated with spore-forming probiotics. | ||||
| Egg weight average, g | A significant increase from 11.03 in the control group to 11.26 in the group treated by spore-forming probiotics in the 9 to 23 weeks period. A significant increase from 12.08 in the control group to 12.82 in the group treated with spore-forming probiotics. | ||||
| Egg mass, g | A significant increase from 9.938 in the control group to 10.807 in the group treated with spore-forming probiotics in the 9 to 23 weeks period. A significant increase from 10.778 in the control group to 11.918 in the group treated with spore-forming probiotics. | ||||
| Feed conversion per egg mass, kg/kg | A significant decrease from 2.292 in the control group to 2.210 in the group treated with spore-forming probiotics in the 9 to 23 weeks period. A significant decrease from 2.362 in the control group to 2.246 in the group treated with spore-forming probiotics. | ||||
| Feed conversion per dozen eggs, kg/dozen | A significant decrease from 0.251 in the control group to 0.228 in the group treated with spore-forming probiotics in the 9 to 23 weeks period. A significant decrease from 0.268 in the control group to 0.238 in the group treated with spore-forming probiotics. | ||||
| Viability of the birds, % | No significant difference. | ||||
| Xuefeng black-bone chicken (Gallus gallus domesticus) | B. subtilis C-3102 at 3.0 × 105 (BS-1), 6.0 × 105 cfu/g (BS-2), and 9.0 × 105 (BS-3) CFU/g. | Supplementing the basal diet | Egg weight, g | A significant increase from 45.00 in the control group to 46.71 (BS-2) and 47.03 (BS-3). | Liu et al., 2019 [251] |
| Egg production, % | No significant difference. | ||||
| Feed conversion ratio, g of feed/g of egg | No significant difference. | ||||
| Egg mass, g/day per hen | No significant difference. | ||||
| Fertility, % | A significant increase from 91.59 in the control group to 96.68 (BS-1) and 97.64 (BS-3). | ||||
| Hatchability, % | No significant difference. | ||||
| Hatchability of fertile eggs, % | No significant difference. | ||||
| Fertile Cobb chicken (Gallus gallus domesticus) | B. subtilis at 107 CFU/0.5 mL | In ovo | Average feed intake g/day | No significant difference. | Majidi-Mosleh et al., 2017 [252] |
| Average weight gain, g/day | No significant difference. | ||||
| Feed conversion ratio | No significant difference. | ||||
| Hi-Sex Brown cross laying hens (Gallus gallus domesticus) | B. subtilis KATMIRA1933 (107–109 CFU viable spores per gram of the probiotic supplement; Group I), B. amyloliquefaciens B-1895 (107–109 CFU viable spores per gram of the probiotic supplement; Group II),and B. subtilis KATMIRA1933 and B. amyloliquefaciens B-1895 (equal amounts, 107–109 CFU viable spores per gram of the probiotic supplement; Group III) | Supplementing the standard diet via solid phase fermentation | The number of eggs, pcs. | A significant increase from 7419 in the control group to 7538 (Group I), 7469 (Group II), and 7482 (Group III). | Mazanko et al., 2017 [184] |
| Egg weight, g | A significant increase from 61.64 ± 0.42 in the control group to 63.49 ± 0.67 (Group I) and 63.11 ± 0.37 (Group III). | ||||
| Hisex Brown laying hens (Gallus gallus domesticus) | B. subtilis at 1.5 × 108 CFU/g of the dried product (and various mixes with distillers, or dried grains with solubles, which were not covered by this review) | Supplementing the standard diet | Feed consumption, g/hen per day | No significant difference. | Abd El-Hack et al., 2016 [253] |
| Egg weight, g | No significant difference. | ||||
| Hen-day egg production, % | No significant difference. | ||||
| Feed conversion, g of feed/g of egg | No significant difference. | ||||
| Egg mass, g | A significant increase from 63.65 in the control group to 67.12 in the group treated with spore-forming probiotics. | ||||
| Viability rate, % | No significant difference. | ||||
| Hy-Line Brown laying hens (Gallus gallus domesticus) | B. subtilis CGMCC 1.921 at 1.0 × 105 CFU/g (B1), 1.0 × 106 CFU/g (B2), 1.0 × 107 CFU/g (B3), and 1.0 × 108 CFU/g (B4) | Supplementing the basal diet | Egg production, % | A significant increase from 90.6 in the control group to 94.0 (B1), 94.2 (B3), 94.9 (B4) in the 5 to 8 weeks period. A significant increase from 89.6 in the control group to 93.6 (B2), 93.5 (B3), 93.7 (B4) in the 9 to 12 weeks period. No significant difference in other dates/periods. | Guo et al., 2017 [254] |
| Feed intake, g/bird/day | No significant difference. | ||||
| Egg weight, g | No significant difference. | ||||
| Feed:egg ratio, g/g | A significant decrease from 2.04 in the control group to 1.97 (B1), 1.95 (B2), 1.95 (B3), 1.94 (B4) in the 13 to 16 weeks period. A significant decrease from 2.01 in the control group to 1.92 (B1), 1.91 (B2), 1.93 (B3), 1.91 (B4) in the 17 to 20 weeks period. A significant decrease from 2.05 in the control group to 1.93 (B1), 1.96 (B2), 1.96 (B3), 1.94 (B4) in the 21 to 24 weeks period. A significant decrease from 2.04 in the control group to 1.93 (B1), 1.95 (B3), 1.94 (B4) in the 1 to 24 weeks period. | ||||
| Japanese quails (Coturnix Coturnix Japonica) | B. subtilis C-3102 at 0.1% level (minimum dose 1.0 × 1010 viable spores per gram) | Supplementing the basal diet | Egg production, hen-day % | A significant increase from 69.09 in the control group to 72.22 in the group treated with spore-forming probiotic. | Manafi et al., 2016 [255] |
| Feed conversion ratio, g feed/g egg produced | A significant decrease from 3.57 in the control group to 3.42 in the group treated with spore-forming probiotic. | ||||
| Feed intake, g/quail/day | No significant difference. | ||||
| Egg weight, g | A significant increase from 11.15 in the control group to 11.26 in the group treated by spore-forming probiotic. | ||||
| Lohmann Brown laying hens (Gallus gallus domesticus) | B. subtilis ATCC PTA-6737 at 1 × 108 CFU/kg feed | Supplementing the standard diet | Body weight at 18 days, kg | No significant difference. | Sobczak et al., 2015 [256] |
| Body weight at 42 days, kg | Significant improvement from 1.954 ± 0.044 in the control group to 2.004 ± 0.050 (1 × 108 CFU/g of probiotic). | ||||
| Body weight gain, from 18 days to 42 days, kg | Significant improvement from 0.359 ± 0.038 in the control group to 0.405 ± 0.037 (1 × 108 CFU/g of probiotic). | ||||
| Egg weight, g | No significant difference. | ||||
| Egg mass, g/hen | No significant difference. | ||||
| Laying rate, % | No significant difference. | ||||
| Feed intake, g/hen | No significant difference. | ||||
| Feed conversion ratio, g feed/g egg mass | No significant difference. | ||||
| Hy-Line layer hybrids (Gallus gallus domesticus) | B. subtilis PB6 at 0.05% dose | Supplementing corn–soybean cake-based diet | Deposition rate | No significant difference. | Forte et al., 2016 [257] |
| Feed efficiency | No significant difference. | ||||
| Egg weight | No significant difference. | ||||
| Hy-Line W-36 (Gallus gallus domesticus) | B. subtilis DSM17299 8 × 105 CFU/g, 4 × 105 CFU/g feed, 3 × 105 CFU/g feed | Delivery in spore form in corn and soybeans | Feed intake, g/hen/day | No significant difference. | Ribeiro Jr. et al., 2014 [258] |
| Egg production, g/kg | Significant improvement from 895 in the control group to 918 (8 × 105 CFU/g of probiotics). No significant difference in other groups. | ||||
| Egg weight, g/hen/day | Significant improvement from 59.9 in the control group to 60.8 (8 × 105 CFU/g of probiotics), 60.7 (4 × 105 CFU/g of probiotics), and 60.5 (3 × 105 CFU/g of probiotics). | ||||
| Egg mass, g/hen/day | Significant improvement from 53.7 in the control group to 55.7 (8 × 105 CFU/g of probiotics) and 55.3 (4 × 105 CFU/g of probiotics). No significant difference in other groups. | ||||
| Feed conversion ratio per dozen eggs, kg/dz | No significant difference. | ||||
| Feed conversion ratio per egg mass, g/g | No significant difference. | ||||
| Excreta dry matter content, g/kg | Significant improvement from 59.9 in the control group to 60.8 (8 × 105 CFU/g of probiotics), 60.7 (4 × 105 CFU/g of probiotics) and 60.5 (3 × 105 CFU/g of probiotics). | ||||
| Ross 308 broiler chicks (Gallus gallus domesticus) | C. butyricum (0 or 1 × 109 CFU/kg (500 mg/kg)) | Supplementing the standard diet | Average daily feed intake, g/day | A significant increase from 83.7 in the control group to 88.0 in the group treated with a spore-forming probiotic in the period from 1 to 42 days. | Zhao et al., 2013 [259] |
| Average daily gain, g/day | A significant increase from 46.7 in the control group to 49.1 in the group treated with a spore-forming probiotic in the period from 1 to 42 days. | ||||
| Feed conversion ratio, g/g | No significant difference. | ||||
| Abdominal fat, % | No significant difference. | ||||
| Intramuscular fat (breast muscle), mg/g | A significant increase from 4.34 in the control group to 7.22 in the group treated with a spore-forming probiotic at 42 days. | ||||
| Intramuscular fat (thigh muscle), mg/g | A significant increase from 4.38 in the control group to 7.20 in the group treated with a spore-forming probiotic at 42 days. | ||||
| White laying hens (Gallus gallus domesticus) | B. subtilis PB6 at 0.5 g/kg and 1.0 g/kg supplement levels | Supplementing the basal diet | Egg production, % | Significant increase in both groups treated with spore-forming probiotics compared to control (no table values provided). | Abdelqader et al., 2013 [183] |
| Egg weight, g | Significant increase in both groups treated with spore-forming probiotics compared to control (no table values provided). | ||||
| Egg mass, g/hen | Significant increase in both groups treated with spore-forming probiotics compared to control (no table values provided). | ||||
| Feed intake, g/day | No significant difference. | ||||
| Feed conversion, kg/kg | Significant decrease from 3.0 in control group to 2.8 (0.5 g/kg) and 2.6 (1.0 g/kg) in groups treated with spore-forming probiotic. | ||||
| Hy-Line Variety W-36 hens (Gallus gallus domesticus) | B. licheniformis at 0.01% (2 × 106 CFU/g), 0.02% (4 × 106 CFU/g), 0.03% (6 × 106 CFU/g), 0.06% (1.2 × 107 CFU/g), and 0.09% (1.8 × 107 CFU/g), | Supplementing the basal diet | Egg production, % | Significant increase from 94.0 ± 0.4 in control group to 98.4 ± 0.6 (0.01%) and 97.9 ± 0.2 (0.06%). | Lei et al., 2013 [182] |
| Egg weight, g | No significant difference. | ||||
| Egg mass, g/hen per day | Significant increase from 54.3 ± 0.6 in control group to 57.0 ± 0.8 (0.01%) and 57.0 ± 0.5 (0.06%). | ||||
| Feed consumption, g/hen per day | No significant difference. | ||||
| Feed conversion, g/g | No significant difference. | ||||
| Lohmann pink laying hens (Gallus gallus domesticus) | B. subtilis at 9 × 109 CFU/g (and various mixes with Lactobacillus bacteria and sodium butyrate, which were not covered by this review) | Supplementing the standard diet | Egg production, % | No significant difference. | Zhang et al., 2012 [260] |
| Egg weight, g | No significant difference. | ||||
| Daily egg yield, g/hen per day | No significant difference. | ||||
| Feed consumption, g/hen per day | A significant decrease from 2.13 ± 0.03 in the control group to 2.03 ± 0.02 in the group treated with spore-forming probiotics. | ||||
| Feed conversion ratio, g/g | No significant difference. | ||||
| Damaged egg ratio, % | No significant difference. | ||||
| Lingnan Yellow broiler chickens (Gallus gallus domesticus) | C. butyricum at 2.5 × 107 CFU/kg (CB1), 5 × 107 CFU/kg (CB2), 1 × 108 CFU/kg (CB3). | Supplementing the basal diet | Body weight, g | Significant increase from 387.80 in control group to 444.70 (CB1), 431.40 (CB2), and 427.60 (CB3) at 21 days. Significant increase from 1327.50 in control group to 1414.30 (CB1) and 1402.30 (CB2) at 42 days. | Cao et al., 2012 [261] |
| Average daily gain, g | Significant increase from 16.50 in control group to 18.50 (CB1), 19.20 (CB2), and 18.40 (CB3) in the 1–21 days period. Significant increase from 30.60 in control group to 32.40 (CB1) and 32.70 (CB2) in the 1–42 days period. | ||||
| Feed conversion ratio (F:G) | Significant decrease from 2.15 in control group to 2.06 (CB1), 2.06 (CB2), and 2.08 (CB3) in the 1–42 days period. | ||||
| Hy-Line Brown laying hens (Gallus gallus domesticus).According to the study design, all animals except the control group were kept at heat–stress conditions (34 °C). There was no positive control group treated with probiotics kept at normal room temperature conditions (21 °C). | B. licheniformis at 106 CFU/g (H + B1) and 107 CFU/g (H + B2). | Supplementing the basal diet | Egg production, % | A significant decrease from 79.51 in the control group to 60.07 (H + B1) and a significant increase from 50.69 in the group kept at heat–stress conditions to 74.35 (H + B2). | Deng et al., 2012 [189] |
| Egg weight, g | No significant difference. | ||||
| Feed intake, g/bird per day | A significant decrease from 124.32 in the control group to 102.35 (H + B1) and 110.28 (H + B2); a significant increase from 95.74 in the group kept at heat–stress conditions to 102.35 (H + B1) and 110.28 (H + B2). | ||||
| Shaoxing ducks (Anas platyrhynchos domesticus) | B. subtilis at 1 × 108 CFU/kg | Supplementing the basal diet | Live body weight, kg | No significant difference. | Li et al., 2011 [262] |
| Egg laying rate, % | A significant increase from 84.767 ± 0.6 in the control group to 88.100 ± 0.9 in the group treated by spore-forming probiotics. | ||||
| Mean egg weight, g | No significant difference. | ||||
| Daily egg mass, g | No significant difference. | ||||
| Feed:egg ratio | No significant difference. | ||||
| Hy-Line W-36 strains of white Leghorn laying hens (Gallus gallus domesticus) | Mix of B. subtilis CH201 and B. licheniformis CH200 at 1000 g/ton and 2000 g/ton | Supplementing the basal diet | Egg production, % | A significant increase from 79.69 in the control group to 84.17 (1000 g ton−1). | Aghaii et al., 2010 [263] |
| Egg weight, g | No significant difference. | ||||
| Feed consumption, g/hen/day | No significant difference. | ||||
| Egg mass, g/hen/day | A significant increase from 45.90 in the control group to 51.03 (1000 g ton−1) and 49.19 (2000 g ton−1). | ||||
| Feed conversion ratio, g/g | A significant increase from 2.263 in the control group to 2.032 (1000 g ton−1). | ||||
| Lohmann Brown layers (Gallus gallus domesticus) | Dried Bacillus subtilis culture at 9.3 × 109 CFU/kg | Supplementing standard diet of 500 mg/kg, 1000 mg/kg, or 1500 mg/kg of probiotic | Egg production, % eggs/hen per day | No significant difference. | Li et al., 2006 [264] |
| Egg weight, g | No significant difference. | ||||
| Egg mass, g/hen per day | A significant increase from 50.58 ± 1.88 in the control group to 51.41 ± 2.51 in the treatment group (500 mg of probiotic/kg) for the 26–42 weeks period. No significant difference in other dates/periods. | ||||
| Feed consumption, g/hen per day | A significant increase from 125.39 ± 5.82 in the control group to 117.42 ± 2.22 (500 mg of probiotic/kg) and 118.62 ± 6.31 (1500 mg of probiotic/kg) for the 43–56 weeks period and from 114.49 ± 2.89 in the control group to 111.14 ± 1.34 (500 mg of probiotic/kg) and 111.52 ± 2.71 (1000 mg of probiotic/kg) for the 26–56 weeks period. No significant difference in other dates/periods. | ||||
| Feed conversion ratio, kg of food/kg of egg | A significant increase from 2.67 ± 0.14 in the control group to 2.41 ± 0.15 (500 mg of probiotic/kg) for the 43–56 weeks period and from 2.35 ± 0.09 in the control group to 2.21 ± 0.07 (500 mg of probiotic/kg) for the 26–56 weeks period. No significant difference in other dates/periods. | ||||
| Damaged egg, % | No significant difference. | ||||
| Mortality, % | No significant difference. | ||||
| Hy-Line White laying hens (Gallus gallus domesticus) | Mix of B. subtilis CH201 and B. licheniformis CH200 at 1.28 × 106 CFU/g (Group I), 3.2 × 106 CFU/g (Group II), 4.6 × 106 CFU/g (Group III) | Supplementing the standard diet | Egg weight, g | No significant difference. | Mahdavi et al., 2005 [265] |
| Egg production, % | No significant difference. | ||||
| Feed consumption, g/hen/day | No significant difference. | ||||
| Feed conversion ratio, g/g | No significant difference. | ||||
| Egg mass, g/hen/day | No significant difference. |
| Animal | Spore-Forming Probiotic Strain, Dose | Way of Probiotic Administration | Egg Quality | Results of the Experimental Group Compared to the Control Group | References |
|---|---|---|---|---|---|
| Hy-Line Brown laying hens (Gallus gallus domesticus) | B. subtilis C-3102 spores 9.3 × 109 CFU/kg | Supplementing the standard diet | Eggshell breaking strength, N | A significant increase from 37.64 in the control group to 38.46 in the treatment group at week 79. | Wang et al., 2021 [239] |
| Eggshell thickness, mm | A significant increase from 37.64 in the control group to 38.46 in the treatment group at week 79. | ||||
| Shell ratio, % | A significant increase from 9.70 in the control group to 9.78 in the treatment group at week 79. | ||||
| Eggshell weight, g | A significant increase from 6.33 in the control group to 6.43 in the treatment group at week 79. | ||||
| Ca content of eggshell, % | A significant increase from 32.49 in the control group to 35.17 in the treatment group at week 79. | ||||
| p content of eggshell, % | No significant difference. | ||||
| Female Pharaon quails (Coturnix japonica) | B. subtilis DSM 32424 at 50 (T1), 75 (T2), and 100 (T3) mg/kg bodyweight (at minimum rate 1 × 106 CFU/g) | Dissolving in drinking water | Yolk acid value, mg of potassium hydroxide per g yolk | No significant difference. | Ermakova et al., 2021 [188] |
| Yolk carotenoid content, mcg/g yolk | A significant decrease from 30.08 ± 0.73 in the control group to 27.32 ± 0.77 (T1), 33.68 ± 0.82 (T2), and 22.06 ± 0.38 (T3). | ||||
| Hy-Line Brown laying hens (Gallus gallus domesticus) | B. velezensis (Group I, 1 × 1010 CFU/kg; Group II, 2 × 1010 CFU/kg) | Supplementing standard diet | Egg weight, g | No significant difference. | Ye et al., 2020 [240] |
| Egg shape index | No significant difference. | ||||
| Eggshell color | No significant difference. | ||||
| Eggshell strength (× 105 Pa) | A significant decrease from 4.227 ± 0.086 in the control group to 3.832 ± 0.117 (Group I) and 3.942 ± 0.103 (Group II) in the first phase (day 2–21). No significant difference in other dates/periods. | ||||
| Yolk weight, g | No significant difference. | ||||
| Eggshell weight, g | No significant difference. | ||||
| Yolk color | A significant increase from 6.834 ± 0.190 in the control group to 7.403 ± 0.099 (Group II) in the second phase (22–42 days). No significant difference in other dates/periods. | ||||
| Albumen height, mm | A significant increase from 6.933 ± 0.021 in the control group to 8.116 ± 0.073 (Group I) and 7.521 ± 0.178 (Group II) in the second phase (22–42 days). No significant difference in other dates/periods. | ||||
| Haugh units | A significant increase from 80.464 ± 1.378 in the control group to 89.454 ± 0.415 (Group I) and 85.036 ± 1.606 (Group II) in the second phase (22–42 days). No significant difference in other dates/periods. | ||||
| Triglyceride, mg/g | No significant difference. | ||||
| Cholesterol, mg/g | No significant difference. | ||||
| Hy-Line Brown laying hens (Gallus gallus domesticus) | B. amyloliquefaciens BLCC1-0238 (2 × 1010 CFU/g) at 0.01%, 0.03%, or 0.06% levels | Supplementing the standard diet | Albumen height, mm | A significant increase from 6.52 ± 0.12 in the control group to 6.96 ± 0.13 (0.03%) and 6.95 ± 0.12 (0.06%) in groups treated with spore-forming probiotics. | Zhou et al., 2020 [241] |
| Yolk color | A significant increase from 6.88 ± 0.10 in the control group to 7.19 ± 0.11 (0.03%) in the group treated with spore-forming probiotics. | ||||
| Haugh units | A significant increase from 79.5 ± 1.3 in the control group to 85.6 ± 1.3 (0.06%) in the group treated with spore-forming probiotics. | ||||
| Eggshell thickness, mm | Significant increase from 0.302 ± 0.003 in control group to 0.331 ± 0.003 (0.01%), 0.343 ± 0.004 (0.03%), and 0.328 ± 0.003 (0.06%) in groups treated with spore-forming probiotics. | ||||
| Eggshell strength, N | Significant increase from 33.92 ± 0.06 in control group to 38.13 ± 0.07 (0.01%), 38.49 ± 0.08 (0.03%), and 38.50 ± 0.09 (0.06%) in groups treated with spore-forming probiotics. | ||||
| Shaver White laying hens (Gallus gallus domesticus) | B. subtilis DSM29784, 1.1. × 108 CFU/kg (low), B. subtilis DSM29784, 2.2 × 108 CFU/kg (medium), B. subtilis DSM29784, 1.1 × 109 CFU/kg (high) | Supplementing corn–soybean meal | Albumen height (mm) | Significant improvement at higher doses of probiotics during the whole layer I and II phases. No significant difference. Age-related impact on decrease. | Neijat et al., 2019 [10] |
| Haugh units | Significant improvement at higher doses of probiotics during the whole layer I and II phases. | ||||
| Yolk color | Significant decreases at week 22 (I phase) and week 40 (II phase) with the lowest value (4,8) due to the inclusion of a high dose of probiotics. | ||||
| Egg shell thickness, mm | No significant difference. | ||||
| Shell-breaking strength (kg) | The lowest at week 20 with the high dose of probiotics (4.518 for high dose vs. 4.889 for control). No significant difference in the layer II phase. | ||||
| Egg component weights (egg yolk, egg shell, and albumen, % of total egg weight) | No significant difference. | ||||
| Shell index | No significant difference. Age related impact to decrease. | ||||
| Total microbial count on egg shell (CFU/mL wash suspension) | No significant difference. | ||||
| Hisex Brown hens (Gallus gallus domesticus) | B. subtilis KATMIRA1933 (Group I; 107–109 CFU viable spores per gram of the probiotic supplement), B. amyloliquefaciens B-1895 (Group II; 107–109 CFU viable spores per gram of the probiotic supplement), both strains (Group III; equal amounts, 107–109 CFU viable spores per gram of the probiotic supplement) | Supplementing the standard diet via solid phase fermentation | Yolk mass, g | The weight of yolk in the experimental groups exceeded the control by 3.49% in Group I (p < 0.05), 1.96% in Group II, and 2.28% in Group III. | Prazdnova et al., 2019 [243] |
| Albumen mass/yolk weight | In the experimental groups, the ratio decreased to 1.90 (p < 0.05), 1.92 (p < 0.05), and 1.91 (p < 0.05) in groups I, II, III, respectively, compared to 1.93 (p < 0.05) in the control. | ||||
| Protein index | In the experimental groups, it was significantly higher than the control at 8.77 (p < 0.01) in Group I, 6.14 (p < 0.05) in Group II, and 7.89 (p < 0.01) in Group III. | ||||
| Haugh units | It was 1.78% (p < 0.01), 1.47% (p < 0.05), and 1.64% (p < 0.05) above the control in Group I, Group II and Group III, respectively. | ||||
| Egg shell thickness, mm | In the experimental groups, it exceeded the control by 3.35% (p < 0.01) in Group I, 1.96% (p < 0.05) in Group II, and 2.79% (p < 0.05) in Group III. | ||||
| Dry matter in the albumen portion, % | A significant increase (p < 0.01) in Group I (12.892) compared to control (12.026). | ||||
| Dry matter in the yolk portion, % | Significant increase in Group I (52.429; p < 0.01), Group II (52.104; p < 0.01) and Group III (52.104; p < 0.05) compared to control (54.412). | ||||
| Protein content in the albumen portion, % | A significant increase (p < 0.01) in Group I (11.414) compared to control (10.605). | ||||
| Protein content in the yolk portion, % | Significant increase in Group I (17.325; p < 0.01), Group II (17.239; p < 0.01), and Group III (17.272; p < 0.01) compared to control (15.820). | ||||
| Fat in the albumen portion, % | No significant difference. | ||||
| Fat in the yolk portion, % | No significant difference. | ||||
| Carbohydrates in the albumen portion, % | No significant difference. | ||||
| Carbohydrates in the yolk portion, % | No significant difference. | ||||
| Amino acid content in eggs, g/100 g | Within the physiological norm. | ||||
| Hy-Line Brown laying hens (Gallus gallus domesticus) | GalliPro Max/B. subtilis, 500 g/ton (GPM) 8 × 105 CFU/g; GalliPro Tect/B. licheniformis, 500 g/ton (GMT) 8 × 105 CFU/g | Supplementing the standard diet | Egg weight, g | No significant difference. | Upadhaya et al., 2019 [244] |
| Albumen height | A significant increase from 8.43 in the control group to 8.73 (GPM). | ||||
| Yolk color | A significant increase from 5.8 in the control group to 6.12 (GPM). | ||||
| Haugh units | No significant difference. | ||||
| Shell color | No significant difference. | ||||
| Eggshell strength, kg/cm2 | No significant difference. | ||||
| Eggshell thickness, mm−2 | No significant difference. | ||||
| Lohmann pink laying hens (Gallus gallus domesticus) | Clostridium butyricum (The dose of the dietary probiotic supplement was added with reference to the company’s recommendations) | Supplementing a standard diet (0.5 g/kg of probiotic) | Egg shape index | No significant difference. | Xiang et al., 2019 [245] |
| Eggshell strength, kg/cm2 | A significant decrease from 4.77 ± 0.27 in the control group to 4.41 ± 0.33 in the treatment group. | ||||
| Haugh units | No significant difference. | ||||
| Albumen height, mm | No significant difference. | ||||
| Yolk color | A significant decrease from 7.33 ± 0.26 in the control group to 7.07 ± 0.27 in the treatment group. | ||||
| Eggshell thickness, um | No significant difference. | ||||
| Yolk percentage, % | No significant difference. | ||||
| Yolk CP%/DM | No significant difference. | ||||
| Albumen CP%/DM | A significant increase from 81.06 ± 1.63 in the control group to 82.65 ± 0.91 in the treatment group. | ||||
| Yolk fat%/DM | No significant difference. | ||||
| Cholesterol content of yolk, % | No significant difference. | ||||
| Hy-Line Brown laying hens (Gallus gallus domesticus) | B. licheniformis yb-214245 (BL; 1.0 × 106 CFU/kg), B. subtilis yb-114246 (BS; 1.0 × 106 CFU/kg), both strains (CB; 6.6 × 105:3.3 × 105 BL:BS) | Supplementing the standard diet via blending with 99.01-kg basic mass feed. | Eggshell thickness, mm | No significant difference. | Yang et al., 2019 [246] |
| Eggshell strength, kg/cm2 | A significant increase from 3.36 in the control group to 3.97 (BL), 4.02 (BS), and 4.19 (CB) for the 60–64 weeks period; from 3.30 in the control group to 3.61 (BS) and 4.19 (CB) for the 64–68 weeks period, and from 3.58 in the control group to 3.88 (CB) for the 68–72 weeks period. | ||||
| Albumen height, mm | No significant difference. | ||||
| Haugh units | A significant increase from 85.1 in the control group to 89.7 (CB) for the 60–64 weeks period; from 85.1 in the control group to 89.6 (CB) for the 64–68 weeks period, and from 84.8 in the control group to 89.9 (CB) for the 68–72 weeks period. | ||||
| Egg yolk color | No significant difference. | ||||
| Total cholesterol, mg/g | A significant decrease from 22.39 in the control group to 18.24 (BL), 15.24 (BS), and 14.28 (CB) at 72 weeks. | ||||
| Triglycerides, mg/g | A significant decrease from 9.67 in the control group to 8.20 (BL), 6.07 (BS), and 5.97 (CB) at 72 weeks. | ||||
| Very low-density lipoprotein cholesterol, mg/g | A significant decrease from 12.58 in the control group to 10.17 (BL), 7.08 (BS), and 6.68 (CB) at 72 weeks. | ||||
| ISA brown laying hens (Gallus gallus domesticus) | Complex strain of spray-dried spores forming B. amyloliquefaciens 1.0 × 107 CFU/kg (P1) and 2.0 × 107 CFU/kg (P2) | Supplementing the standard diet | Yolk height, mm | No significant difference. | Tang et al., 2018 [248] |
| Yolk color | No significant difference. | ||||
| Haugh units | No significant difference. | ||||
| Eggshell strength, kg/cm2 | Significant improvement from 3.21 in the control group to 3.55 (P1) and 3.61 (P2) at 3 weeks, and from 3.27 in the control group to 3.47 (P1) and 3.79 (P2) at 6 weeks. | ||||
| Eggshell thickness, mm | Significant improvement from 0.381 in the control group to 0.413 (P1) and 0.426 (P2) at 3 weeks, and from 0.387 in the control group to 0.420 (P1) and 0.441 (P2) at 6 weeks. | ||||
| Jinghong-1 strain laying hens (Gallus gallus domesticus) | C. butyricum at 2.5 × 104 (CB1), 5 × 104 (CB2), 1 × 105 (CB3), and 2 × 105 (CB4) CFU/g | Supplementing standard corn–soybean basal diet | Albumen height, mm | No significant difference. | Zhan et al., 2018 [249] |
| Haugh units | No significant difference. | ||||
| Yolk color | No significant difference. | ||||
| Eggshell strength, kgf3 | A significant increase from 2.40 in the control group to 3.55 (CB2). | ||||
| Eggshell thickness, mm | No significant difference. | ||||
| Xuefeng black-bone chicken (Coturnix coturnix japonica) | B. subtilis C-3102 at 3.0 × 105 (BS-1), 6.0 × 105 cfu/g (BS-2), and 9.0 × 105 (BS-3) CFU/g. | Supplementing the basal diet | Eggshell-breaking strength, kgf | No significant difference. | Liu et al., 2019 [251] |
| Eggshell thickness, mm | Significant increase from 0.33 in control group to 0.35 (BS-1) and 0.36 (BS-2). | ||||
| Egg-shape index | No significant difference. | ||||
| Yolk index | No significant difference. | ||||
| Yolk percentage, % | No significant difference. | ||||
| Yolk color | A significant increase from 5.61 in the control group to 6.50 (BS-1) and 6.97 (BS-3). | ||||
| Haugh units | No significant difference. | ||||
| Hisex Brown cross laying hens (Gallus gallus domesticus) | B. subtilis KATMIRA1933 (107–109 CFU viable spores per gram of the probiotic supplement; Group I); B. amyloliquefaciens B-1895 (107–109 CFU viable spores per gram of the probiotic supplement; Group II) and B. subtilis KATMIRA1933 and B. amyloliquefaciens B-1895 (equal amounts, 107–109 CFU viable spores per gram of the probiotic supplement; Group III) | Supplementing the standard diet via solid phase fermentation | Weight of egg albumen g | No significant difference. | Mazanko et al., 2017 [184] |
| Weight of egg yolk, g | A significant increase from 18.89 ± 0.17 in the control group to 19.55 ± 0.19 (Group I). | ||||
| Weight of eggshell, g | A significant increase from 6.27 ± 0.09 in the control group to 6.79 ± 0.08 (Group I), 6.61 ± 0.07 (Group II), and 6.73 ± 0.08 (Group III). | ||||
| Shape index, % | No significant difference. | ||||
| Albumen index, % | A significant increase from 9.12 ± 0.14 in the control group to 9.92 ± 0.16 (Group I), 9.68 ± 0.11 (Group II), and 9.84 ± 0.15 (Group III). | ||||
| Yolk index, % | A significant increase from 44.85 ± 0.69 in the control group to 48.83 ± 0.54 (Group I), 48.18 ± 0.61 (Group II) and 48.51 ± 0.47 (Group III). | ||||
| Haugh units | Significant increase from 81.47 ± 0.27 in control group to 82.92 ± 0.33 (Group I), 82.67 ± 0.28 (Group II), and 82.81 ± 0.36 (Group III). | ||||
| Shell thickness, μm | A significant increase from 358.00 ± 2.14 in the control group to 370.00 ± 2.28 (Group I), 365.00 ± 2.11 (Group II), and 368.00 ± 1.99 (Group III). | ||||
| The ratio of egg albumen, % | No significant difference. | ||||
| The ratio of egg yolk, % | No significant difference. | ||||
| Ratio of egg shell, % | No significant difference. | ||||
| The ratio of albumen to yolk | A significant increase from 1.93 ± 0.015 in the control group to 1.90 ± 0.018 (Group I). | ||||
| Hisex Brown laying hens (Gallus gallus domesticus) | B. subtilis at 1.5 × 108 CFU/g of the dried product (and various mixes with distillers, or dried grains with solubles, which were not covered by this review) | Supplementing the standard diet | Egg shape index | No significant difference. | Abd El-Hack et al., 2016 [252] |
| Yolk index | No significant difference. | ||||
| Egg shell thickness | A significant increase from 0.370 in the control group to 0.385 in the group treated with spore-forming probiotics. | ||||
| Haugh units | No significant difference. | ||||
| Yolk color, lightness (with a greater value indicating a lighter color) | A significant decrease from 62.13 in the control group to 60.60 in the group treated with spore-forming probiotics. | ||||
| Yolk color, redness (with a greater value indicating a redder color) | A significant increase from 8.88 in the control group to 10.53 in the group treated with spore-forming probiotics. | ||||
| Yolk color, yellowness (with a greater value indicating a more yellow color) | A significant increase from 38.85 in the control group to 43.22 in the group treated with spore-forming probiotics. | ||||
| Hy-Line Brown laying hens (Gallus gallus domesticus) | B. subtilis CGMCC 1.921 at 1.0 × 105 CFU/g (B1), 1.0 × 106 CFU/g (B2), 1.0 × 107 CFU/g (B3), and 1.0 × 108 CFU/g (B4). | Supplementing the basal diet | Eggshell strength | A significant increase from 45.66 in the control group to 52.31 (B1), 51.05 (B3) at 8 weeks. A significant increase from 48.45 in the control group to 53.24 (B1) at 16 weeks. A significant increase from 48.18 in the control group to 51.42 (B1), 51.24 (B2), and 51.79 (B3) at 16 weeks. A significant increase from 48.18 in the control group to 51.42 (B1), 51.24 (B2), and 51.79 (B3) at 20 weeks. A significant increase from 49.40 in the control group to 54.84 (B1) at 24 weeks. No significant difference in other dates/periods. | Guo et al., 2017 [254] |
| Albumen height | A significant decrease from 7.8 in the control group to 6.9 (B3) and 7.0 (B4) at 4 weeks. A significant increase from 8.1 in the control group to 8.3 (B1) at 20 weeks. No significant difference in other dates/periods. | ||||
| Yolk color | A significant decrease from 7.8 in the control group to 6.8 (B4) at 0 weeks. A significant increase from 6.7 in the control group to 7.6 (B2), 7.4 (B3), 7.4 (B4) at 1 week. A significant increase from 6.4 in the control group to 7.2 (B2) at 2 weeks. A significant increase from 6.7 in the control group to 6.1 (B2) at 4 weeks. A significant increase from 6.7 in the control group to 7.2 (B2) at 8 weeks. A significant increase from 6.2 in the control group to 6.7 (B3) and 6.5 (B4) at 12 weeks. A significant decrease from 7.1 to 6.7 (B1) and 6.7 (B3) at 16 weeks. A significant increase from 6.3 in the control group to 6.7 (B2) and 6.6 (B4) at 24 weeks. No significant difference in other dates/periods. | ||||
| Haugh units | A significant increase from 88.1 in the control group to 82.6 (B3) and 83.6 (B4) at 4 weeks. No significant difference in other dates/periods. | ||||
| Japanese quails (Coturnix Coturnix Japonica) | B. subtilis C-3102 at 0.1% level (minimum dose 1.0 × 1010 viable spores per gram) | Supplementing the basal diet | Eggshell thickness, mm | No significant difference. | Manafi et al., 2016 [255] |
| Eggshell-breaking strength, kg | No significant difference. | ||||
| Haugh units | No significant difference. | ||||
| Eggshell, % | No significant difference. | ||||
| Lohmann Brown laying hens (Gallus gallus domesticus) | B. subtilis ATCC PTA- 6737 at 1 × 108 CFU/kg feed | Supplementing the standard diet | Shell thickness, mm | Significant improvement from 0.355 ± 0.008 in the control group to 0.365 ± 0.008 (1 × 108 CFU/g of probiotic). | Sobczak et al., 2015 [256] |
| Shell strength, N | Significant improvement from 45.12 ± 2.30 in the control group to 47.63 ± 2.78 (1 × 108 CFU/g of probiotic). | ||||
| Yolk color, points | Significant improvement from 7.83 ± 0.83 in the control group to 9.01 ± 0.71 (1 × 108 CFU/g of probiotic). | ||||
| Haugh units | Significant improvement from 70.45 ± 3.45 in the control group to 72.95 ± 2.59 (1 × 108 CFU/g of probiotic). | ||||
| Egg composition–yolk, % | No significant difference. | ||||
| Egg composition–albumen, % | No significant difference. | ||||
| Egg composition–shell, % | A significant increase from 9.79 ± 0.18 in the control group to 10.04 ± 0.15 (1 × 108 CFU/g of probiotic). | ||||
| Fatty acid profile of egg yolk, (% of total fatty acid content) | Significant increase of oleic acid content from 1.78 ± 0.12 in the control group to 1.93 ± 0.15 (1 × 108 CFU/g of probiotic). No significant difference in other fatty acids. | ||||
| Content in egg yolk fat (cholesterol), mg/g | A significant decrease from 28.1 ± 2.0 in the control group to 24.8 ± 4.6 (1 × 108 CFU/g of probiotic). | ||||
| Hy-Line layer hybrids (Gallus gallus domesticus) | B. subtilis PB6 at 0.05% dose | Supplementing corn–soybean cake-based diet | Yolk weight, g | No significant difference. No significant difference. | Forte et al., 2016 [257] |
| Albumen weight, g | No significant difference. | ||||
| Shell weight, g | No significant difference. | ||||
| Shell ash, % | No significant difference. | ||||
| Yolk, % | No significant difference. | ||||
| Albumen, % | No significant difference. | ||||
| Shell, % | No significant difference. | ||||
| Edible, % | No significant difference. | ||||
| Albumen/yolk | No significant difference. | ||||
| Color (Roche scale) | No significant difference. | ||||
| Haugh units | No significant difference. | ||||
| Color, lightness | No significant difference. | ||||
| Color, redness | No significant difference. | ||||
| Color, yellowness | No significant difference. | ||||
| Ash, % | No significant difference. | ||||
| Crude protein, % | No significant difference. | ||||
| Lipid, % | No significant difference. | ||||
| Cholesterol, mg/g yolk | No significant difference. | ||||
| Cholesterol, mg/egg | No significant difference. | ||||
| Hy-Line W-36 (Gallus gallus domesticus) | B. subtilis GalliPro® patented by Chr. Hansen 8 × 105 CFU/g (T2), 4 × 105 CFU/g feed (T3), 3 × 105 CFU/g feed (T4) | Delivery in spore form in corn and soybeans | Yolk weight, g/kg | No significant difference. | Ribeiro Jr. et al., 2014 [258] |
| Eggshell weight, g/kg | No significant difference. | ||||
| Albumen weight, g/kg | No significant difference. | ||||
| White laying hens (Gallus gallus domesticus) | B. subtilis PB6 at 0.5 g/kg and 1.0 g/kg supplement levels | Supplementing the basal diet | Eggshell weight, % of egg weight | Significant increase in both groups treated with spore-forming probiotics compared to control (no table values provided). | Abdelqader et al., 2013 [183] |
| Eggshell thickness, mm | Significant increase in both groups treated with spore-forming probiotics compared to control (no table values provided). | ||||
| Eggshell density, mg/cm2 | Significant increase in both groups treated with spore-forming probiotics compared to control (no table values provided). | ||||
| Unmarketable eggs, % | Significant decrease in both groups treated with spore-forming probiotics compared to control (no table values provided). | ||||
| Hy-Line Variety W-36 hens (Gallus gallus domesticus) | B. licheniformis at 0.01% (2 × 106 CFU/g), 0.02% (4 × 106 CFU/g), 0.03% (6 × 106 CFU/g), 0.06% (1.2 × 107 CFU/g), and 0.09% (1.8 × 107 CFU/g), | Supplementing the basal diet | Albumen height, mm | Significant increase from 6.50 ± 0.15 in control group to 6.95 ± 0.12 (0.03%) and 6.93 ± 0.13 (0.06%). | Lei et al., 2013 [182] |
| Yolk color | Significant increase from 6.50 ± 0.15 in control group to 6.95 ± 0.12 (0.03%) and 6.93 ± 0.13 (0.06%). | ||||
| Haugh units | Significant increase from 6.86 ± 0.09 in control group to 7.20 ± 0.10 (0.03%) and 6.51 ± 0.0 (0.09%). | ||||
| Eggshell thickness, mm | Significant increase from 0.303 ± 0.004 in control group to 0.332 ± 0.004 (0.01%), 0.324 ± 0.003 (0.02%), 0.342 ± 0.005 (0.03%), 0.327 ± 0.004 (0.06%), and 0.319 ± 0.003 (0.09%). | ||||
| Eggshell strength, N | Significant increase from 33.91 ± 0.08 in control group to 38.12 ± 0.08 (0.01%), 37.44 ± 0.08 (0.02%), 38.51 ± 0.09 (0.03%), 38.51 ± 0.10 (0.06%), and 36.85 ± 0.06 (0.09%). | ||||
| Lohmann pink layer hens (Gallus gallus domesticus) | B. subtilis at 9 × 109 CFU/g (and various mixes with Lactobacillus bacteria and sodium butyrate, which were not covered by this review) | Supplementing the standard diet | Yolk color | No significant difference. | Zhang et al., 2012 [261] |
| Yolk relative weight, % | A significant decrease from 28.38 ± 0.50 in the control group to 26.49 ± 0.64 in the group treated with spore-forming probiotics. | ||||
| Yolk cholesterol, mg/g yolk | No significant difference. | ||||
| Haugh unit | No significant difference. | ||||
| Shape index | No significant difference. | ||||
| Shell thickness, mm | No significant difference. | ||||
| Shaoxing ducks (Anas platyrhynchos domesticus) | B. subtilis at 1 × 108 CFU/kg | Supplementing the basal diet | Egg weight, kg | No significant difference. | Li et al., 2011 [262] |
| Shell thickness, mm | No significant difference. | ||||
| Horizontal–vertical | No significant difference. | ||||
| Egg yolk color | No significant difference. | ||||
| Haugh units | No significant difference. | ||||
| Triglyceride, mmol/L | A significant decrease from 712.45 ± 22.12 to 622.66 ± 28.95 in the group treated with spore-forming probiotics. | ||||
| Total cholesterol, mmol/L | A significant decrease from 126.96 ± 2.79 to 97.09 ± 2.29 in the group treated with spore-forming probiotics. | ||||
| Malondialdehyde | A significant decrease from 943.92 ± 38.68 to 564.99 ± 39.99 in the group treated with spore-forming probiotics. | ||||
| Hy-Line W-36 strains of white Leghorn laying hens (Gallus gallus domesticus) | Mix of B. subtilis CH201 and B. licheniformis CH200 at 1000 g/ton and 2000 g/ton | Supplementing the basal diet | Shell thickness, mm | No significant difference. | Aghaii et al., 2010 [263] |
| Shell hardness, kg cm−1 | No significant difference. | ||||
| Haugh units | No significant difference. | ||||
| Yolk index | A significant increase from 0.402 in the control group to 0.420 (1000 g ton−1). | ||||
| Lohmann Brown layers (Gallus gallus domesticus) | Dried B. subtilis culture at 9.3 × 109 CFU/kg | Supplementing standard diet with 500 mg/kg, 1000 mg/kg, or 1500 mg/kg of probiotics | Shell strength, kg/cm2 | No significant difference. | Li et al., 2006 [264] |
| Shell thickness, um | No significant difference. | ||||
| Yolk color | No significant difference. | ||||
| Haugh units | No significant difference. | ||||
| Yolk cholesterol (mg/yolk) | A significant decrease from 251.80 ± 13.11 in the control group to 221.05 ± 16.23 in the treatment group (500 mg of probiotic/kg). | ||||
| Hy-Line White laying hens (Gallus gallus domesticus) | Mix of B. subtilis CH201 and B. licheniformis CH200 at 1.28 × 106 CFU/g (Group I), 3.2 × 106 CFU/g (Group II), 4.6 × 106 CFU/g (Group III) | Supplementing the standard diet | Shell thickness, mm | No significant difference. | Mahdavi et al., 2005 [265] |
| Shell hardness, kg cm−1 | No significant difference. | ||||
| Haugh unit | No significant difference. | ||||
| Egg cholesterol, mg gr−1 yolk | Significant decrease from10.73 in the control group to 10.27 in Group II and 0.23 in Group III. |
| Animal | Spore-Forming Probiotic Strain, Dose | Way of Probiotic Administration | Sperm Quality | Results of the Experimental Group Compared to Control Group | References |
|---|---|---|---|---|---|
| Hisex Brown hens (Gallus gallus domesticus) | B. subtilis KATMIRA1933 (Group I), B. amyloliquefaciens B-1895 (Group II), both strains (Group III) | Supplementing standard diet via solid phase fermentation | Color | Within the physiological norm (white among all groups). | Prazdnova et al., 2019 [243] |
| The volume of ejaculate, ml | No significant difference. | ||||
| Total number of spermatozoa in the ejaculate, billions | A significant increase from 1.47 to 1.64 (Group I) observed in cocks of age 82 weeks. | ||||
| Concentration of spermatozoa, billion/ml | A significant increase from 2.49 to 2.89 (Group I) observed in cocks of age 82 weeks. | ||||
| The number of morphologically abnormal germ cells in the ejaculate, % | A significant decrease from 15.40 to 11.50 (Group I), 11.98 (Group II), and 12.01 (Group III) observed in cocks of age 82 weeks. | ||||
| Amino acid content in rooster’s semen, g/100 g | The content of amino acids in the sperm of the experimental groups was higher than in the control. A more significant difference in the amino acid composition of the sperm of roosters with respect to the control was observed in Group I with aspartic acid (17.69%, p < 0.05), glutamic acid (9.47%, p < 0.05), serine (25.87%, p < 0.05), and alanine (31.09%, p < 0.05). | ||||
| Hisex Brown cross laying hens (Gallus gallus domesticus) | B. subtilis KATMIRA1933 (107–109 CFU viable spores per gram of the probiotic supplement; Group I); B. amyloliquefaciens B-1895 (107–109 CFU viable spores per gram of the probiotic supplement; Group II) and B. subtilis KATMIRA1933 and B. amyloliquefaciens B-1895 (equal amounts, 107–109 CFU viable spores per gram of the probiotic supplement; Group III) | Supplementing the standard diet via solid phase fermentation | Color | Within the physiological norm (white among all groups). | Mazanko et al., 2017 [184] |
| The volume of ejaculate, ml | A significant increase from 18.89 ± 0.17 in the control group to 19.55 ± 0.19 (Group I). | ||||
| Total number of spermatozoa in the ejaculate, 109 | A significant increase from 1.49 ± 0.05 in the control group to 1.75 ± 0.06 (Group I). | ||||
| Concentration of spermatozoa, 109/mL | A significant increase from 2.56 ± 0.08 in the control group to 3.29 ± 0.07 (Group I), 3.01 ± 0.09 (Group II), and 3.17 ± 0.09 (Group III). | ||||
| The number of morphologically abnormal germ cells in the ejaculate, % | A significant decrease from 14.7 ± 0.40 in control group to 10.4 ± 0.51 (Group I), 11.7 ± 0.43 (Group II), and 10.1 ± 0.62 (Group III). | ||||
| White Leghorn roosters (Gallus gallus domesticus) | 4.5 × 104 CFU of B. subtilis/g of feed | Supplementing the standard diet | Sperm quality index | No significant difference. | dos Santos et al., 2018 [266] |
| Dead sperm, % | No significant difference. | ||||
| Sperm concentration (total), billion sperm/mL | No significant difference. | ||||
| Sperm concentration (live), billion sperm/mL | No significant difference. | ||||
| Volume, mL | No significant difference. | ||||
| Ejaculated sperm (total), billion sperm/ejaculate | No significant difference. | ||||
| Ejaculated sperm (live), billion sperm/ejaculate | No significant difference. | ||||
| pH | No significant difference. | ||||
| O2, nmol/mL | No significant difference. | ||||
| CO2, nmol/mL | No significant difference. | ||||
| Na+, μmol/mL | No significant difference. | ||||
| K+, μmol/mL | No significant difference. | ||||
| Ca2+, μmol/mL | No significant difference. | ||||
| Cl−, μmol/mL | No significant difference. | ||||
| Cobb male broiler breeders (Gallus gallus domesticus) | Bacillus amyloliquefaciens TOA5001 at 1 × 108 CFU/g | Probiotic in rice supplemented to the standard diet | Sperm count, million/mL | A significant increase from 22.8 ± 2.55 in the control group to 26.5 ± 2.90 in the group treated with spore-forming probiotics. | Inatomi et al., 2018 [267] |
| Live sperm, % | A significant increase from 94.1 ± 2.63 in the control group to 95.2 ± 1.06 in group treated with spore-forming probiotics |
References
- Ruiz Sella, S.R.B.; Bueno, T.; de Oliveira, A.A.B.; Karp, S.G.; Soccol, C.R. Bacillus subtilis natto as a potential probiotic in animal nutrition. Crit. Rev. Biotechnol. 2021, 41, 355–369. [Google Scholar] [CrossRef]
- Angelakis, E. Weight gain by gut microbiota manipulation in productive animals. Microb. Pathog. 2017, 106, 162–170. [Google Scholar] [CrossRef]
- Zommiti, M.; Chikindas, M.L.; Ferchichi, M. Probiotics-Live Biotherapeutics: A Story of Success, Limitations, and Future Prospects-Not Only for Humans. Probiotics Antimicrob. Proteins 2020, 12, 1266–1289. [Google Scholar] [CrossRef]
- Mingmongkolchai, S.; Panbangred, W. Bacillus probiotics: An alternative to antibiotics for livestock production. J. Appl. Microbiol. 2018, 124, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Elisashvili, V.; Kachlishvili, E.; Chikindas, M.L. Recent Advances in the Physiology of Spore Formation for Bacillus Probiotic Production. Probiotics Antimicrob. Proteins 2019, 11, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Berikashvili, V.; Sokhadze, K.; Kachlishvili, E.; Elisashvili, V.; Chikindas, M.L. Bacillus amyloliquefaciens Spore Production Under Solid-State Fermentation of Lignocellulosic Residues. Probiotics Antimicrob. Proteins 2018, 10, 755–761. [Google Scholar] [CrossRef]
- McAllister, T.A.; Wang, Y.; Diarra, M.S.; Alexander, T.; Stanford, K. Challenges of a one-health approach to the development of alternatives to antibiotics. Anim. Front. 2018, 8, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Demin, K.A.; Refeld, A.G.; Bogdanova, A.A.; Prazdnova, E.V.; Popov, I.V.; Kutsevalova, O.Y.; Ermakov, A.M.; Bren, A.B.; Rudoy, D.V.; Chistyakov, V.A.; et al. Mechanisms of Candida Resistance to Antimycotics and Promising Ways to Overcome It: The Role of Probiotics. Probiotics Antimicrob. Proteins 2021. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.; Gay, C.G.; Lillehoj, H.S. Bacillus spp. as direct-fed microbial antibiotic alternatives to enhance growth, immunity, and gut health in poultry. Avian Pathol. 2018, 47, 339–351. [Google Scholar] [CrossRef]
- Neijat, M.; Shirley, R.B.; Barton, J.; Thiery, P.; Welsher, A.; Kiarie, E. Effect of dietary supplementation of Bacillus subtilis DSM29784 on hen performance, egg quality indices, and apparent retention of dietary components in laying hens from 19 to 48 weeks of age. Poult. Sci. 2019, 98, 5622–5635. [Google Scholar] [CrossRef] [PubMed]
- Rougière, N.; Carré, B. Comparison of gastrointestinal transit times between chickens from D+ and D− genetic lines selected for divergent digestion efficiency. Animal 2010, 4, 1861–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scanes, C.G. Sturkie’s Avian Physiology, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2014; p. 1056. [Google Scholar]
- Grist, A. Poultry Inspection: Anatomy, Physiology, and Disease Conditions, 2nd ed.; Nottingham University Press: Nottingham, UK, 2006; p. 262. [Google Scholar]
- Feye, K.M.; Baxter, M.F.A.; Tellez-Isaias, G.; Kogut, M.H.; Ricke, S.C. Influential factors on the composition of the conventionally raised broiler gastrointestinal microbiomes. Poult. Sci. 2020, 99, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Hughes, R.J.; Moore, R.J. Microbiota of the chicken gastrointestinal tract: Influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014, 98, 4301–4310. [Google Scholar] [CrossRef]
- Han, G.G.; Kim, E.B.; Lee, J.; Lee, J.Y.; Jin, G.; Park, J.; Huh, C.S.; Kwon, I.K.; Kil, D.Y.; Choi, Y.J.; et al. Relationship between the microbiota in different sections of the gastrointestinal tract, and the body weight of broiler chickens. Springerplus 2016, 5, 911. [Google Scholar] [CrossRef] [Green Version]
- Saxena, S.; Saxena, V.K.; Tomar, S.; Sapcota, D.; Gonmei, G. Characterisation of caecum and crop microbiota of Indian indigenous chicken targeting multiple hypervariable regions within 16S rRNA gene. Br. Poult. Sci. 2016, 57, 381–389. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiang, Y.; Zhou, W.; Chen, J.; Li, K.; Yang, H. Microbial community mapping in intestinal tract of broiler chicken. Poult. Sci. 2017, 96, 1387–1393. [Google Scholar] [CrossRef]
- Mohd Shaufi, M.A.; Sieo, C.C.; Chong, C.W.; Gan, H.M.; Ho, Y.W. Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut Pathog. 2015, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Idris, U.; Harmon, B.; Hofacre, C.; Maurer, J.J.; Lee, M.D. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl. Environ. Microbiol. 2003, 69, 6816–6824. [Google Scholar] [CrossRef] [Green Version]
- Clavijo, V.; Flórez, M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: A review. Poult. Sci. 2018, 97, 1006–1021. [Google Scholar] [CrossRef]
- Apajalahti, J.; Kettunen, A. Microbes of the chicken gastrointestinal tract. In Avian Gut Function in Health and Disease; Graham, C.P., Ed.; CABI: Wallingford, UK, 2006; Volume 28, pp. 124–137. [Google Scholar]
- Gong, J.; Forster, R.J.; Yu, H.; Chambers, J.R.; Wheatcroft, R.; Sabour, P.M.; Chen, S. Molecular analysis of bacterial populations in the ileum of broiler chickens and comparison with bacteria in the cecum. FEMS Microbiol. Ecol. 2002, 41, 171–179. [Google Scholar] [CrossRef]
- Józefiak, D.; Rutkowski, A.; Martin, S.A. Carbohydrate fermentation in the avian ceca: A review. Anim. Feed Sci. Technol. 2004, 113, 1–15. [Google Scholar] [CrossRef]
- Wei, S.; Morrison, M.; Yu, Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013, 92, 671–683. [Google Scholar] [CrossRef]
- Glendinning, L.; Stewart, R.D.; Pallen, M.J.; Watson, K.A.; Watson, M. Assembly of hundreds of novel bacterial genomes from the chicken caecum. Genome Biol. 2020, 21, 34. [Google Scholar] [CrossRef] [Green Version]
- Waite, D.W.; Taylor, M.W. Exploring the avian gut microbiota: Current trends and future directions. Front Microbiol. 2015, 6, 673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanley, D.; Geier, M.S.; Hughes, R.J.; Denman, S.E.; Moore, R.J. Highly variable microbiota development in the chicken gastrointestinal tract. PLoS ONE 2013, 8, e84290. [Google Scholar] [CrossRef] [Green Version]
- Ballou, A.L.; Ali, R.A.; Mendoza, M.A.; Ellis, J.C.; Hassan, H.M.; Croom, W.J.; Koci, M.D. Development of the Chick Microbiome: How Early Exposure Influences Future Microbial Diversity. Front Vet. Sci. 2016, 3, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awad, W.A.; Mann, E.; Dzieciol, M.; Hess, C.; Schmitz-Esser, S.; Wagner, M.; Hess, M. Age-Related Differences in the Luminal and Mucosa-Associated Gut Microbiome of Broiler Chickens and Shifts Associated with Campylobacter jejuni Infection. Front Cell Infect. Microbiol. 2016, 6, 154. [Google Scholar] [CrossRef] [Green Version]
- Apajalahti, J.H.; Kettunen, A.; Bedford, M.R.; Holben, W.E. Percent G+C profiling accurately reveals diet-related differences in the gastrointestinal microbial community of broiler chickens. Appl. Environ. Microbiol. 2001, 67, 5656–5667. [Google Scholar] [CrossRef] [Green Version]
- Engberg, R.M.; Hedemann, M.S.; Jensen, B.B. The influence of grinding and pelleting of feed on the microbial composition and activity in the digestive tract of broiler chickens. Br. Poult. Sci. 2002, 43, 569–579. [Google Scholar] [CrossRef]
- Tellez, G.; Latorre, J.D.; Kuttappan, V.A.; Kogut, M.H.; Wolfenden, A.; Hernandez-Velasco, X.; Hargis, B.M.; Bottje, W.G.; Bielke, L.R.; Faulkner, O.B. Utilization of rye as energy source affects bacterial translocation, intestinal viscosity, microbiota composition, and bone mineralization in broiler chickens. Front. Genet. 2014, 5, 339. [Google Scholar] [CrossRef]
- Haberecht, S.; Bajagai, Y.S.; Moore, R.J.; Van, T.T.H.; Stanley, D. Poultry feeds carry diverse microbial communities that influence chicken intestinal microbiota colonisation and maturation. AMB Express 2020, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Li, H.; Li, M.; Gao, R.; Guo, C.; Mi, S. Disequilibrium in chicken gut microflora with avian colibacillosis is related to microenvironment damaged by antibiotics. Sci. Total Environ. 2021, 762, 143058. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Huang, K.; Bai, Y.; Feng, X.; Gong, L.; Wei, C.; Huang, H.; Zhang, H. Dietary supplementation with berberine improves growth performance and modulates the composition and function of cecal microbiota in yellow-feathered broilers. Poult. Sci. 2021, 100, 1034–1048. [Google Scholar] [CrossRef]
- De Cesare, A.; Caselli, E.; Lucchi, A.; Sala, C.; Parisi, A.; Manfreda, G.; Mazzacane, S. Impact of a probiotic-based cleaning product on the microbiological profile of broiler litters and chicken caeca microbiota. Poult. Sci. 2019, 98, 3602–3610. [Google Scholar] [CrossRef]
- De Toledo, T.D.S.; Roll, A.A.P.; Rutz, F.; Dallmann, H.M.; Dai Prá, M.A.; Leite, F.P.L.; Roll, V.F.B. An assessment of the impacts of litter treatments on the litter quality and broiler performance: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0232853. [Google Scholar] [CrossRef] [PubMed]
- Pin Viso, N.; Redondo, E.; Díaz Carrasco, J.M.; Redondo, L.; Sabio, Y.; Garcia, J.; Fernández Miyakawa, M.; Farber, M.D. Geography as non-genetic modulation factor of chicken cecal microbiota. PLoS ONE 2021, 16, e0244724. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. The Gut Microbiota of Laying Hens and Its Manipulation with Prebiotics and Probiotics To Enhance Gut Health and Food Safety. Appl. Environ. Microbiol. 2020, 86, e00600-20. [Google Scholar] [CrossRef] [PubMed]
- Ricke, S.C.; Lee, S.I.; Kim, S.A.; Park, S.H.; Shi, Z. Prebiotics and the poultry gastrointestinal tract microbiome. Poult. Sci. 2020, 99, 670–677. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. Biomed. Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.; Daly, K.; Moran, A.W.; Ryan, S.; Bravo, D.; Shirazi-Beechey, S.P. Composition and diversity of mucosa-associated microbiota along the entire length of the pig gastrointestinal tract; dietary influences. Environ. Microbiol. 2017, 19, 1425–1438. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, B.; Kwon, Y.M. Characterization of the Culturable Subpopulations of Lactobacillus in the Chicken Intestinal Tract as a Resource for Probiotic Development. Front. Microbiol. 2017, 8, 1389. [Google Scholar] [CrossRef]
- Nicholson, W.L. Roles of Bacillus endospores in the environment. Cell Mol. Life Sci. 2002, 59, 410–416. [Google Scholar] [CrossRef]
- Lee, D.H.; Cha, I.H.; Woo, D.S.; Ohba, M. Microbial ecology of Bacillus thuringiensis: Fecal populations recovered from wildlife in Korea. Can. J. Microbiol. 2003, 49, 465–471. [Google Scholar] [CrossRef]
- Salzman, N.H.; de Jong, H.; Paterson, Y.; Harmsen, H.J.M.; Welling, G.W.; Bos, N.A. Analysis of 16S libraries of mouse gastrointestinal microflora reveals a large new group of mouse intestinal bacteria. Microbiology 2002, 148, 3651–3660. [Google Scholar] [CrossRef] [Green Version]
- Park, K.Y.; Jung, H.Y.; Woo, K.L.; Jun, K.D.; Kang, J.S.; Paik, H.D. Effects of Bacillus polyfermenticus SCD administration on fecal microflora and putrefactive metabolites in healthy adults. J. Mol. Microbiol. Biotechnol. 2002, 12, 657–663. [Google Scholar]
- Hisanga, S. Studies on the germination of genus Bacillus spores in rabbit and canine intestines. J. Nagoya City Med. Assoc. 1980, 30, 456–469. [Google Scholar]
- Hoa, T.T.; Duc, L.H.; Isticato, R.; Baccigalupi, L.; Ricca, E.; Van, P.H.; Cutting, S.M. Fate and dissemination of Bacillus subtilis spores in a murine model. Appl. Environ. Microbiol. 2001, 67, 3819–3823. [Google Scholar] [CrossRef] [Green Version]
- Casula, G.; Cutting, S.M. Bacillus probiotics: Spore germination in the gastrointestinal tract. Appl. Environ. Microbiol. 2002, 68, 2344–2352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leser, T.D.; Knarreborg, A.; Worm, J. Germination and outgrowth of Bacillus subtilis and Bacillus licheniformis spores in the gastrointestinal tract of pigs. J. Appl. Microbiol. 2008, 104, 1025–1033. [Google Scholar] [CrossRef]
- Keller, D.; Verbruggen, S.; Cash, H.; Farmer, S.; Venema, K. Spores of Bacillus coagulans GBI-30, 6086 show high germination, survival and enzyme activity in a dynamic, computer-controlled in vitro model of the gastrointestinal tract. Benef. Microbes 2019, 10, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Kashikar, M.S.; Madempudi, R.S. Survival and Germination of Bacillus clausii UBBC07 Spores in in vitro Human Gastrointestinal Tract Simulation Model and Evaluation of Clausin Production. Front. Microbiol. 2020, 11, 1010. [Google Scholar] [CrossRef] [PubMed]
- Latorre, J.D.; Hernandez-Velasco, X.; Kallapura, G.; Menconi, A.; Pumford, N.R.; Morgan, M.J.; Layton, S.L.; Bielke, L.R.; Hargis, B.M.; Téllez, G. Evaluation of germination, distribution, and persistence of Bacillus subtilis spores through the gastrointestinal tract of chickens. Poult. Sci. 2014, 93, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.M.; Zuber, P. Anaerobic growth of a “strict aerobe” (Bacillus subtilis). Ann. Rev. Microbiol. 1998, 52, 165–190. [Google Scholar] [CrossRef]
- Jadamus, A.; Vahjen, W.; Simon, O. Growth behaviour of a spore forming probiotic strain in the gastrointestinal tract of broiler chicken and piglets. Arch. Tierernahr 2001, 54, 1–17. [Google Scholar] [CrossRef]
- Tam, N.K.; Uyen, N.Q.; Hong, H.A.; Duc, L.H.; Hoa, T.T.; Serra, C.R.; Henriques, A.O.; Cutting, S.M. The intestinal life cycle of Bacillus subtilis and close relatives. J. Bacteriol. 2006, 188, 2692–2700. [Google Scholar] [CrossRef] [Green Version]
- Faille, C.; Tauveron, G.; Le Gentil-Lelièvre, C.; Slomianny, C. Occurrence of Bacillus cereus spores with a damaged exosporium: Consequences on the spore adhesion on surfaces of food processing lines. J. Food Prot. 2007, 70, 2346–2353. [Google Scholar] [CrossRef]
- Andersson, A.; Granum, P.E.; Rönner, U. The adhesion of Bacillus cereus spores to epithelial cells might be an additional virulence mechanism. Int. J. Food Microbiol. 1998, 39, 93–99. [Google Scholar] [CrossRef]
- Hong, H.A.; Khaneja, R.; Tam, N.M.; Cazzato, A.; Tan, S.; Urdaci, M.; Brisson, A.; Gasbarrini, A.; Barnes, I.; Cutting, S.M. Bacillus subtilis isolated from the human gastrointestinal tract. Res. Microbiol. 2009, 160, 134–143. [Google Scholar] [CrossRef]
- Rohith, H.S.; Halami, P.M. In vitro validation studies for adhesion factor and adhesion efficiency of probiotic Bacillus licheniformis MCC 2514 and Bifidobacterium breve NCIM 5671 on HT-29 cell lines. Arch. Microbiol. 2021. [Google Scholar] [CrossRef]
- Sánchez, B.; Arias, S.; Chaignepain, S.; Denayrolles, M.; Schmitter, J.M.; Bressollier, P.; Urdaci, M.C. Identification of surface proteins involved in the adhesion of a probiotic Bacillus cereus strain to mucin and fibronectin. Microbiology 2009, 155, 1708–1716. [Google Scholar] [CrossRef] [Green Version]
- Auger, S.; Ramarao, N.; Faille, C.; Fouet, A.; Aymerich, S.; Gohar, M. Biofilm formation and cell surface properties among pathogenic and nonpathogenic strains of the Bacillus cereus group. Appl. Environ. Microbiol. 2009, 75, 6616–6618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayaraman, S.; Thangavel, G.; Kurian, H.; Mani, R.; Mukkalil, R.; Chirakkal, H. Bacillus subtilis PB6 improves intestinal health of broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Poult. Sci. 2013, 92, 370–374. [Google Scholar] [CrossRef]
- Applegate, T.J.; Klose, V.; Steiner, T.; Ganner, A.; Schatzmayr, G. Probiotics and phytogenics for poultry: Myth or reality? J. Appl. Poult. Res. 2010, 19, 194–210. [Google Scholar] [CrossRef]
- Amerah, A.M.; Jansen van Rensburg, C.; Plumstead, P.W.; Kromm, C.; Dunham, S. Effect of feeding diets containing a probiotic or antibiotic on broiler performance, intestinal mucosa-associated avian pathogenic E. coli and litter water-soluble phosphorus. J. Appl. Anim. Nutr. 2013, 1, 1–7. [Google Scholar] [CrossRef]
- World Health Organisation. Guidelines for the Evaluation of Probiotics in Food. Report of a Joint Food and Agriculture Organisation (FAO)/World Health Organisation (WHO) Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food. Available online: http://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed on 7 May 2021).
- Lee, K.W.; Lillehoj, H.S.; Siragusa, G.R. Review: Direct-fed microbials and their impact on the intestinal microflora and immune system of chickens. J. Poult. Sci. 2010, 47, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.W.; Lee, S.H.; Lillehoj, H.S.; Li, G.X.; Jang, S.I.; Babu, U.S.; Park, M.S.; Kim, D.K.; Lillehoj, E.P.; Neumann, A.P.; et al. Effects of direct-fed microbials on growth performance, gut morphometry, and immune characteristics in broiler chickens. Poult. Sci. 2010, 89, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Lutful Kabir, S.M. The role of probiotics in the poultry industry. Int. J. Mol. Sci. 2009, 10, 3531–3546. [Google Scholar] [CrossRef]
- Kiarie, E.; Romero, L.F.; Nyachoti, C.M. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr. Res. Rev. 2013, 26, 71–88. [Google Scholar] [CrossRef] [Green Version]
- Reddy, P.V.M.; Kondal, R.K.; Kuhad, R.C.; Shashi, K.M.; Gnana, P.M. Effect of supplementation of enzymes and probiotics on performance of broiler chicken. Ind. J. Poult. Sci. 2010, 45, 361–363. [Google Scholar]
- Momtazan, R.; Moravej, H.; Zaghari, M.; Taheri, H.R. A note on the effects of a combination of an enzyme complex and probiotic in the diet on performance of broiler chickens. Irish J. Agric. Food Res. 2011, 50, 249–254. [Google Scholar] [CrossRef]
- Dersjant-Li, Y.; Awati, A.; Kromm, C.; Evans, C. A direct fed microbial containing a combination of three-strain Bacillus sp. can be used as an alternative to feed antibiotic growth promoters in broiler production. J. Appl. Anim. Nutr. 2013, 2, E11. [Google Scholar] [CrossRef] [Green Version]
- Honey, C.C.; Summaya, J.; Keerthi, T.R. Probiotic effect of Bacillus coagulans, mbtu-p1f2 from in fant faeces with a known probiotic. Eur. J. Biomed. Pharm. Sci. 2016, 3, 298–304. [Google Scholar]
- Jensen, G.B.; Hansen, B.M.; Eilenberg, J.; Mahillon, J. The hidden lifestyles of Bacillus cereus and relatives. Environ. Microbiol. 2003, 5, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Morelli, L.; Tompkins, T. Sporeformers as Human Probiotics: Bacillus, Sporolactobacillus, and Brevibacillus. Compr. Rev. Food Sci. Food Saf. 2003, 2, 101–110. [Google Scholar] [CrossRef]
- Duc, L.H.; Hong, H.A.; Fairweather, N.; Ricca, E.; Cutting, S.M. Bacterial spores as vaccine vehicles. Infect. Immun. 2003, 71, 2810–2818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duc, L.H.; Hong, H.A.; Barbosa, T.M.; Henriques, A.O.; Cutting, S.M. Characterisation of Bacillus probiotics available for human use. Appl. Environ. Microbiol. 2004, 68, 2344–2352. [Google Scholar] [CrossRef] [Green Version]
- Sebastian, A.P.; Keerthi, T.R. Adhesion and cellsurface properties of wild species of spore formers against enteric pathogens. Asian Pac. J. Trop. Med. 2013, 6, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Takimoto, T.; Sato, K.; Akiba, Y.; Takahashi, K. Role of chicken TL1A on inflammatory responses and partial characterization of its receptor. J. Immunol. 2008, 180, 8327–8332. [Google Scholar] [CrossRef] [Green Version]
- Lillehoj, H.S.; Choi, K.D. Recombinant chicken interferon-gamma-mediated inhibition of Eimeria tenella development in vitro and reduction of oocyst production and body weight loss following Eimeria acervulina challenge infection. Avian Dis. 1998, 42, 307–314. [Google Scholar] [CrossRef]
- Park, S.S.; Lillehoj, H.S.; Allen, P.C.; Park, D.W.; FitzCoy, S.; Bautista, D.A.; Lillehoje, E.P. Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis. Avian Dis. 2008, 52, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Platzer, C.; Meisel, C.; Vogt, K.; Platzer, M.; Volk, H.D. Up-regulation of monocytic IL-10 by tumor necrosis factor-alpha and cAMP elevating drugs. Int. Immunol. 1995, 7, 517–523. [Google Scholar] [CrossRef]
- Xu, X.; Huang, Q.; Mao, Y.; Cui, Z.; Li, Y.; Huang, Y.; Rajput, I.R.; Yu, D.; Li, W. Immunomodulatory effects of Bacillus subtilis (natto) B4 spores on murine macrophages. Microbiol. Immunol. 2012, 56, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Huyghebaert, G.; Ducatelle, R.; Van Immerseel, F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011, 187, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.W.; Lillehoj, H.S.; Jang, S.I.; Li, G.; Lee, S.H.; Lillehoj, E.P.; Siragusa, G.R. Effect of Bacillus-based direct-fed microbials on Eimeria maxima infection in broiler chickens. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, e105–e110. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Tsen, H.Y.; Lin, C.L.; Yu, B.; Chen, C.S. Oral administration of a combination of select lactic acid bacteria strains to reduce the Salmonella invasion and inflammation of broiler chicks. Poult. Sci. 2012, 91, 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- El Kady, M.F.; Hassan, E.R.; Radwan, I.A.E.; Rabie, N.S.; Rady, M.M. Effect of probiotic on necrotic enteritis in chickens with the presence of immunsuppressive factors. Glob. Vet. 2012, 9, 345–351. [Google Scholar] [CrossRef]
- Levkut, M.; Revajová, V.; Lauková, A.; Ševčíková, Z.; Spišáková, V.; Faixová, Z.; Levkutová, M.; Strompfová, V.; Pistl, J.; Levkut, M. Leukocytic responses and intestinal mucin dynamics of broilers protected with Enterococcus faecium EF55 and challenged with Salmonella Enteritidis. Res. Vet. Sci. 2012, 93, 195–201. [Google Scholar] [CrossRef]
- Higgins, S.E.; Wolfenden, A.D.; Tellez, G.; Hargis, B.M.; Porter, T.E. Transcriptional profiling of cecal gene expression in probiotic- and Salmonella-challenged neonatal chicks. Poult. Sci. 2011, 90, 901–913. [Google Scholar] [CrossRef]
- Rajput, I.R.; Li, L.Y.; Xin, X.; Wu, B.B.; Juan, Z.L.; Cui, Z.W.; Yu, D.Y.; Li, W.F. Effect of Saccharomyces boulardii and Bacillus subtilis B10 on intestinal ultrastructure modulation and mucosal immunity development mechanism in broiler chickens. Poult. Sci. 2013, 92, 956–965. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, D.K.; Lillehoj, H.S.; Jang, S.I.; Lee, S.H. Immune modulation by Bacillus subtilis-based direct-fed microbials in commercial broiler chickens. Anim. Feed Sci. Technol. 2015, 200, 76–85. [Google Scholar] [CrossRef]
- Naura, A.S.; Zerfaoui, M.; Kim, H.; Abd Elmageed, Z.Y.; Rodriguez, P.C.; Hans, C.P.; Ju, J.; Errami, Y.; Park, J.; Ochoa, A.C.; et al. Requirement for inducible nitric oxide synthase in chronic allergen exposure-induced pulmonary fibrosis but not inflammation. J. Immunol. 2010, 185, 3076–3085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Kim, H.Y. Lantibiotics, class I bacteriocins from the genus Bacillus. J. Microbiol. Biotechnol. 2011, 21, 229–235. [Google Scholar] [CrossRef]
- Lee, K.W.; Lillehoj, H.S.; Jang, S.I.; Lee, S.H.; Bautista, D.A.; Siragusa, G.R. Effect of Bacillus Subtilis-based Direct-fed Microbials on Immune Status in Broiler Chickens Raised on Fresh or Used Litter. Asian-Australas. J. Anim. Sci. 2013, 26, 1592–1597. [Google Scholar] [CrossRef] [Green Version]
- Korhonen, R.; Korpela, R.; Saxelin, M.; Mäki, M.; Kankaanranta, H.; Moilanen, E. Induction of nitric oxide synthesis by probiotic Lactobacillus rhamnosus GG in J774 macrophages and human T84 intestinal epithelial cells. Inflammation 2001, 25, 223–232. [Google Scholar] [CrossRef]
- Kim, D.W.; Cho, S.B.; Lee, H.J.; Chung, W.T.; Kim, K.H.; Hwangbo, J.; Nam, I.S.; Cho, Y.I.; Yang, M.P.; Chung, I.B. Comparison of cytokine and nitric oxide induction in murine macrophages between whole cell and enzymatically digested Bifidobacterium sp. obtained from monogastric animals. J. Microbiol. 2007, 45, 305–310. [Google Scholar] [PubMed]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Privett, B.J.; Broadnax, A.D.; Bauman, S.J.; Riccio, D.A.; Schoenfisch, M.H. Examination of bacterial resistance to exogenous nitric oxide. Nitric. Oxide 2012, 26, 169–173. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, L.Y. TNFSF15 Modulates Neovascularization and Inflammation. Cancer Microenviron. 2012, 5, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Fanzani, A.; Conraads, V.M.; Penna, F.; Martinet, W. Molecular and cellular mechanisms of skeletal muscle atrophy: An update. J. Cachexia Sarcopenia Muscle 2012, 3, 163–179. [Google Scholar] [CrossRef]
- Kaushik, J.K.; Kumar, A.; Duary, R.K.; Mohanty, A.K.; Grover, S.; Batish, V.K. Functional and probiotic attributes of an indigenous isolate of Lactobacillus plantarum. PLoS ONE 2009, 4, e8099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliakbarpour, H.R.; Chamani, M.; Rahimi, G.; Sadeghi, A.A.; Qujeq, D. The Bacillus subtilis and Lactic Acid Bacteria Probiotics Influences Intestinal Mucin Gene Expression, Histomorphology and Growth Performance in Broilers. Asian-Australas. J. Anim. Sci. 2012, 25, 1285–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Adlerberth, I.; Cerquetti, M.; Poilane, I.; Wold, A.; Collignon, A. Mechanisms of Colonisation and Colonisation Resistance of the Digestive Tract Part 1: Bacteria/host Interactions. Microb. Ecol. Health Dis. 2000, 12, 223–239. [Google Scholar]
- Mohan, V. The role of probiotics in the inhibition of Campylobacter jejuni colonization and virulence attenuation. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1503–1513. [Google Scholar] [CrossRef]
- Flint, J.F.; Garner, M.R. Feeding beneficial bacteria: A natural solution for increasing efficiency and decreasing pathogens in animal agriculture. J. Appl. Poult. Res. 2009, 18, 367–378. [Google Scholar] [CrossRef]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef]
- Dharmani, P.; Srivastava, V.; Kissoon-Singh, V.; Chadee, K. Role of intestinal mucins in innate host defense mechanisms against pathogens. J. Innate Immun. 2009, 1, 123–135. [Google Scholar] [CrossRef]
- Horn, N.L.; Donkin, S.S.; Applegate, T.J.; Adeola, O. Intestinal mucin dynamics: Response of broiler chicks and White Pekin ducklings to dietary threonine. Poult. Sci. 2009, 88, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Deplancke, B.; Gaskins, H.R. Microbial modulation of innate defense: Goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 2001, 73, 1131S–1141S. [Google Scholar] [CrossRef] [Green Version]
- Smirnov, A.; Perez, R.; Amit-Romach, E.; Sklan, D.; Uni, Z. Mucin dynamics and microbial population in chicken small intestine are changed by dietary probiotic and antibiotic growth promoter supplementation. J. Nutr. 2005, 135, 187–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, H.; Wathes, C. Ammonia and poultry welfare: A review. World’s Poult. Sci. J. 2000, 56, 235–245. [Google Scholar] [CrossRef]
- Wang, Y.; Cho, J.H.; Chen, Y.J.; Yoo, J.S.; Huang, Y.; Kim, H.J.; Kim, I.H. The effect of probiotic BioPlus 2B® on growth performance, dry matter and nitrogen digestibility and slurry noxious gas emission in growing pigs. Livest. Sci. 2009, 120, 35–42. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Kim, I.H. Effects of probiotic supplementation in different energy and nutrient density diets on performance, egg quality, excreta microflora, excreta noxious gas emission, and serum cholesterol concentrations in laying hens. J. Anim. Sci. 2013, 91, 4781–4787. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, I.H. Effect of Bacillus subtilis C-3102 spores as a probiotic feed supplement on growth performance, noxious gas emission, and intestinal microflora in broilers. Poult. Sci. 2014, 93, 3097–3103. [Google Scholar] [CrossRef]
- Adeola, O.; Cowieson, A.J. BOARD-INVITED REVIEW: Opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 2011, 89, 3189–3218. [Google Scholar] [CrossRef]
- Lee, J.; Park, I.; Ho, C.Y.; Cho, J. Bacillus strains as feed additives: In vitro evaluation of its potential probiotic properties. Rev. Colomb. Cienc. Pec. 2012, 25, 577–585. [Google Scholar]
- Hosoi, T.; Ametani, A.; Kiuchi, K.; Kaminogawa, S. Improved growth and viability of lactobacilli in the presence of Bacillus subtilis (natto), catalase, or subtilisin. Can. J. Microbiol. 2000, 46, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, T.M.; Serra, C.R.; La Ragione, R.M.; Woodward, M.J.; Henriques, A.O. Screening for bacillus isolates in the broiler gastrointestinal tract. Appl. Environ. Microbiol. 2005, 71, 968–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Z.; Imai, F.; Kim, I.J.; Fujita, H.; Katayama, K.; Mori, K.; Yoshihara, Y.; Yoshida, Y. Expression of the immunoglobulin superfamily cell adhesion molecules in the developing spinal cord and dorsal root ganglion. PLoS ONE 2015, 10, e0121550. [Google Scholar] [CrossRef]
- Larsen, N.; Thorsen, L.; Kpikpi, E.N.; Stuer-Lauridsen, B.; Cantor, M.D.; Nielsen, B.; Brockmann, E.; Derkx, P.M.; Jespersen, L. Characterization of Bacillus spp. strains for use as probiotic additives in pig feed. Appl. Microbiol. Biotechnol. 2014, 98, 1105–1118. [Google Scholar] [CrossRef]
- Chistyakov, V.; Melnikov, V.; Chikindas, M.L.; Khutsishvili, M.; Chagelishvili, A.; Bren, A.; Kostina, N.; Cavera, V.; Elisashvili, V. Poultry-beneficial solid-state Bacillus amyloliquefaciens B-1895 fermented soybean formulation. Biosci. Microbiota Food Health 2015, 34, 25–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Zhang, Q.; Dang, H.; Liu, X.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Screening for potential new probiotic based on probiotic properties and α-glucosidase inhibitory activity. Food Control 2014, 35, 65–72. [Google Scholar] [CrossRef]
- Farhat-Khemakhem, A.; Blibech, M.; Boukhris, I.; Makni, M.; Chouayekh, H. Assessment of the potential of the multi-enzyme producer Bacillus amyloliquefaciens US573 as alternative feed additive. J. Sci. Food Agric. 2018, 98, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Endo, T.; Nakano, M.; Shimizu, S.; Fukushima, M.; Miyoshi, S. Effects of a probiotic on the lipid metabolism of cocks fed on a cholesterol-enriched diet. Biosci. Biotechnol. Biochem. 1999, 63, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Abriouel, H.; Franz, C.M.; Ben Omar, N.; Gálvez, A. Diversity and applications of Bacillus bacteriocins. FEMS Microbiol. Rev. 2011, 35, 201–232. [Google Scholar] [CrossRef] [Green Version]
- Stein, T.; Heinzmann, S.; Düsterhus, S.; Borchert, S.; Entian, K.D. Expression and functional analysis of the subtilin immunity genes spaIFEG in the subtilin-sensitive host Bacillus subtilis MO1099. J. Bacteriol. 2005, 187, 822–828. [Google Scholar] [CrossRef] [Green Version]
- Hong, H.A.; Duc, L.H.; Cutting, S.M. The use of bacterial spore formers as probiotics. FEMS Microbiol. Rev. 2005, 29, 813–835. [Google Scholar] [CrossRef] [Green Version]
- The True Cost of Necrotic Enteritis. Available online: https://www.poultryworld.net/Meat/Articles/2015/10/The-true-cost-of-necrotic-enteritis-2699819W (accessed on 9 May 2021).
- Johansson, M.E.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef] [Green Version]
- Stein, T. Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Mol. Microbiol. 2005, 56, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Sumi, C.D.; Yang, B.W.; Yeo, I.C.; Hahm, Y.T. Antimicrobial peptides of the genus Bacillus: A new era for antibiotics. Can. J. Microbiol. 2015, 61, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Suva, M.; Sureja, V.; Kheni, D. Novel insight on probiotic Bacillus subtilis: Mechanism of action and clinical applications. J. Curr. Res. Sci. Med. 2016, 2, 65–72. [Google Scholar] [CrossRef]
- Sang, Y.; Blecha, F. Antimicrobial peptides and bacteriocins: Alternatives to traditional antibiotics. Anim. Health Res. Rev. 2008, 9, 227–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazza, P. The use of Bacillus subtilis as an antidiarrhoeal microorganism. Boll. Chim Farm. 1994, 133, 3–18. [Google Scholar] [PubMed]
- Fuller, R. Probiotics in human medicine. Gut 1991, 32, 439–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teo, A.Y.; Tan, H.M. Evaluation of the performance and intestinal gut microflora of broilers fed on corn-soy diets supplemented with Bacillus subtilis PB6 (CloSTAT). J. Appl. Poult. Res. 2007, 16, 296–303. [Google Scholar] [CrossRef]
- Knap, I.; Lund, B.; Kehlet, A.B.; Hofacre, C.; Mathis, G. Bacillus licheniformis prevents necrotic enteritis in broiler chickens. Avian Dis. 2010, 54, 931–935. [Google Scholar] [CrossRef]
- Craven, S.E. Colonization of the intestinal tract by Clostridium perfringens and fecal shedding in diet-stressed and unstressed broiler chickens. Poult. Sci. 2000, 79, 843–849. [Google Scholar] [CrossRef]
- Kaldhusdal, M.; Lovland, A. The economic impact of Clostridium perfringens is greater than anticipated. World Poult. Sci. J. 2000, 16, 50–51. [Google Scholar]
- Park, J.H.; Kim, I.H. Supplemental effect of Probiotic Bacillus subtilis B2A on productivity, organ weight, intestinal Salmonella microflora, and breast meat quality of growing broiler chicks. Poult. Sci. 2014, 93, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- Kadaikunnan, S.; Rejiniemon, T.; Khaled, J.M.; Alharbi, N.S.; Mothana, R. In-vitro antibacterial, antifungal, antioxidant and functional properties of Bacillus amyloliquefaciens. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 9. [Google Scholar] [CrossRef] [Green Version]
- La Ragione, R.M.; Casula, G.; Cutting, S.M.; Woodward, M.J. Bacillus subtilis spores competitively exclude Escherichia coli O78:K80 in poultry. Vet. Microbiol. 2001, 79, 133–142. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Hossiendoust, A.; Kim, I.H. Probiotics in Salmonella-challenged Hy-Line brown layers. Poult. Sci. 2016, 95, 1894–1897. [Google Scholar] [CrossRef]
- Bizani, D.; Brandelli, A. Characterization of a bacteriocin produced by a newly isolated Bacillus sp. Strain 8 A. J. Appl. Microbiol. 2002, 93, 512–519. [Google Scholar] [CrossRef]
- Rea, M.C.; Sit, C.S.; Clayton, E.; O’Connor, P.M.; Whittal, R.M.; Zheng, J.; Vederas, J.C.; Ross, R.P.; Hill, C. Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc. Natl. Acad. Sci. USA 2010, 107, 9352–9357. [Google Scholar] [CrossRef] [Green Version]
- Teo, A.Y.; Tan, H.M. Inhibition of Clostridium perfringens by a novel strain of Bacillus subtilis isolated from the gastrointestinal tracts of healthy chickens. Appl. Environ. Microbiol. 2005, 71, 4185–4190. [Google Scholar] [CrossRef] [Green Version]
- Grilli, E.; Messina, M.R.; Catelli, E.; Morlacchini, M.; Piva, A. Pediocin A improves growth performance of broilers challenged with Clostridium perfringens. Poult. Sci. 2009, 88, 2152–2158. [Google Scholar] [CrossRef] [PubMed]
- Udompijitkul, P.; Paredes-Sabja, D.; Sarker, M.R. Inhibitory effects of nisin against Clostridium perfringens food poisoning and nonfood-borne isolates. J. Food Sci. 2012, 77, M51–M56. [Google Scholar] [CrossRef] [PubMed]
- Dabard, J.; Bridonneau, C.; Phillipe, C.; Anglade, P.; Molle, D.; Nardi, M.; Ladiré, M.; Girardin, H.; Marcille, F.; Gomez, A.; et al. Ruminococcin A, a new lantibiotic produced by a Ruminococcus gnavus strain isolated from human feces. Appl. Environ. Microbiol. 2001, 67, 4111–4118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crost, E.H.; Ajandouz, E.H.; Villard, C.; Geraert, P.A.; Puigserver, A.; Fons, M. Ruminococcin C, a new anti-Clostridium perfringens bacteriocin produced in the gut by the commensal bacterium Ruminococcus gnavus E1. Biochimie 2011, 93, 1487–1494. [Google Scholar] [CrossRef]
- Sharma, N.; Gupta, A.; Gautam, N. Characterization of Bacteriocin like inhibitory substance produced by a new Strain Brevibacillus borstelensis AG1 Isolated from ‘Marcha’. Braz. J. Microbiol. 2014, 45, 1007–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lillehoj, H.S.; Trout, J.M. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin. Microbiol. Rev. 1996, 9, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M.W.; Lillehoj, H.S. The long view: A selective review of 40 years of coccidiosis research. Avian Pathol. 2012, 41, 111–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadde, U.D.; Oh, S.; Lee, Y.; Davis, E.; Zimmerman, N.; Rehberger, T.; Lillehoj, H.S. Dietary Bacillus subtilis-based direct-fed microbials alleviate LPS-induced intestinal immunological stress and improve intestinal barrier gene expression in commercial broiler chickens. Res. Vet. Sci. 2017, 114, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E. Peptide antibiotics. Lancet 1997, 349, 418–422. [Google Scholar] [CrossRef]
- Deleu, M.; Paquot, M.; Nylander, T. Effect of fengycin, a lipopeptide produced by Bacillus subtilis, on model biomembranes. Biophys. J. 2008, 94, 2667–2679. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.K.; Singh, S.S.; Verma, S.; Kumar, S. Antifungal activity of anthraquinone derivatives from Rheum emodi. J. Ethnopharmacol. 2000, 72, 43–46. [Google Scholar] [CrossRef]
- Athukorala, S.N.; Fernando, W.G.; Rashid, K.Y. Identification of antifungal antibiotics of Bacillus species isolated from different microhabitats using polymerase chain reaction and MALDI-TOF mass spectrometry. Can. J. Microbiol. 2009, 55, 1021–1032. [Google Scholar] [CrossRef]
- Haraguchi, H.; Kataoka, S.; Okamoto, S.; Hanafi, M.; Shibata, K. Antimicrobial triterpenes from Ilex integra and the mechanism of antifungal action. Phytother. Res. 1999, 13, 151–156. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Valerio, F.; Visconti, A. Antifungal activity of phenyllactic acid against molds isolated from bakery products. Appl. Environ. Microbiol. 2003, 69, 634–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjögren, J.; Magnusson, J.; Broberg, A.; Schnürer, J.; Kenne, L. Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Appl. Environ. Microbiol. 2003, 69, 7554–7667. [Google Scholar] [CrossRef] [Green Version]
- Sögaard, H.; Suhr-Jessen, T. Microbials for feed: Beyond lactic acid bacteria. Feed Int. Ernational. 1990, 11, 32–38. [Google Scholar]
- Jayaraman, S.; Das, P.P.; Saini, P.C.; Roy, B.; Chatterjee, P.N. Use of Bacillus Subtilis PB6 as a potential antibiotic growth promoter replacement in improving performance of broiler birds. Poult. Sci. 2017, 96, 2614–2622. [Google Scholar] [CrossRef]
- Li, Y.B.; Xu, Q.Q.; Yang, C.J.; Yang, X.; Lv, L.; Yin, C.H.; Liu, X.L.; Yan, H. Effects of probiotics on the growth performance and intestinal micro flora of broiler chickens. Pak. J. Pharm. Sci. 2014, 27, 713–717. [Google Scholar] [PubMed]
- Timmerman, H.M.; Veldman, A.; van den Elsen, E.; Rombouts, F.M.; Beynen, A.C. Mortality and growth performance of broilers given drinking water supplemented with chicken-specific probiotics. Poult. Sci. 2006, 85, 1383–1388. [Google Scholar] [CrossRef]
- Liu, X.; Yan, H.; Lv, L.; Xu, Q.; Yin, C.; Zhang, K.; Wang, P.; Hu, J. Growth Performance and Meat Quality of Broiler Chickens Supplemented with Bacillus licheniformis in Drinking Water. Asian-Australas. J. Anim. Sci. 2012, 25, 682–689. [Google Scholar] [CrossRef]
- Bodinga, B.M.; Hayat, K.; Liu, X.; Zhou, J.; Yang, X.; Ismaila, A.; Soomro, R.N.; Ren, Z.; Zhang, W.; Yang, X. Effects of Bacillus Subtilis DSM 32315 on Immunity, Nutrient Transporters and Functional Diversity of Cecal Microbiome of Broiler Chickens in Necrotic Enteritis Challenge. J. World Poult. Res. 2020, 10, 527–544. [Google Scholar] [CrossRef]
- Cetin, N.; Güçlü, B.K.; Cetin, E. The effects of probiotic and mannanoligosaccharide on some haematological and immunological parameters in turkeys. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2005, 52, 263–267. [Google Scholar] [CrossRef]
- Rahman, M.; Mustari, A.; Salauddin, M.; Rahman, M. Effects of probiotics and enzymes on growth performance and haematobiochemical parameters in broilers. J. Bangladesh Agric. Univ. 2014, 11, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Keerqin, C.; Rhayat, L.; Zhang, Z.H.; Gharib-Naseri, K.; Kheravii, S.K.; Devillard, E.; Crowley, T.M.; Wu, S.B. Probiotic Bacillus subtilis 29,784 improved weight gain and enhanced gut health status of broilers under necrotic enteritis condition. Poult. Sci. 2021, 100, 100981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhong, G.; Shao, D.; Wang, Q.; Hu, Y.; Wu, T.; Ji, C.; Shi, S. Dietary supplementation with Bacillus subtilis promotes growth performance of broilers by altering the dominant microbial community. Poult. Sci. 2021, 100, 100935. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, H.; Zhang, J.; Tang, X.; Raheem, A.; Wang, M.; Lin, W.; Liang, L.; Qi, Y.; Zhu, Y.; et al. Modulatory Effects of Bacillus subtilis on the Performance, Morphology, Cecal Microbiota and Gut Barrier Function of Laying Hens. Animals 2021, 11, 1523. [Google Scholar] [CrossRef] [PubMed]
- Neveling, D.P.; Dicks, L.M.T. Probiotics: An Antibiotic Replacement Strategy for Healthy Broilers and Productive Rearing. Probiotics Antimicrob. Proteins 2021, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Duskaev, G.; Rakhmatullin, S.; Kvan, O. Effects of Bacillus cereus and coumarin on growth performance, blood biochemical parameters, and meat quality in broilers. Vet. World 2020, 13, 2484–2492. [Google Scholar] [CrossRef]
- Lei, K.; Li, Y.L.; Yu, D.Y.; Rajput, I.R.; Li, W.F. Influence of dietary inclusion of Bacillus licheniformis on laying performance, egg quality, antioxidant enzyme activities, and intestinal barrier function of laying hens. Poult. Sci. 2013, 92, 2389–2395. [Google Scholar] [CrossRef]
- Abdelqader, A.; Irshaid, R.; Al-Fataftah, A.R. Effects of dietary probiotic inclusion on performance, eggshell quality, cecal microflora composition, and tibia traits of laying hens in the late phase of production. Trop. Anim. Health Prod. 2013, 45, 1017–1024. [Google Scholar] [CrossRef]
- Mazanko, M.S.; Gorlov, I.F.; Prazdnova, E.V.; Makarenko, M.S.; Usatov, A.V.; Bren, A.B.; Chistyakov, V.A.; Tutelyan, A.V.; Komarova, Z.B.; Mosolova, N.I.; et al. Bacillus Probiotic Supplementations Improve Laying Performance, Egg Quality, Hatching of Laying Hens, and Sperm Quality of Roosters. Probiotics Antimicrob. Proteins 2018, 10, 367–373. [Google Scholar] [CrossRef]
- Chaucheyras-Durand, F.; Durand, H. Probiotics in animal nutrition and health. Benef. Microbes 2010, 1, 3–9. [Google Scholar] [CrossRef]
- Bai, W.K.; Zhang, F.J.; He, T.J.; Su, P.W.; Ying, X.Z.; Zhang, L.L.; Wang, T. Dietary Probiotic Bacillus subtilis Strain fmbj Increases Antioxidant Capacity and Oxidative Stability of Chicken Breast Meat during Storage. PLoS ONE 2016, 11, e0167339. [Google Scholar] [CrossRef]
- Percie Du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Ermakova, L.P.; Nozdrin, G.A.; Tishkov, S.N.; Novik, Y.V.; Gotovchikov, N.A.; Mensh, I.K. Effects of a probiotic containing Bacillus subtilis on the gut microflora, yolk quality and blood lipid concentrations of laying Pharaon quails. Vet. Stanica 2021, 52, 297–306. [Google Scholar] [CrossRef]
- Deng, W.; Dong, X.F.; Tong, J.M.; Zhang, Q. The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult. Sci. 2012, 91, 575–582. [Google Scholar] [CrossRef]
- Global Probiotics Market Size 2019, by Application (Functional Food & Beverages [Dairy Products, Non-dairy Beverages, Infant Formula, Cereals], Dietary Supplements, Feed), Ingredient (Bacteria, Yeast), End-User (Human, Animal), Region and Forecast to 2025. Available online: https://www.adroitmarketresearch.com/industry-reports/probiotics-market (accessed on 9 May 2021).
- Monteiro, S.M.; Clemente, J.J.; Henriques, A.O.; Gomes, R.J.; Carrondo, M.J.; Cunha, A.E. A procedure for high-yield spore production by Bacillus subtilis. Biotechnol. Prog. 2005, 21, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Cutting, S.M. Bacillus probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Posada-Uribe, L.F.; Romero-Tabarez, M.; Villegas-Escobar, V. Effect of medium components and culture conditions in Bacillus subtilis EA-CB0575 spore production. Bioprocess. Biosyst. Eng. 2015, 38, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.R.; Vakil, B.V. Development of bioprocess for high density cultivation yield of the probiotic Bacillus coagulans and its spores. J. BioSci. Biotechnol. 2016, 5, 173–181. [Google Scholar]
- Ren, H.; Su, Y.T.; Guo, X.H. Rapid optimization of spore production from Bacillus amyloliquefaciens in submerged cultures based on dipicolinic acid fluorimetry assay. AMB Express 2018, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Ballardo, C.; Barrena, R.; Artola, A.; Sánchez, A. A novel strategy for producing compost with enhanced biopesticide properties through solid-state fermentation of biowaste and inoculation with Bacillus thuringiensis. Waste Manag. 2017, 70, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Martelli, F.; Favari, C.; Mena, P.; Guazzetti, S.; Ricci, A.; Del Rio, D.; Lazzi, C.; Neviani, E.; Bernini, V. Antimicrobial and Fermentation Potential of Himanthalia elongata in Food Applications. Microorganisms 2020, 8, 248. [Google Scholar] [CrossRef] [Green Version]
- Aslam, F.; Ansari, A.; Aman, A.; Baloch, G.; Nisar, G.; Baloch, A.H.; Rehman, H.U. Production of commercially important enzymes from Bacillus licheniformis KIBGE-IB3 using date fruit wastes as substrate. J. Genet. Eng. Biotechnol. 2020, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Su, B.; Chen, X.; Pian, R. Solid state fermentation of Moringa oleifera leaf meal by mixed strains for the protein enrichment and the improvement of nutritional value. PeerJ 2020, 8, e10358. [Google Scholar] [CrossRef]
- Monteiro, S.M.S.; Clemente, J.J.; Carrondo, M.J.T.; Cunha, A.E. Enhanced spore production of Bacillus subtilis grown in a chemically defined medium. Adv. Microbiol. 2014, 4, 444–454. [Google Scholar] [CrossRef] [Green Version]
- Khardziani, T.; Kachlishvili, E.; Sokhadze, K.; Elisashvili, V.; Weeks, R.; Chikindas, M.L.; Chistyakov, V. Elucidation of Bacillus subtilis KATMIRA 1933 Potential for Spore Production in Submerged Fermentation of Plant Raw Materials. Probiotics Antimicrob. Proteins 2017, 9, 435–443. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, F.; Wei, Z.; Ran, W.; Shen, Q. The usage of rice straw as a major substrate for the production of surfactin by Bacillus amyloliquefaciens XZ-173 in solid-state fermentation. J. Environ. Manag. 2013, 127, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Gowdhaman, D.; Manaswini, V.S.; Jayanthi, V.; Dhanasri, M.; Jeyalakshmi, G.; Gunasekar, V.; Sugumaran, K.R.; Ponnusami, V. Xylanase production from Bacillus aerophilus KGJ2 and its application in xylooligosaccharides preparation. Int. J. Biol. Macromol. 2014, 64, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Viayaraghavan, P.; Jeba Kumar, S.; Valan Arasu, M.; Al-Dhabi, N.A. Simultaneous production of commercial enzymes using agro industrial residues by statistical approach. J. Sci. Food Agric. 2019, 99, 2685–2696. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, Z.; Yu, W.; Zheng, L.; Li, L.; Gu, W.; Xu, H.; Wei, B.; Yan, X. Nutritional quality improvement of soybean meal by Bacillus velezensis and Lactobacillus plantarum during two-stage solid- state fermentation. AMB Express 2021, 11, 23. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Horng, Y.B.; Dybus, A.; Yu, Y.H. Bacillus licheniformis-Fermented Products Improve Growth Performance and Intestinal Gut Morphology in Broilers under Clostridium perfringens Challenge. J. Poult. Sci. 2021, 58, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, L.; Khan, A.; Zhao, R.; Wei, S.; Jing, X. Fermented wheat bran by xylanase-producing Bacillus cereus boosts the intestinal microflora of broiler chickens. Poult. Sci. 2020, 99, 263–271. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, S.Y.; Weon, H.-Y.; Sang, M.K.; Song, J. Complete genome sequence of Bacillus velezensis M75, a biocontrol agent against fungal plant pathogens, isolated from cotton waste. J. Biotechnol. 2017, 241, 112–115. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Rao, Y.K.; Tsay, K.J.; Wu, W.S.; Tzeng, Y.M. Medium optimization of carbon and nitrogen sources for the production of spores from Bacillus amyloliquefaciens B128 using response surface methodology. Process. Biochem. 2007, 42, 535–541. [Google Scholar] [CrossRef]
- Chen, Z.M.; Li, Q.; Liu, H.M.; Yu, N.; Xie, T.J.; Yang, M.Y.; Shen, P.; Chen, X.D. Greater enhancement of Bacillus subtilis spore yields in submerged cultures by optimization of medium composition through statistical experimental designs. Appl. Microbiol. Biotechnol. 2010, 85, 1353–1360. [Google Scholar] [CrossRef]
- Gangadharan, D.; Sivaramakrishnan, S.; Nampoothiri, K.M.; Pandey, A. Solid culturing of Bacillus amyloliquefaciens for alpha amylase production. Food Technol. Biotechnol. 2006, 44, 269–274. [Google Scholar]
- Hölker, U.; Höfer, M.; Lenz, J. Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl. Microbiol. Biotechnol. 2004, 64, 175–186. [Google Scholar] [CrossRef]
- Schultze, M.O. Nutritional value of plant materials; growth of rats on purified rations containing soybean protein. J. Nutr. 1950, 41, 103–113. [Google Scholar] [CrossRef]
- Zhao, S.; Hu, N.; Huang, J.; Liang, Y.; Zhao, B. High-yield spore production from Bacillus licheniformis by solid state fermentation. Biotechnol. Lett. 2008, 30, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.H.; Shinde, P.L.; Choi, J.Y.; Kim, J.S.; Seo, D.K.; Pak, J.I.; Chae, B.J.; Kwon, I.K. Evaluation of multi-microbial probiotics produced by submerged liquid and solid substrate fermentation methods in broilers. Asian-Australas. J. Anim. Sci. 2010, 23, 521–529. [Google Scholar] [CrossRef]
- Su, Y.T.; Liu, C.; Long, Z.; Ren, H.; Guo, X.H. Improved Production of Spores and Bioactive Metabolites from Bacillus amyloliquefaciens in Solid-state Fermentation by a Rapid Optimization Process. Probiotics Antimicrob. Proteins 2019, 11, 921–930. [Google Scholar] [CrossRef]
- Mahoney, R.; Weeks, R.; Zheng, T.; Huang, Q.; Dai, W.; Cao, Y.; Liu, G.; Guo, Y.; Chistyakov, V.; Chikindas, M.L. Evaluation of an Industrial Soybean Byproduct for the Potential Development of a Probiotic Animal Feed Additive with Bacillus Species. Probiotics Antimicrob. Proteins 2020, 12, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Kragh, K.N.; Hutchison, J.B.; Melaugh, G.; Rodesney, C.; Roberts, A.E.; Irie, Y.; Jensen, P.Ø.; Diggle, S.P.; Allen, R.J.; Gordon, V.; et al. Role of Multicellular Aggregates in Biofilm Formation. mBio 2016, 7, e00237. [Google Scholar] [CrossRef] [Green Version]
- Rossi, E.; Cimdins, A.; Lüthje, P.; Brauner, A.; Sjöling, Å.; Landini, P.; Römling, U. “It’s a gut feeling”—Escherichia coli biofilm formation in the gastrointestinal tract environment. Crit. Rev. Microbiol. 2018, 44, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Ushakova, N.A.; Abramov, V.M.; Khlebnikov, V.S.; Semenov, A.M.; Kuznetsov, B.B.; Kozlova, A.A.; Nifatov, A.V.; Sakulin, V.K.; Kosarev, I.V.; Vasilenko, R.N.; et al. Properties of the Probiotic Strain Lactobacillus plantarum 8-RA-3 Grown in a Biofilm by Solid Substrate Cultivation Method. Probiotics Antimicrob. Proteins 2012, 4, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Hesseltine, C.W. Biotechnology report. Solid state fermentations. Biotechnol. Bioeng. 1972, 14, 517–532. [Google Scholar] [CrossRef]
- Anderson, R.L.; Wolf, W.J. Compositional changes in trypsin inhibitors, phytic acid, saponins and isoflavones related to soybean processing. J. Nutr. 1995, 125, 581S–588S. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, T. Effect of solid-state fermentation with Bacillus subtilis lwo on the proteolysis and the antioxidative properties of chickpeas. Int. J. Food Microbiol. 2021, 338, 108988. [Google Scholar] [CrossRef] [PubMed]
- McGovern, E.P.; Bentley, R. Biosynthesis of flaviolin and 5,8-dihydroxy-2,7-dimethoxy-1,4-naphthoquinone. Biochemistry 1975, 14, 3138–3143. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Chiou, J.Y.; Wang, J.Y.; Hong, C.Y.; Tsen, W.C. Cephalosporin C production by solid state fermentation with rice grains. Zhonghua Min. Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi 1984, 17, 55–69. [Google Scholar]
- Yang, S.S.; Ling, M.Y. Tetracycline production with sweet potato residue by solid state fermentation. Biotechnol. Bioeng. 1989, 33, 1021–1028. [Google Scholar] [CrossRef]
- Barrios-González, J.; Mejía, A. Production of secondary metabolites by solid-state fermentation. Biotechnol. Annu. Rev. 1996, 2, 85–121. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Ahluwalia, V.; Saran, S.; Kumar, J.; Patel, A.K.; Singhania, R.R. Recent developments on solid-state fermentation for production of microbial secondary metabolites: Challenges and solutions. Bioresour. Technol. 2021, 323, 124566. [Google Scholar] [CrossRef]
- Jang, C.H.; Oh, J.; Lim, J.S.; Kim, H.J.; Kim, J.S. Fermented Soy Products: Beneficial Potential in Neurodegenerative Diseases. Foods 2021, 10, 636. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Chakraborty, R.; Dutta, A. Role of Fermentation in Improving Nutritional Quality of Soybean Meal—A Review. Asian-Australas. J. Anim. Sci. 2016, 29, 1523–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, U.; Nagao, K.; Tosa, Y.; Yoshioka, Y. Relationship between food containing “Natto” (fermented soybeans) and the blood pressure of SHR. JPN Heart J. 1976, 17, 343–344. [Google Scholar] [CrossRef]
- Chou, H.Y.; Liu, L.H.; Chen, C.Y.; Lin, I.F.; Ali, D.; Yueh-Luen Lee, A.; David Wang, H.M. Bifunctional mechanisms of autophagy and apoptosis regulations in melanoma from Bacillus subtilis natto fermentation extract. Food Chem. Toxicol. 2021, 150, 112020. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Sato, M.; Yoshihara, A.; Saito, T.; Kitamura, K.; Ansai, T.; Nakamura, K. A 5-year longitudinal association between dietary fermented soya bean (natto) intake and tooth loss through bone mineral density in postmenopausal women: The Yokogoshi cohort study. Gerodontology 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ding, H.; Liu, Q.; Wei, Y.; Zhang, Y.; Wang, Y.; Lu, Y.; Ma, A.; Li, Z.; Hu, Y. Effects of peanut meal extracts fermented by Bacillus natto on the growth performance, learning and memory skills and gut microbiota modulation in mice. Br. J. Nutr. 2020, 123, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Babu, K.S. Modelling and optimization of the process conditions for biomass production and sporulation of a probiotic culture. Process. Biochem. 2005, 40, 2531–2538. [Google Scholar] [CrossRef]
- Riesenberg, D.; Guthke, R. High-cell-density cultivation of microorganisms. Appl. Microbiol. Biotechnol. 1999, 51, 422–430. [Google Scholar] [CrossRef]
- Oladokun, S.; Koehler, A.; MacIsaac, J.; Ibeagha-Awemu, E.M.; Adewole, D.I. Bacillus subtilis delivery route: Effect on growth performance, intestinal morphology, cecal short-chain fatty acid concentration, and cecal microbiota in broiler chickens. Poult. Sci. 2021, 100, 100809. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.W.; Qi, G.H.; Cui, C.F.; Wu, S.G.; Zhang, H.J.; Xu, L.; Wang, J. Effects of dietary Bacillus subtilis supplementation and calcium levels on performance and eggshell quality of laying hens in the late phase of production. Poult. Sci. 2021, 100, 100970. [Google Scholar] [CrossRef]
- Ye, M.; Wei, C.; Khalid, A.; Hu, Q.; Yang, R.; Dai, B.; Cheng, H.; Wang, Z. Effect of Bacillus velezensis to substitute in-feed antibiotics on the production, blood biochemistry and egg quality indices of laying hens. BMC Vet. Res. 2020, 16, 400. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, S.; Pang, Q.; Miao, Z. Bacillus amyloliquefaciens BLCC1-0238 Can Effectively Improve Laying Performance and Egg Quality Via Enhancing Immunity and Regulating Reproductive Hormones of Laying Hens. Probiotics Antimicrob. Proteins 2020, 12, 246–252. [Google Scholar] [CrossRef]
- Chen, J.F.; Xu, M.M.; Kang, K.L.; Tang, S.G.; He, C.Q.; Qu, X.Y.; Guo, S.C. The effects and combinational effects of Bacillus subtilis and montmorillonite on the intestinal health status in laying hens. Poult. Sci. 2020, 99, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Prazdnova, E.V.; Mazanko, M.S.; Chistyakov, V.A.; Denisenko, Y.V.; Makarenko, M.S.; Usatov, A.V.; Bren, A.B.; Tutelyan, A.V.; Komarova, Z.B.; Gorlov, I.F.; et al. Effect of Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B-1895 on the productivity, reproductive aging, and physiological characteristics of hens and roosters. Benef. Microbes 2019, 10, 395–412. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Rudeaux, F.; Kim, I.H. Efficacy of dietary Bacillus subtilis and Bacillus licheniformis supplementation continuously in pullet and lay period on egg production, excreta microflora, and egg quality of Hyline-Brown birds. Poult. Sci. 2019, 98, 4722–4728. [Google Scholar] [CrossRef]
- Xiang, Q.; Wang, C.; Zhang, H.; Lai, W.; Wei, H.; Peng, J. Effects of Different Probiotics on Laying Performance, Egg Quality, Oxidative Status, and Gut Health in Laying Hens. Animals 2019, 9, 1110. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhan, K.; Zhang, M. Effects of the Use of a Combination of Two Bacillus Species on Performance, Egg Quality, Small Intestinal Mucosal Morphology, and Cecal Microbiota Profile in Aging Laying Hens. Probiotics Antimicrob. Proteins 2020, 12, 204–213. [Google Scholar] [CrossRef]
- Bai, K.; Feng, C.; Jiang, L.; Zhang, L.; Zhang, J.; Zhang, L.; Wang, T. Dietary effects of Bacillus subtilis fmbj on growth performance, small intestinal morphology, and its antioxidant capacity of broilers. Poult. Sci. 2018, 97, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.Y.; Wu, Z.L.; Wang, G.Z.; Liu, W.C. The effect of Bacillus amyloliquefaciens on productive performance of laying hens. Ital. J. Anim. Sci. 2018, 17, 436–441. [Google Scholar] [CrossRef] [Green Version]
- Zhan, H.Q.; Dong, X.Y.; Li, L.L.; Zheng, Y.X.; Gong, Y.J.; Zou, X.T. Effects of dietary supplementation with Clostridium butyricum on laying performance, egg quality, serum parameters, and cecal microflora of laying hens in the late phase of production. Poult. Sci. 2019, 98, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Lemos, M.J.; Calixto, L.F.L.; Souza, D.S.; Reis, T.L.; Nascimento, A.A.; Oliveira, C.A. Comparative Effect of The Inclusion of Zootechnical Additives in the Feed of Japanese Quails in Two Productive Phases. An. Acad. Bras. Ciências 2018, 90, 3313–3325. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Peng, C.; Qu, X.; Guo, S.; Chen, J.F.; He, C.; Zhou, X.; Zhu, S. Effects of Bacillus subtilis C-3102 on production, hatching performance, egg quality, serum antioxidant capacity and immune response of laying breeders. J. Anim. Physiol. Anim. Nutr. 2019, 103, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Majidi-Mosleh, A.; Sadeghi, A.; Mousavi, S.; Chamani, M.; Zarei, A. Effects of in Ovo Infusion of Probiotic Strains on Performance Parameters, Jejunal Bacterial Population and Mucin Gene Expression in Broiler Chicken. Rev. Bras. Ciência Avícola 2017, 19, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Hack, M.E.; Mahgoub, S.A.; Alagawany, M.; Ashour, E.A. Improving productive performance and mitigating harmful emissions from laying hen excreta via feeding on graded levels of corn DDGS with or without Bacillus subtilis probiotic. J. Anim. Physiol. Anim. Nutr. 2017, 101, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.R.; Dong, X.F.; Liu, S.; Tong, J.M. Effects of long-term Bacillus subtilis CGMCC 1.921 supplementation on performance, egg quality, and fecal and cecal microbiota of laying hens. Poult. Sci. 2017, 96, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Manafi, M.; Khalaji, S.; Hedayati, M. Assessment of a probiotic Containing Bacillus Subtilis on the Performance and Gut Health of Laying Japanese Quails (Coturnix Coturnix Japonica). Rev. Bras. Ciência Avícola 2016, 18, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Sobczak, A.; Kozłowski, K. The effect of a probiotic preparation containing Bacillus subtilis ATCC PTA-6737 on egg production and physiological parameters of laying hens. Ann. Anim. Sci. 2015, 15, 711–723. [Google Scholar] [CrossRef] [Green Version]
- Forte, C.; Moscati, L.; Acuti, G.; Mugnai, C.; Franciosini, M.P.; Costarelli, S.; Cobellis, G.; Trabalza-Marinucci, M. Effects of dietary Lactobacillus acidophilus and Bacillus subtilis on laying performance, egg quality, blood biochemistry and immune response of organic laying hens. J. Anim. Physiol. Anim. Nutr. 2016, 100, 977–987. [Google Scholar] [CrossRef]
- Ribeiro, V.; Albino, L.F.T.; Rostagno, H.S.; Barreto, S.L.T.; Hannas, M.I.; Harrington, D.; de Araujo, F.A.; Ferreira, H.C.; Ferreira, M.A. Effects of the dietary supplementation of Bacillus subtilis levels on performance, egg quality and excreta moisture of layers. Anim. Feed Sci. Technol. 2014, 209, 142–146. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, Y.; Guo, S.; Tan, J. Effects of Clostridium butyricum and Enterococcus faecium on growth performance, lipid metabolism, and cecal microbiota of broiler chickens. Appl. Microbiol. Biotechnol. 2013, 97, 6477–6488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Xie, Q.M.; Ji, J.; Yang, W.H.; Wu, Y.B.; Li, C.; Ma, J.Y.; Bi, Y.Z. Different combinations of probiotics improve the production performance, egg quality, and immune response of layer hens. Poult. Sci. 2012, 91, 2755–2760. [Google Scholar] [CrossRef]
- Cao, G.T.; Xiao, Y.P.; Yang, C.M.; Chen, A.G.; Liu, T.T.; Zhou, L.; Zhang, L.; Ferket, P.R. Effects of Clostridium butyricum on Growth Performance, Nitrogen Metabolism, Intestinal Morphology and Cecal Microflora in Broiler Chickens. J. Anim. Vet. Adv. 2012, 11, 2665–2671. [Google Scholar] [CrossRef]
- Li, W.F.; Rajput, I.R.; Xu, X.; Li, Y.L.; Lei, J.; Huang, Q.; Wang, M.Q. Effects of Probiotic (Bacillus subtilis) on Laying Performance, Blood Biochemical Properties and Intestinal Microflora of Shaoxing Duck. Int. J. Poult. Sci. 2011, 10, 583–589. [Google Scholar] [CrossRef] [Green Version]
- Aghaii, A.; Chaji, M.; Mohammadabadi, T.; Sari, M. The effect of probiotic supplementation on production performance, egg quality and serum and egg chemical composition of lying hens. J. Anim. Vet. Adv. 2010, 9, 2774–2777. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Xu, C.L.; Ji, C.; Ma, Q.; Hao, K.; Jin, Z.Y.; Li, K. Effects of a dried Bacillus subtilis culture on egg quality. Poult. Sci. 2006, 85, 364–368. [Google Scholar] [CrossRef]
- Mahdavi, A.H.; Rahmani, H.R.; Pourreza, J. Effect of Probiotic Supplements on Egg Quality and Laying Hen’s Performance. Int. J. Poult. Sci. 2005, 4, 488–492. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, M.N.; Ramachandran, R.; Kiess, A.S.; Wamsley, K.G.S.; Mcdaniel, C.D. Impact of in vitro inoculation and dietary supplementation with Bacillus subtilis on sperm quality of aged White Leghorn roosters. J. Appl. Poult. Res. 2018, 27, 304–315. [Google Scholar] [CrossRef]
- Inatomi, T.; Otomaru, K. Effect of dietary probiotics on the semen traits and antioxidative activity of male broiler breeders. Sci. Rep. 2018, 8, 5874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
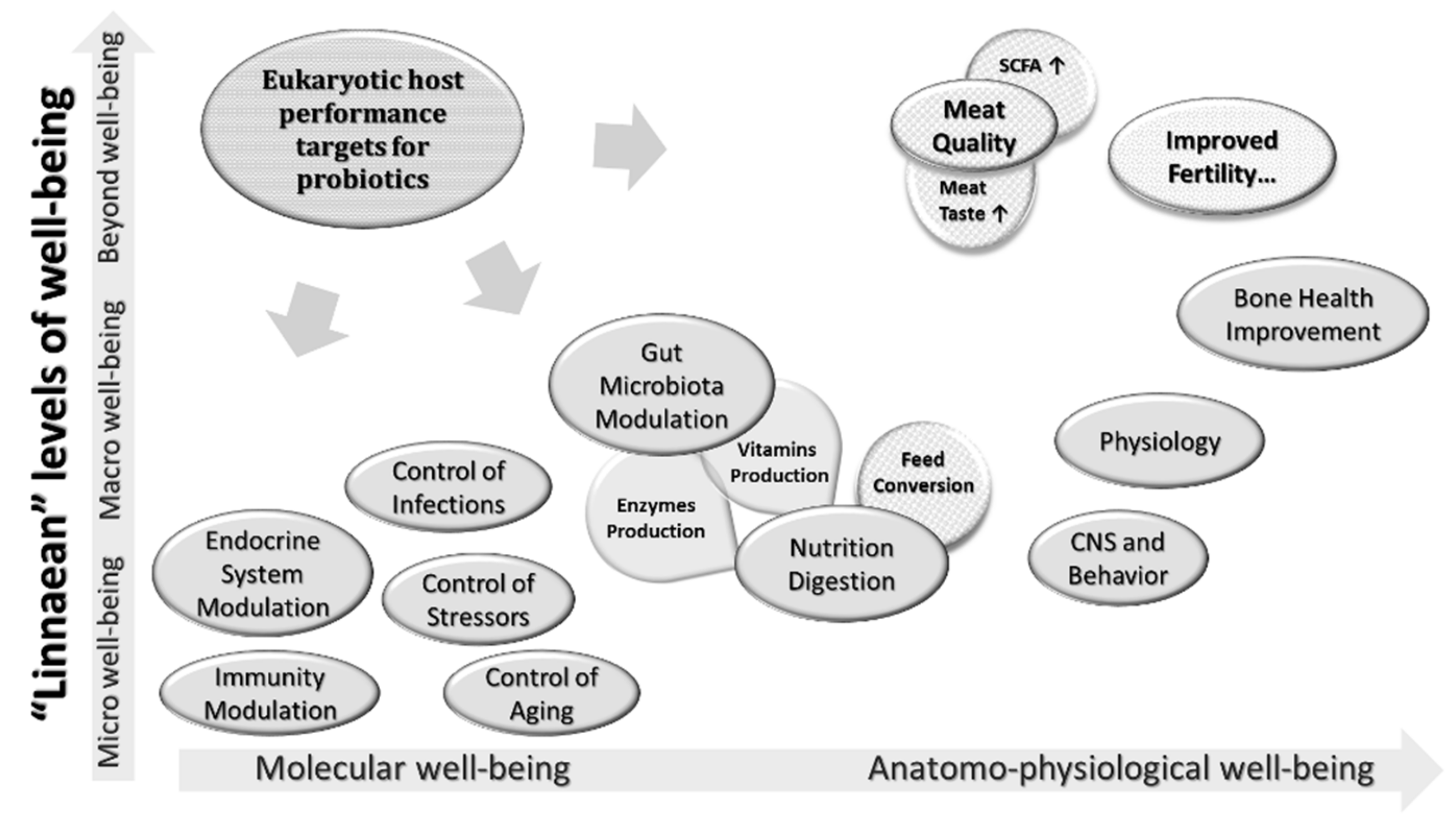
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, I.V.; Algburi, A.; Prazdnova, E.V.; Mazanko, M.S.; Elisashvili, V.; Bren, A.B.; Chistyakov, V.A.; Tkacheva, E.V.; Trukhachev, V.I.; Donnik, I.M.; et al. A Review of the Effects and Production of Spore-Forming Probiotics for Poultry. Animals 2021, 11, 1941. https://doi.org/10.3390/ani11071941
Popov IV, Algburi A, Prazdnova EV, Mazanko MS, Elisashvili V, Bren AB, Chistyakov VA, Tkacheva EV, Trukhachev VI, Donnik IM, et al. A Review of the Effects and Production of Spore-Forming Probiotics for Poultry. Animals. 2021; 11(7):1941. https://doi.org/10.3390/ani11071941
Chicago/Turabian StylePopov, Igor V., Ammar Algburi, Evgeniya V. Prazdnova, Maria S. Mazanko, Vladimir Elisashvili, Anzhelica B. Bren, Vladimir A. Chistyakov, Elizaveta V. Tkacheva, Vladimir I. Trukhachev, Irina M. Donnik, and et al. 2021. "A Review of the Effects and Production of Spore-Forming Probiotics for Poultry" Animals 11, no. 7: 1941. https://doi.org/10.3390/ani11071941
APA StylePopov, I. V., Algburi, A., Prazdnova, E. V., Mazanko, M. S., Elisashvili, V., Bren, A. B., Chistyakov, V. A., Tkacheva, E. V., Trukhachev, V. I., Donnik, I. M., Ivanov, Y. A., Rudoy, D., Ermakov, A. M., Weeks, R. M., & Chikindas, M. L. (2021). A Review of the Effects and Production of Spore-Forming Probiotics for Poultry. Animals, 11(7), 1941. https://doi.org/10.3390/ani11071941