An Overview of the Use of Genotyping Techniques for Assessing Genetic Diversity in Local Farm Animal Breeds
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. General Information with Special Consideration of Investigated Breeds
3.2. Non Target Breeds
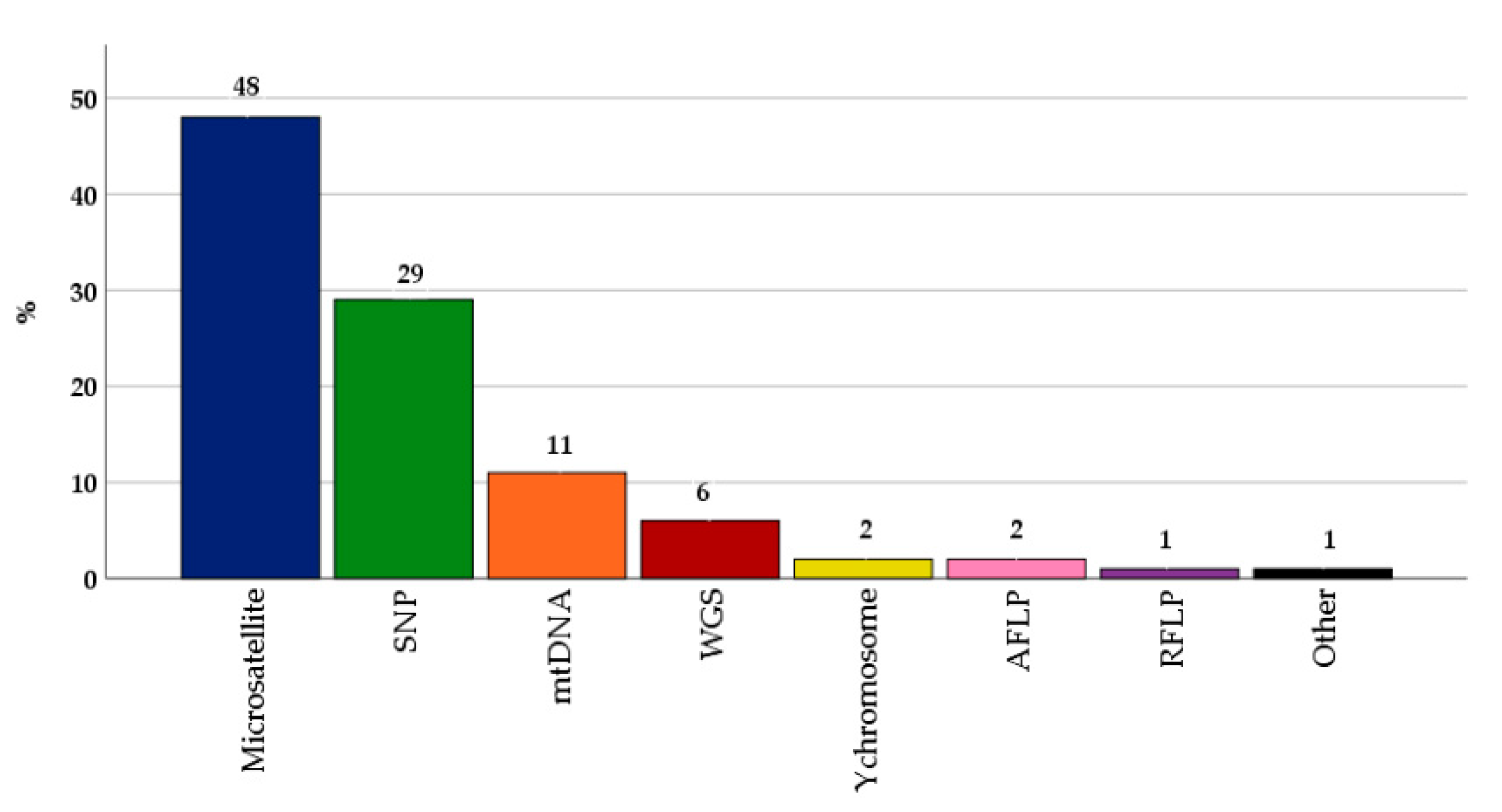
3.3. Genotyping Techniques
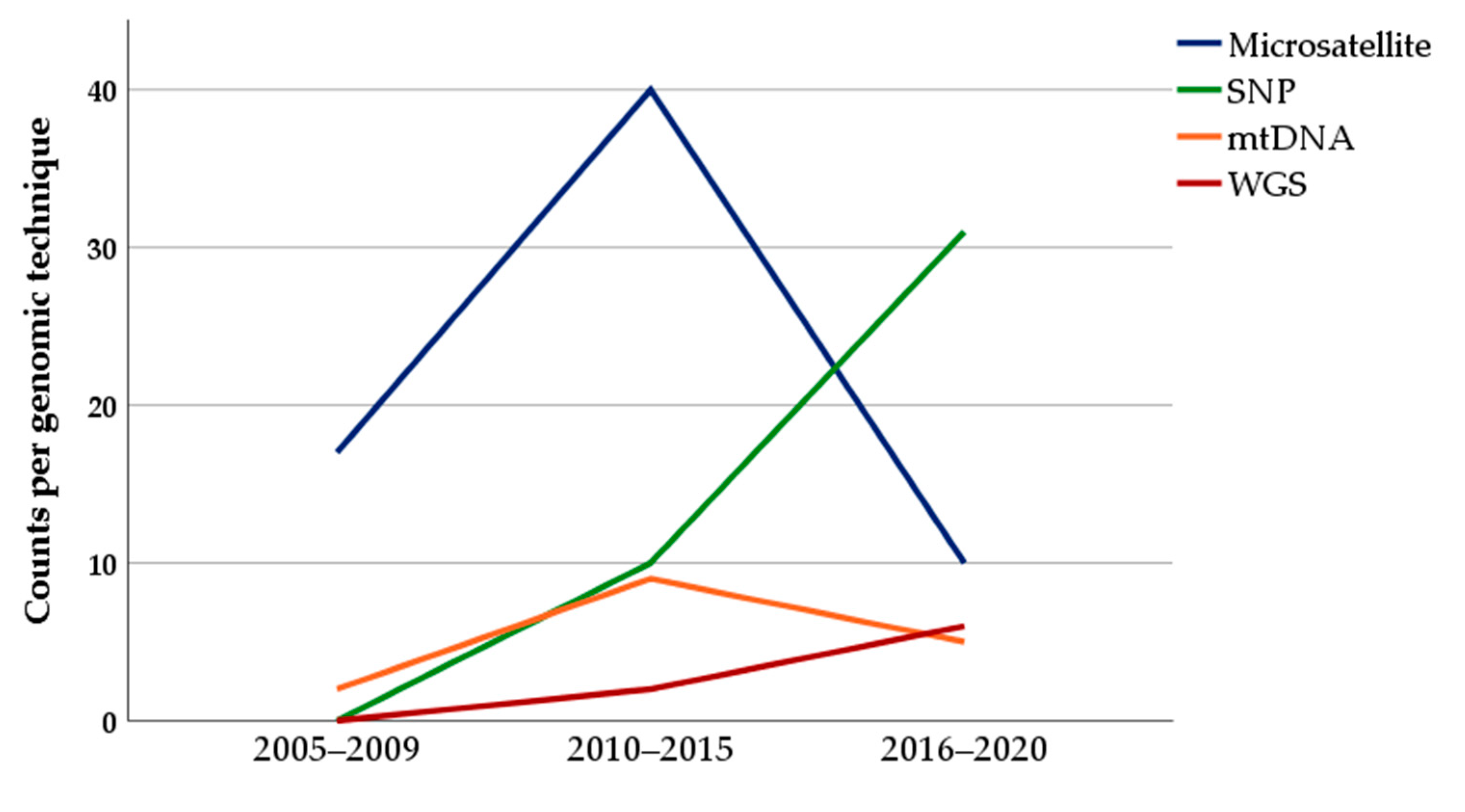
3.4. Changes of the Use of Genotyping Techniques over Time
3.5. Diversity Parameters and Evaluation Software
3.6. Availability of Genomic Data and Phenotype
4. Discussion
4.1. Target and Non Target Breeds
4.2. Genotyping Techniques and Their Changed Use over Time
4.3. Diversity Parameters and Software Programs
4.4. Availability of Genomic Data and Phenotype
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- FAO. The State of the World’s Biodiversity for Food and Agriculture; FAO: Rome, Italy, 2019; Available online: http://www.fao.org/3/CA3129EN/CA3129EN.pdf (accessed on 23 March 2020).
- Zhang, M.; Peng, W.-F.; Hu, X.-J.; Zhao, Y.-X.; Lv, F.-H.; Yang, J. Global genomic diversity and conservation priorities for domestic animals are associated with the economies of their regions of origin. Sci. Rep. 2018, 8, 11677. [Google Scholar] [CrossRef] [Green Version]
- FAO. The Second Report on the State of the World’s Animal Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2015; Available online: http://www.fao.org/3/a-i4787e/index.html (accessed on 23 March 2020).
- Boettcher, P.J.; Tixier-Boichard, M.; Toro, M.A.; Simianer, H.; Eding, H.; Gandini, G.; Joost, S.; Garcia, D.; Colli, L.; Ajmone-Marsan, P. Objectives, criteria and methods for using molecular genetic data in priority setting for conservation of animal genetic resources. Anim. Genet. 2010, 41 (Suppl. 1), 64–77. [Google Scholar] [CrossRef] [Green Version]
- Eusebi, P.G.; Martinez, A.; Cortes, O. Genomic Tools for Effective Conservation of Livestock Breed Diversity. Diversity 2020, 12, 8. [Google Scholar] [CrossRef] [Green Version]
- Yaro, M.; Munyard, K.A.; Stear, M.J.; Groth, D.M. Molecular identification of livestock breeds: A tool for modern conservation biology. Biol. Rev. Camb. Philos. Soc. 2017, 92, 993–1010. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Enciso, M.; Rincón, J.C.; Legarra, A. Sequence- vs. chip-assisted genomic selection: Accurate biological information is advised. Genet. Sel. Evol. 2015, 47, 43. [Google Scholar] [CrossRef] [Green Version]
- Eding, H.; Meuwissen, T.H.E. Marker-based estimates of between and within population kinships for the conservation of genetic diversity. J. Anim. Breed Genet. 2001, 118, 141–159. [Google Scholar] [CrossRef]
- Toro, M.A.; Fernández, J.; Caballero, A. Molecular characterization of breeds and its use in conservation. Livest. Sci. 2009, 120, 174–195. [Google Scholar] [CrossRef]
- Weitzman, M.L. On Diversity. Q. J. Econ. 1992, 107, 363–405. [Google Scholar] [CrossRef]
- Marsjan, P.A.; Oldenbroek, J.K. Molecular markers, a tool for exploring genetic diversity (Section C in part 4). In The State of the World’s Animal Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2007; pp. 359–379. [Google Scholar]
- Caballero, A.; García-Dorado, A. Allelic diversity and its implications for the rate of adaptation. Genetics 2013, 195, 1373–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, T.; Han, J.L.; Guo, J.; Zhao, Q.J.; Fu, B.L.; Pu, Y.B.; He, X.H.; Jeon, J.T.; Guan, W.J.; Ma, Y.-H. Tracing genetic differentiation of Chinese Mongolian sheep using microsatellites. Anim. Genet. 2011, 42, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Zerabruk, M.; Li, M.-H.; Kantanen, J.; Olsaker, I.; Ibeagha-Awemu, E.M.; Erhardt, G.; Vangen, O. Genetic diversity and admixture of indigenous cattle from North Ethiopia: Implications of historical introgressions in the gateway region to Africa. Anim. Genet. 2012, 43, 257–266. [Google Scholar] [CrossRef]
- Zhang, G.X.; Wang, Z.G.; Chen, W.S.; Wu, C.X.; Han, X.; Chang, H.; Zan, L.S.; Li, R.L.; Wang, J.H.; Song, W.T.; et al. Genetic diversity and population structure of indigenous yellow cattle breeds of China using 30 microsatellite markers. Anim. Genet. 2007, 38, 550–559. [Google Scholar] [CrossRef]
- Yilmaz, O.; Cemal, I.; Karaca, O. Genetic diversity in nine native Turkish sheep breeds based on microsatellite analysis. Anim. Genet. 2014, 45, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.; Wiener, P.; Teverson, D.; Haley, C.S.; Hocking, P.M. Characterization of the genetic diversity, structure and admixture of British chicken breeds. Anim. Genet. 2012, 43, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Z.; Chen, S.S.; Chao, T.L.; Ji, Z.B.; Hou, L.; Qin, Z.J.; Wang, J.M. Analysis of genetic diversity of Chinese dairy goats via microsatellite markers. J. Anim. Sci. 2017, 95, 2304–2313. [Google Scholar] [CrossRef] [PubMed]
- Vicente, A.A.; Carolino, M.I.; Sousa, M.C.O.; Ginja, C.; Silva, F.S.; Martinez, A.M.; Vega-Pla, J.L.; Carolino, N.; Gama, L.T. Genetic diversity in native and commercial breeds of pigs in Portugal assessed by microsatellites. J. Anim. Sci. 2008, 86, 2496–2507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahidi, S.M.F.; Tarang, A.R.; Naqvi, A.-N.; Falahati Anbaran, M.; Boettcher, P.; Joost, S.; Colli, L.; Garcia, J.F.; Ajmone-Marsan, P. Investigation of the genetic diversity of domestic Capra hircus breeds reared within an early goat domestication area in Iran. Genet. Sel. Evol. 2014, 46, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahidi, S.M.F.; Faruque, M.O.; Falahati Anbaran, M.; Afraz, F.; Mousavi, S.M.; Boettcher, P.; Joost, S.; Han, J.L.; Colli, L.; Periasamy, K.; et al. Multilocus genotypic data reveal high genetic diversity and low population genetic structure of Iranian indigenous sheep. Anim. Genet. 2016, 47, 463–470. [Google Scholar] [CrossRef]
- Tantia, M.S.; Vijh, R.K.; Mishra, B.; Bharani Kumar, S.T. Genetic diversity among four short stature cattle populations of India. Anim. Genet. Resour. Inf. 2008, 43, 15–23. [Google Scholar] [CrossRef]
- Sollero, B.P.; Paiva, S.R.; Faria, D.A.; Guimarães, S.; Castro, S.; Egito, A.A.; Albuquerque, M.; Piovezan, U.; Bertani, G.R.; Mariante, A.d.S. Genetic diversity of Brazilian pig breeds evidenced by microsatellite markers. Livest. Sci. 2009, 123, 8–15. [Google Scholar] [CrossRef]
- Serrano, M.; Calvo, J.H.; Martínez, M.; Marcos-Carcavilla, A.; Cuevas, J.; González, C.; Jurado, J.J.; deTejada, P.D. Microsatellite based genetic diversity and population structure of the endangered Spanish Guadarrama goat breed. BMC Genet. 2009, 10, 61. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Kishore, A.; Mukesh, M.; Ahlawat, S.; Maitra, A.; Pandey, A.K.; Tantia, M.S. Genetic diversity and relationship of Indian cattle inferred from microsatellite and mitochondrial DNA markers. BMC Genet. 2015, 16, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, T.M.; Patel, J.S.; Bhong, C.D.; Doiphode, A.; Umrikar, U.D.; Parmar, S.S.; Rank, D.N.; Solanki, J.V.; Joshi, C.G. Evaluation of genetic diversity and population structure of West-Central Indian cattle breeds. Anim. Genet. 2013, 44, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Larrañaga, O.; Asadollahpour Nanaei, H.; Montes, I.; Ayatollahi Mehrgardi, A.; Abdolmohammadi, A.; Kharrati-Koopaee, H.; Sohrabi, S.S.; Rendo, F.; Manzano, C.; Estonba, A.; et al. Genetic structure of Iranian indigenous sheep breeds: Insights for conservation. Trop. Anim. Health Prod. 2020. [Google Scholar] [CrossRef]
- Revidatti, M.A.; Delgado Bermejo, J.V.; Gama, L.T.; Landi Periati, V.; Ginja, C.; Alvarez, L.A.; Vega-Pla, J.L.; Martínez, A.M. Genetic characterization of local Criollo pig breeds from the Americas using microsatellite markers. J. Anim. Sci. 2014, 92, 4823–4832. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.H.; Berthouly-Salazar, C.; Tran, X.H.; Chang, W.H.; Crooijmans, R.P.M.A.; Lin, D.Y.; Hoang, V.T.; Lee, Y.P.; Tixier-Boichard, M.; Chen, C.F. Genetic diversity of Vietnamese domestic chicken populations as decision-making support for conservation strategies. Anim. Genet. 2013, 44, 509–521. [Google Scholar] [CrossRef]
- Osei-Amponsah, R.; Kayang, B.B.; Naazie, A.; Osei, Y.D.; Youssao, I.A.K.; Yapi-Gnaore, V.C.; Tixier-Boichard, M.; Rognon, X. Genetic diversity of Forest and Savannah chicken populations of Ghana as estimated by microsatellite markers. Anim. Sci. J. 2010, 81, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Nishimaki, T.; Ibi, T.; Tanabe, Y.; Miyazaki, Y.; Kobayashi, N.; Matsuhashi, T.; Akiyama, T.; Yoshida, E.; Imai, K.; Matsui, M.; et al. The assessment of genetic diversity within and among the eight subpopulations of Japanese Black cattle using 52 microsatellite markers. Anim. Sci. J. 2013, 84, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Nigussie, H.; Mwacharo, J.M.; Osama, S.; Agaba, M.; Mekasha, Y.; Kebede, K.; Abegaz, S.; Pal, S.K. Genetic diversity and matrilineal genetic origin of fat-rumped sheep in Ethiopia. Trop. Anim. Health Prod. 2019, 51, 1393–1404. [Google Scholar] [CrossRef]
- Ngeno, K.; van der Waaij, E.H.; Megens, H.J.; Kahi, A.K.; van Arendonk, J.; Crooijmans, R. Genetic diversity of different indigenous chicken ecotypes using highly polymorphic MHC-linked and non-MHC microsatellite markers. Anim. Genet. Resour. 2015, 56, 1–7. [Google Scholar] [CrossRef]
- Mwambene, P.L.; Katule, A.M.; Chenyambuga, S.W.; Plante, Y.; Mwakilembe, P. Fipa cattle in the southwestern highlands of Tanzania: Molecular characterization. Anim. Genet. Resour. 2012, 51, 31–43. [Google Scholar] [CrossRef]
- Mtileni, B.J.; Muchadeyi, F.C.; Maiwashe, A.; Groeneveld, E.; Groeneveld, L.F.; Dzama, K.; Weigend, S. Genetic diversity and conservation of South African indigenous chicken populations. J. Anim. Breed. Genet. 2011, 128, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Mukesh, M.; Sodhi, M.; Bhatia, S. Microsatellite-based diversity analysis and genetic relationships of three Indian sheep breeds. J. Anim. Breed. Genet. 2006, 123, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Ali, A.S.; Aggarwal, R.; Dixit, S.P.; Kawitkar, V.S.; Dangi, P.S.; Verma, N.K. Genetic diversity and bottleneck analysis of Konkan Kanyal goats. Anim. Genet. Resour. 2012, 50, 43–48. [Google Scholar] [CrossRef]
- Mishra, P.; Ali, A.S.; Dixit, S.P.; Aggarwal, R.; Dangi, P.S.; Tyagi, N.; Dash, S.K.; Verma, N.K. Microsatellite-based genetic evaluation of Ghumusar goats of Orissa, India. Anim. Genet. Resour. 2013, 52, 59–64. [Google Scholar] [CrossRef]
- Michailidou, S.; Kalivas, A.; Ganopoulos, I.; Stea, E.; Michailidis, G.; Tsaftaris, A.; Argiriou, A. A multi-farm assessment of Greek black pig genetic diversity using microsatellite molecular markers. Genet. Mol. Res. 2014, 13, 2752–2765. [Google Scholar] [CrossRef]
- Menéndez, J.; Goyache, F.; Beja-Pereira, A.; Fernández, I.; Menéndez-Arias, N.A.; Godinho, R.; Álvarez, I. Genetic characterisation of the endangered Gochu Asturcelta pig breed using microsatellite and mitochondrial markers: Insights for the composition of the Iberian native pig stock. Livest. Sci. 2016, 187, 162–167. [Google Scholar] [CrossRef]
- Medugorac, I.; Medugorac, A.; Russ, I.; Veit-Kensch, C.E.; Taberlet, P.; Luntz, B.; Mix, H.M.; Förster, M. Genetic diversity of European cattle breeds highlights the conservation value of traditional unselected breeds with high effective population size. Mol. Ecol. 2009, 18, 3394–3410. [Google Scholar] [CrossRef]
- Mateus, J.C.; Russo-Almeida, P.A. Exploring the genetic diversity and substructure of the Portuguese cattle breed “Brava de Lide” using microsatellites. Anim. Genet. Resour. 2014, 55, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Martín-Burriel, I.; Rodellar, C.; Cañón, J.; Cortés, O.; Dunner, S.; Landi, V.; Martínez-Martínez, A.; Gama, L.T.; Ginja, C.; Penedo, M.C.T.; et al. Genetic diversity, structure, and breed relationships in Iberian cattle. J. Anim. Sci. 2011, 89, 893–906. [Google Scholar] [CrossRef] [Green Version]
- Mahgoub, O.; Babiker, H.A.; Kadim, I.T.; Al-Kindi, M.; Hassan, S.; Al-Marzooqi, W.; Eltahir, Y.E.; Al-Abri, M.A.; Al-Khayat, A.; Al-Sinani, K.R.; et al. Disclosing the origin and diversity of Omani cattle. Anim. Genet. 2013, 44, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Leroy, G.; Kayang, B.B.; Youssao, I.A.K.; Yapi-Gnaoré, C.V.; Osei-Amponsah, R.; Loukou, N.E.; Fotsa, J.-C.; Benabdeljelil, K.; Bed’hom, B.; Tixier-Boichard, M.; et al. Gene diversity, agroecological structure and introgression patterns among village chicken populations across North, West and Central Africa. BMC Genet. 2012, 13, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenstra, J.A.; Tigchelaar, J.; Biebach, I.; Hallsson, J.H.; Kantanen, J.; Nielsen, V.H.; Pompanon, F.; Naderi, S.; Rezaei, H.-R.; Saether, N.; et al. Microsatellite diversity of the Nordic type of goats in relation to breed conservation: How relevant is pure ancestry? J. Anim. Breed. Genet. 2017, 134, 78–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koudandé, O.D.; Dossou-Gbété, G.; Mujibi, F.; Kibogo, H.; Mburu, D.; Mensah, G.A.; Hanotte, O.; van Arendonk, J. Genetic diversity and zebu genes introgression in cattle population along the coastal region of the Bight of Benin. Anim. Genet. Resour. Inf. 2009, 44, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Jordana, J.; Ferrando, A.; Marmi, J.; Avellanet, R.; Aranguren-Méndez, J.A.; Goyache, F. Molecular, genealogical and morphometric characterisation of the Pallaresa, a Pyrenean relic cattle breed: Insights for conservation. Livest. Sci. 2010, 132, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Goraga, Z.; Weigend, S.; Brockmann, G. Genetic diversity and population structure of five Ethiopian chicken ecotypes. Anim. Genet. 2012, 43, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Glowatzki-Mullis, M.-L.; Muntwyler, J.; Bäumle, E.; Gaillard, C. Genetic diversity of Swiss sheep breeds in the focus of conservation research. J. Anim. Breed. Genet. 2009, 126, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Ginja, C.; Gama, L.T.; Martínez, A.; Sevane, N.; Martin-Burriel, I.; Lanari, M.R.; Revidatti, M.A.; Aranguren-Méndez, J.A.; Bedotti, D.O.; Ribeiro, M.N.; et al. Genetic diversity and patterns of population structure in Creole goats from the Americas. Anim. Genet. 2017, 48, 315–329. [Google Scholar] [CrossRef]
- Ginja, C.; Gama, L.T.; Cortes, O.; Delgado, J.V.; Dunner, S.; García, D.; Landi, V.; Martín-Burriel, I.; Martínez-Martínez, A.; Penedo, M.C.T.; et al. Analysis of conservation priorities of Iberoamerican cattle based on autosomal microsatellite markers. Genet. Sel. Evol. 2013, 45, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaouar, S.B.S.; Da Silva, A.; Ciani, E.; Kdidi, S.; Aouissat, M.; Dhimi, L.; Lafri, M.; Maftah, A.; Mehtar, N. Admixture and Local Breed Marginalization Threaten Algerian Sheep Diversity. PLoS ONE 2015, 10, e0122667. [Google Scholar] [CrossRef] [Green Version]
- Eusebi, P.G.; Cortés, O.; Dunner, S.; Cañón, J. Genetic diversity of the Mexican Lidia bovine breed and its divergence from the Spanish population. J. Anim. Breed. Genet. 2017, 134, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Eltanany, M.; Philipp, U.; Weigend, S.; Distl, O. Genetic diversity of ten Egyptian chicken strains using 29 microsatellite markers. Anim. Genet. 2011, 42, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Egito, A.A.; Paiva, S.R.; Albuquerque, M.d.S.M.; Mariante, A.S.; Almeida, L.D.; Castro, S.R.; Grattapaglia, D. Microsatellite based genetic diversity and relationships among ten Creole and commercial cattle breeds raised in Brazil. BMC Genet. 2007, 8, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Druml, T.; Salajpal, K.; Dikic, M.; Urosevic, M.; Grilz-Seger, G.; Baumung, R. Genetic diversity, population structure and subdivision of local Balkan pig breeds in Austria, Croatia, Serbia and Bosnia-Herzegovina and its practical value in conservation programs. Genet. Sel. Evol. 2012, 44, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorji, T.; Jianlin, H.; Wafula, P.; Yamamoto, Y.; Sasazaki, S.; Oyama, K.; Hanotte, O.; Lin, B.-Z.; Mannen, H. Sheep genetic diversity in Bhutan using microsatellite markers. Anim. Sci. J. 2010, 81, 145–151. [Google Scholar] [CrossRef]
- Di, R.; Vahidi, S.M.F.; Ma, Y.H.; He, X.H.; Zhao, Q.J.; Han, J.L.; Guan, W.J.; Chu, M.X.; Sun, W.; Pu, Y.P. Microsatellite analysis revealed genetic diversity and population structure among Chinese cashmere goats. Anim. Genet. 2011, 42, 428–431. [Google Scholar] [CrossRef]
- Delgado, J.V.; Martínez, A.M.; Acosta, A.; Alvarez, L.A.; Armstrong, E.; Camacho, E.; Cañón, J.; Cortés, O.; Dunner, S.; Landi, V.; et al. Genetic characterization of Latin-American Creole cattle using microsatellite markers. Anim. Genet. 2012, 43, 2–10. [Google Scholar] [CrossRef]
- Dalvit, C.; de Marchi, M.; Dal Zotto, R.; Zanetti, E.; Meuwissen, T.; Cassandro, M. Genetic characterization of the Burlina cattle breed using microsatellites markers. J. Anim. Breed. Genet. 2008, 125, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Dalvit, C.; de Marchi, M.; Zanetti, E.; Cassandro, M. Genetic variation and population structure of Italian native sheep breeds undergoing in situ conservation. J. Anim. Sci. 2009, 87, 3837–3844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadi, H.; Tibbo, M.; Takahashi, Y.; Nomura, K.; Hanada, H.; Amano, T. Microsatellite analysis reveals high genetic diversity but low genetic structure in Ethiopian indigenous cattle populations. Anim. Genet. 2008, 39, 425–431. [Google Scholar] [CrossRef]
- Ćurković, M.; Ramljak, J.; Ivanković, S.; Mioč, B.; Ivanković, A.; Pavić, V.; Brka, M.; Veit-Kensch, C.; Medugorac, I. The genetic diversity and structure of 18 sheep breeds exposed to isolation and selection. J. Anim. Breed. Genet. 2016, 133, 71–80. [Google Scholar] [CrossRef]
- Cuc, N.T.K.; Simianer, H.; Eding, H.; Tieu, H.V.; Cuong, V.C.; Wollny, C.B.A.; Groeneveld, L.F.; Weigend, S. Assessing genetic diversity of Vietnamese local chicken breeds using microsatellites. Anim. Genet. 2010, 41, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, M.C.; Colombo, E.; Zaniboni, L.; Madeddu, M.; Mosca, F.; Strillacci, M.G.; Longeri, M.; Bagnato, A.; Cerolini, S. Phenotypic and genetic characterization of the Italian bantam chicken breed Mericanel della Brianza. Livest. Sci. 2017, 205, 56–63. [Google Scholar] [CrossRef]
- Chang, C.S.; Chen, C.F.; Berthouly-Salazar, C.; Chazara, O.; Lee, Y.P.; Chang, C.M.; Chang, K.H.; Bed’Hom, B.; Tixier-Boichard, M. A global analysis of molecular markers and phenotypic traits in local chicken breeds in Taiwan. Anim. Genet. 2012, 43, 172–182. [Google Scholar] [CrossRef]
- Chang, W.H.; Chu, H.P.; Jiang, Y.N.; Li, S.H.; Wang, Y.; Chen, C.H.; Chen, K.J.; Lin, C.Y.; Ju, Y.T. Genetic variation and phylogenetics of Lanyu and exotic pig breeds in Taiwan analyzed by nineteen microsatellite markers. J. Anim. Sci. 2009, 87, 1–8. [Google Scholar] [CrossRef]
- Ceccobelli, S.; Di Lorenzo, P.; Lancioni, H.; Monteagudo Ibáñez, L.V.; Tejedor, M.T.; Castellini, C.; Landi, V.; Martínez Martínez, A.; Delgado Bermejo, J.V.; Vega Pla, J.L.; et al. Genetic diversity and phylogeographic structure of sixteen Mediterranean chicken breeds assessed with microsatellites and mitochondrial DNA. Livest. Sci. 2015, 175, 27–36. [Google Scholar] [CrossRef]
- Carvalho, G.M.C.; Paiva, S.R.; Araújo, A.M.; Mariante, A.; Blackburn, H.D. Genetic structure of goat breeds from Brazil and the United States: Implications for conservation and breeding programs. J. Anim. Sci. 2015, 93, 4629–4636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cañón, J.; Tupac-Yupanqui, I.; García-Atance, M.A.; Cortés, O.; García, D.; Fernández, J.; Dunner, S. Genetic variation within the Lidia bovine breed. Anim. Genet. 2008, 39, 439–445. [Google Scholar] [CrossRef]
- Bruno-de-Sousa, C.; Martinez, A.M.; Ginja, C.; Santos-Silva, F.; Carolino, M.I.; Delgado, J.V.; Gama, L.T. Genetic diversity and population structure in Portuguese goat breeds. Livest. Sci. 2011, 135, 131–139. [Google Scholar] [CrossRef]
- Bodzsar, N.; Eding, H.; Revay, T.; Hidas, A.; Weigend, S. Genetic diversity of Hungarian indigenous chicken breeds based on microsatellite markers. Anim. Genet. 2009, 40, 516–523. [Google Scholar] [CrossRef]
- Berthouly, C.; Bed’Hom, B.; Tixier-Boichard, M.; Chen, C.F.; Lee, Y.P.; Laloë, D.; Legros, H.; Verrier, E.; Rognon, X. Using molecular markers and multivariate methods to study the genetic diversity of local European and Asian chicken breeds. Anim. Genet. 2008, 39, 121–129. [Google Scholar] [CrossRef]
- Berthouly, C.; Maillard, J.C.; Pham Doan, L.; Nhu Van, T.; Bed’Hom, B.; Leroy, G.; Hoang Thanh, H.; Laloë, D.; Bruneau, N.; Vu Chi, C.; et al. Revealing fine scale subpopulation structure in the Vietnamese H’Mong cattle breed for conservation purposes. BMC Genet. 2010, 11, 45. [Google Scholar] [CrossRef] [Green Version]
- van Ba, N.; Le Nam, Q.; Do, D.N.; van Hau, N.; Pham, L.D. An assessment of genetic diversity and population structures of fifteen Vietnamese indigenous pig breeds for supporting the decision making on conservation strategies. Trop. Anim. Health Prod. 2019. [Google Scholar] [CrossRef]
- Arora, R.; Bhatia, S.; Jain, A. Morphological and genetic characterization of Ganjam sheep. Anim. Genet. Resour. 2010, 46, 1–9. [Google Scholar] [CrossRef]
- Acosta, A.C.; Uffo, O.; Sanz, A.; Ronda, R.; Osta, R.; Rodellar, C.; Martin-Burriel, I.; Zaragoza, P. Genetic diversity and differentiation of five Cuban cattle breeds using 30 microsatellite loci. J. Anim. Breed. Genet. 2013, 130, 79–86. [Google Scholar] [CrossRef]
- Abebe, A.S.; Mikko, S.; Johansson, A.M. Genetic diversity of five local Swedish chicken breeds detected by microsatellite markers. PLoS ONE 2015, 10, e0120580. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.Z.; Kato, T.; Kaneda, M.; Matsumoto, H.; Sasazaki, S.; Mannen, H. Genetic diversity and structure in Asian native goat analyzed by newly developed SNP markers. Anim. Sci. J. 2013, 84, 579–584. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [Green Version]
- Abied, A.; Bagadi, A.; Bordbar, F.; Pu, Y.; Augustino, S.M.A.; Xue, X.; Xing, F.; Gebreselassie, G.; Mwacharo, J.-L.H.J.M.; Ma, Y.; et al. Genomic Diversity, Population Structure, and Signature of Selection in Five Chinese Native Sheep Breeds Adapted to Extreme Environments. Genes 2020, 11, 494. [Google Scholar] [CrossRef]
- Browett, S.; McHugo, G.; Richardson, I.W.; Magee, D.A.; Park, S.D.E.; Fahey, A.G.; Kearney, J.F.; Correia, C.N.; Randhawa, I.A.S.; MacHugh, D.E. Genomic Characterisation of the Indigenous Irish Kerry Cattle Breed. Front. Genet. 2018, 9, 51. [Google Scholar] [CrossRef] [Green Version]
- Burren, A.; Signer-Hasler, H.; Neuditschko, M.; Tetens, J.; Kijas, J.; Drögemüller, C.; Flury, C. Fine-scale population structure analysis of seven local Swiss sheep breeds using genome-wide SNP data. Anim. Genet. Resour. 2014, 55, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Burren, A.; Neuditschko, M.; Signer-Hasler, H.; Frischknecht, M.; Reber, I.; Menzi, F.; Drögemüller, C.; Flury, C. Genetic diversity analyses reveal first insights into breed-specific selection signatures within Swiss goat breeds. Anim. Genet. 2016, 47, 727–739. [Google Scholar] [CrossRef]
- Cañas-Álvarez, J.J.; González-Rodríguez, A.; Munilla, S.; Varona, L.; Díaz, C.; Baro, J.A.; Altarriba, J.; Molina, A.; Piedrafita, J. Genetic diversity and divergence among Spanish beef cattle breeds assessed by a bovine high-density SNP chip. J. Anim. Sci. 2015, 93, 5164–5174. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, J.; Xiao, Q.; Pan, Y.; Zhang, X.; Lo, L.J.; Xu, N. The genetic diversity and population structures of indigenous pig breeds in Zhejiang Province revealed by GGRS sequencing. Anim. Genet. 2018, 49, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, X.; Cheng, D.; Chen, K.; Fan, Y.; Wu, G.; You, J.; Liu, S.; Mao, H.; Ren, J. Population genetic analyses of seven Chinese indigenous chicken breeds in a context of global breeds. Anim. Genet. 2018, 50, 82–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciani, E.; Crepaldi, P.; Nicoloso, L.; Lasagna, E.; Sarti, F.M.; Moioli, B.; Napolitano, F.; Carta, A.; Usai, G.; D’Andrea, M.; et al. Genome-wide analysis of Italian sheep diversity reveals a strong geographic pattern and cryptic relationships between breeds. Anim. Genet. 2014, 45, 256–266. [Google Scholar] [CrossRef]
- Colli, L.; Milanesi, M.; Talenti, A.; Bertolini, F.; Chen, M.; Crisà, A.; Daly, K.G.; Del Corvo, M.; Guldbrandtsen, B.; Lenstra, J.A.; et al. Genome-wide SNP profiling of worldwide goat populations reveals strong partitioning of diversity and highlights post-domestication migration routes. Genet. Sel. Evol. 2018, 50, 58. [Google Scholar] [CrossRef] [Green Version]
- Deniskova, T.E.; Dotsev, A.V.; Selionova, M.I.; Kunz, E.; Medugorac, I.; Reyer, H.; Wimmers, K.; Barbato, M.; Traspov, A.A.; Brem, G.; et al. Population structure and genetic diversity of 25 Russian sheep breeds based on whole-genome genotyping. Genet. Sel. Evol. 2018, 50, 29. [Google Scholar] [CrossRef] [Green Version]
- Deniskova, T.; Dotsev, A.; Lushihina, E.; Shakhin, A.; Kunz, E.; Medugorac, I.; Reyer, H.; Wimmers, K.; Khayatzadeh, N.; Sölkner, J.; et al. Population Structure and Genetic Diversity of Sheep Breeds in the Kyrgyzstan. Front. Genet. 2019, 10, 1311. [Google Scholar] [CrossRef] [Green Version]
- Edea, Z.; Dessie, T.; Dadi, H.; Do, K.-T.; Kim, K.-S. Genetic Diversity and Population Structure of Ethiopian Sheep Populations Revealed by High-Density SNP Markers. Front. Genet. 2017, 8, 218. [Google Scholar] [CrossRef] [Green Version]
- Eydivandi, S.; Sahana, G.; Momen, M.; Moradi, M.H.; Schönherz, A.A. Genetic diversity in Iranian indigenous sheep vis-à-vis selected exogenous sheep breeds and wild mouflon. Anim. Genet. 2020, 51, 772–787. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Medrano, J.M.; Megens, H.-J.; Groenen, M.A.M.; Ramis, G.; Bosse, M.; Pérez-Enciso, M.; Crooijmans, R.P.M.A. Conservation genomic analysis of domestic and wild pig populations from the Iberian Peninsula. BMC Genet. 2013, 14, 106. [Google Scholar] [CrossRef] [Green Version]
- Iso-Touru, T.; Tapio, M.; Vilkki, J.; Kiseleva, T.; Ammosov, I.; Ivanova, Z.; Popov, R.; Ozerov, M.; Kantanen, J. Genetic diversity and genomic signatures of selection among cattle breeds from Siberia, eastern and northern Europe. Anim. Genet. 2016, 47, 647–657. [Google Scholar] [CrossRef]
- Johansson, A.M.; Nelson, R.M. Characterization of genetic diversity and gene mapping in two Swedish local chicken breeds. Front. Genet. 2015, 6, 44. [Google Scholar] [CrossRef] [Green Version]
- Karimi, K.; Esmailizadeh Koshkoiyeh, A.; Asadi Fozi, M.; Porto-Neto, L.R.; Gondro, C. Prioritization for conservation of Iranian native cattle breeds based on genome-wide SNP data. Conserv. Genet. 2016, 17, 77–89. [Google Scholar] [CrossRef]
- Khanyile, K.S.; Dzomba, E.F.; Muchadeyi, F.C. Population genetic structure, linkage disequilibrium and effective population size of conserved and extensively raised village chicken populations of Southern Africa. Front. Genet. 2015, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Kumar, C.; Song, S.; Dewani, P.; Kumar, M.; Parkash, O.; Ma, Y.; Malhi, K.K.; Yang, N.; Mwacharo, J.M.; He, X.; et al. Population structure, genetic diversity and selection signatures within seven indigenous Pakistani goat populations. Anim. Genet. 2018, 49, 592–604. [Google Scholar] [CrossRef]
- Lukić, B.; Ferenčaković, M.; Šalamon, D.; Čačić, M.; Orehovački, V.; Iacolina, L.; Curik, I.; Cubric-Curik, V. Conservation Genomic Analysis of the Croatian Indigenous Black Slavonian and Turopolje Pig Breeds. Front. Genet. 2020, 11, 1212. [Google Scholar] [CrossRef]
- Lwin, M.; Mon, S.L.Y.; Yamanaka, H.; Nagano, Y.; Mannen, H.; Faruque, M.O.; Kawabe, K.; Okamoto, S.; Shimogiri, T. Genetic diversities and population structures of four popular Myanmar local cattle breeds. Anim. Sci. J. 2018, 89, 1648–1655. [Google Scholar] [CrossRef]
- Makina, S.O.; Muchadeyi, F.C.; van Marle-Köster, E.; MacNeil, M.D.; Maiwashe, A. Genetic diversity and population structure among six cattle breeds in South Africa using a whole genome SNP panel. Front. Genet. 2014, 5, 333. [Google Scholar] [CrossRef] [Green Version]
- Manunza, A.; Noce, A.; Serradilla, J.M.; Goyache, F.; Martínez, A.; Capote, J.; Delgado, J.V.; Jordana, J.; Muñoz, E.; Molina, A.; et al. A genome-wide perspective about the diversity and demographic history of seven Spanish goat breeds. Genet. Sel. Evol. 2016, 48, 52. [Google Scholar] [CrossRef] [Green Version]
- Mastrangelo, S.; Di Gerlando, R.; Tolone, M.; Tortorici, L.; Sardina, M.T.; Portolano, B. Genome wide linkage disequilibrium and genetic structure in Sicilian dairy sheep breeds. BMC Genet. 2014, 15, 108. [Google Scholar] [CrossRef] [Green Version]
- Mastrangelo, S.; Portolano, B.; Di Gerlando, R.; Ciampolini, R.; Tolone, M.; Sardina, M.T. Genome-wide analysis in endangered populations: A case study in Barbaresca sheep. Animal 2017, 11, 1107–1116. [Google Scholar] [CrossRef]
- Mdladla, K.; Dzomba, E.F.; Huson, H.J.; Muchadeyi, F.C. Population genomic structure and linkage disequilibrium analysis of South African goat breeds using genome-wide SNP data. Anim. Genet. 2016, 47, 471–482. [Google Scholar] [CrossRef]
- Meyermans, R.; Gorssen, W.; Wijnrocx, K.; Lenstra, J.A.; Vellema, P.; Buys, N.; Janssens, S. Unraveling the genetic diversity of Belgian Milk Sheep using medium-density SNP genotypes. Anim. Genet. 2020, 51, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Mujibi, F.D.; Okoth, E.; Cheruiyot, E.K.; Onzere, C.; Bishop, R.P.; Fèvre, E.M.; Thomas, L.; Masembe, C.; Plastow, G.; Rothschild, M. Genetic diversity, breed composition and admixture of Kenyan domestic pigs. PLoS ONE 2018, 13, e0190080. [Google Scholar] [CrossRef]
- Nicoloso, L.; Bomba, L.; Colli, L.; Negrini, R.; Milanesi, M.; Mazza, R.; Sechi, T.; Frattini, S.; Talenti, A.; Coizet, B.; et al. Genetic diversity of Italian goat breeds assessed with a medium-density SNP chip. Genet. Sel. Evol. 2015, 47, 62. [Google Scholar] [CrossRef] [PubMed]
- Oget, C.; Servin, B.; Palhière, I. Genetic diversity analysis of French goat populations reveals selective sweeps involved in their differentiation. Anim. Genet. 2019, 50, 54–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papachristou, D.; Koutsouli, P.; Laliotis, G.P.; Kunz, E.; Upadhyay, M.; Seichter, D.; Russ, I.; Gjoko, B.; Kostaras, N.; Bizelis, I.; et al. Genomic diversity and population structure of the indigenous Greek and Cypriot cattle populations. Genet. Sel. Evol. 2020, 52. [Google Scholar] [CrossRef]
- Pertoldi, C.; Purfield, D.C.; Berg, P.; Jensen, T.H.; Bach, O.S.; Vingborg, R.; Kristensen, T.N. Genetic characterization of a herd of the endangered Danish Jutland cattle. J. Anim. Sci. 2014, 92, 2372–2376. [Google Scholar] [CrossRef]
- Rochus, C.M.; Jonas, E.; Johansson, A.M. Population structure of five native sheep breeds of Sweden estimated with high density SNP genotypes. BMC Genet. 2020, 21, 27. [Google Scholar] [CrossRef]
- Roberts, K.S.; Lamberson, W.R. Relationships among and variation within rare breeds of swine. J. Anim. Sci. 2015, 93, 3810–3813. [Google Scholar] [CrossRef]
- Sermyagin, A.A.; Dotsev, A.V.; Gladyr, E.A.; Traspov, A.A.; Deniskova, T.E.; Kostyunina, O.V.; Reyer, H.; Wimmers, K.; Barbato, M.; Paronyan, I.A.; et al. Whole-genome SNP analysis elucidates the genetic structure of Russian cattle and its relationship with Eurasian taurine breeds. Genet. Sel. Evol. 2018, 50, 37. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Lee, S.-H.; Lim, D.; Chai, H.-H.; Choi, B.-H.; Cho, Y. A genome-wide assessment of genetic diversity and population structure of Korean native cattle breeds. BMC Genet. 2016, 17, 139. [Google Scholar] [CrossRef] [Green Version]
- Traspov, A.; Deng, W.; Kostyunina, O.; Ji, J.; Shatokhin, K.; Lugovoy, S.; Zinovieva, N.; Yang, B.; Huang, L. Population structure and genome characterization of local pig breeds in Russia, Belorussia, Kazakhstan and Ukraine. Genet. Sel. Evol. 2016, 48, 16. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, M.; Eriksson, S.; Mikko, S.; Strandberg, E.; Stålhammar, H.; Groenen, M.A.M.; Crooijmans, R.P.M.A.; Andersson, G.; Johansson, A.M. Genomic relatedness and diversity of Swedish native cattle breeds. Genet. Sel. Evol. 2019, 51, 56. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Wang, X.; Ni, L.; Zhang, W.; Lu, C.; Zhao, X.; Zhao, X.; Ren, J. Genome-wide genotyping uncovers genetic diversity, phylogeny, signatures of selection, and population structure of Chinese Jiangquhai pigs in a global perspective1. J. Anim. Sci. 2019, 97, 1491–1500. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, X.; Zhang, Y.; Zhao, Y.; Zhang, J.; Jia, Y.; Zhu, B.; Xu, L.; Zhang, L.; Gao, H.; et al. Genome-wide assessment of genetic diversity and population structure insights into admixture and introgression in Chinese indigenous cattle. BMC Genet. 2018, 19, 114. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Nie, C.; Li, X.; Ning, Z.; Chen, Y.; Jia, Y.; Han, J.; Wang, L.; Lv, X.; Yang, W.; et al. Genome-Wide Population Genetic Analysis of Commercial, Indigenous, Game, and Wild Chickens Using 600K SNP Microarray Data. Front. Genet. 2020, 11, 543294. [Google Scholar] [CrossRef]
- Agaviezor, B.O.; Adefenwa, M.A.; Peters, S.O.; Yakubu, A.; Adebambo, O.A.; Ozoje, M.O.; Ikeobi, C.; Ilori, B.M.; Wheto, M.; Ajayi, O.O.; et al. Genetic diversity analysis of the mitochondrial D-loop of Nigerian indigenous sheep. Anim. Genet. Resour. 2012, 50, 13–20. [Google Scholar] [CrossRef]
- Amills, M.; Ramírez, O.; Tomàs, A.; Badaoui, B.; Marmi, J.; Acosta, J.; Sànchez, A.; Capote, J. Mitochondrial DNA diversity and origins of South and Central American goats. Anim. Genet. 2009, 40, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Berthouly-Salazar, C.; Rognon, X.; Van, T.N.; Gély, M.; Chi, C.V.; Tixier-Boichard, M.; Bed’Hom, B.; Bruneau, N.; Verrier, E.; Maillard, J.C.; et al. Vietnamese chickens: A gate towards Asian genetic diversity. BMC Genet. 2010, 11, 53. [Google Scholar] [CrossRef]
- Bemji, M.N.; Awotunde, E.O.; Olowofeso, O.; James, I.J.; Oduguwa, B.O.; Okwelum, N.; Osinowo, O.A. Maintenance of mtDNA diversity in Kalahari Red goat of South Africa imported to Nigeria. Anim. Genet. Resour. 2014, 55, 39–46. [Google Scholar] [CrossRef]
- Benjelloun, B.; Alberto, F.J.; Streeter, I.; Boyer, F.; Coissac, E.; Stucki, S.; BenBati, M.; Ibnelbachyr, M.; Chentouf, M.; Bechchari, A.; et al. Characterizing neutral genomic diversity and selection signatures in indigenous populations of Moroccan goats (Capra hircus) using WGS data. Front. Genet. 2015, 6, 107. [Google Scholar] [CrossRef]
- Mannen, H.; Yonesaka, R.; Noda, A.; Shimogiri, T.; Oshima, I.; Katahira, K.; Kanemaki, M.; Kunieda, T.; Inayoshi, Y.; Mukai, F.; et al. Low mitochondrial DNA diversity of Japanese Polled and Kuchinoshima feral cattle. Anim. Sci. J. 2017, 88, 739–744. [Google Scholar] [CrossRef]
- Okpeku, M.; Peters, S.O.; Imumorin, I.G.; Caires, K.C.; Sharma, V.K.; Wheto, M.; Tamang, R.; Adenaike, A.S.; Ozoje, M.O.; Thangaraj, K. Mitochondrial DNA hypervariable region 1 diversity in Nigerian goats. Anim. Genet. Resour. 2016, 59, 47–54. [Google Scholar] [CrossRef]
- Pardeshi, V.C.; Kadoo, N.Y.; Sainani, M.N.; Meadows, J.R.S.; Kijas, J.W.; Gupta, V.S. Mitochondrial haplotypes reveal a strong genetic structure for three Indian sheep breeds. Anim. Genet. 2007, 38, 460–466. [Google Scholar] [CrossRef]
- Quan, J.; Gao, C.; Cai, Y.; Ge, Q.; Jiao, T.; Zhao, S. Population genetics assessment model reveals priority protection of genetic resources in native pig breeds in China. Glob. Ecol. Conserv. 2020, 21, e00829. [Google Scholar] [CrossRef]
- Wang, G.Z.; Pi, X.S.; Ji, Z.B.; Qin, Z.J.; Hou, L.; Chao, T.L.; Wang, J.M. Investigation of the diversity and origins of Chinese dairy goats via the mitochondrial DNA D-loop. J. Anim. Sci. 2015, 93, 949–955. [Google Scholar] [CrossRef]
- Qiao, R.; Li, X.; Han, X.; Wang, K.; Lv, G.; Ren, G. Population structure and genetic diversity of four Henan pig populations. Anim. Genet. 2019, 50, 262–265. [Google Scholar] [CrossRef] [Green Version]
- Qin, M.; Li, C.; Li, Z.; Chen, W.; Zeng, Y. Genetic Diversities and Differentially Selected Regions Between Shandong Indigenous Pig Breeds and Western Pig Breeds. Front. Genet. 2020, 10, 1351. [Google Scholar] [CrossRef] [PubMed]
- Reimer, C.; Ha, N.-T.; Sharifi, A.R.; Geibel, J.; Mikkelsen, L.F.; Schlather, M.; Weigend, S.; Simianer, H. Assessing breed integrity of Göttingen Minipigs. BMC Genom. 2020, 21, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Chen, Q.; Yang, Y.; Liao, R.; Zhao, J.; Zhang, Z.; Chen, Z.; Zhang, X.; Xue, M.; Yang, H.; et al. Genetic diversity and population structure of six Chinese indigenous pig breeds in the Taihu Lake region revealed by sequencing data. Anim. Genet. 2015, 46, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Weldenegodguad, M.; Popov, R.; Pokharel, K.; Ammosov, I.; Ming, Y.; Ivanova, Z.; Kantanen, J. Whole-Genome Sequencing of Three Native Cattle Breeds Originating From the Northernmost Cattle Farming Regions. Front. Genet. 2018, 9, 728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Sun, H.; Lu, S.; Gou, X.; Yan, D.; Xu, Z.; Zhang, Z.; Qadri, Q.R.; Zhang, Z.; Wang, Z.; et al. Genetic Diversity and Selection Signatures Within Diannan Small-Ear Pigs Revealed by Next-Generation Sequencing. Front. Genet. 2020, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Han, W.; Tang, H.; Li, G.; Zhang, M.; Xu, R.; Liu, Y.; Yang, T.; Li, W.; Zou, J.; et al. Genomic diversity dynamics in conserved chicken populations are revealed by genome-wide SNPs. BMC Genom. 2018, 19, 598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Cortes, O.; Tupac-Yupanqui, I.; Dunner, S.; Fernández, J.; Cañón, J. Y chromosome genetic diversity in the Lidia bovine breed: A highly fragmented population. J. Anim. Breed. Genet. 2011, 128, 491–496. [Google Scholar] [CrossRef]
- Xia, X.; Yao, Y.; Li, C.; Zhang, F.; Qu, K.; Chen, H.; Huang, B.; Lei, C. Genetic diversity of Chinese cattle revealed by Y-SNP and Y-STR markers. Anim. Genet. 2019, 50, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Yeh, F.C.; Boyle, T.J.B. Population genetic analysis of co-dominant and dominant markers and quantitative traits. Belg. J. Bot. 1997, 129, 157. [Google Scholar]
- Felsenstein, J. PHYLIP—Phylogeny Inference Package (Version 3.2). Cladistics 1989, 5, 164–166. [Google Scholar]
- SanCristobal, M.; Chevalet, C.; Peleman, J.; Heuven, H.; Brugmans, B.; van Schriek, M.; Joosten, R.; Rattink, A.P.; Harlizius, B.; Groenen, M.A.M.; et al. Genetic diversity in European pigs utilizing amplified fragment length polymorphism markers. Anim. Genet. 2006, 37, 232–238. [Google Scholar] [CrossRef]
- de Marchi, M.; Dalvit, C.; Targhetta, C.; Cassandro, M. Assessing genetic diversity in indigenous Veneto chicken breeds using AFLP markers. Anim. Genet. 2006, 37, 101–105. [Google Scholar] [CrossRef]
- Mekchay, S.; Supakankul, P.; Assawamakin, A.; Wilantho, A.; Chareanchim, W.; Tongsima, S. Population structure of four Thai indigenous chicken breeds. BMC Genet. 2014, 15, 40. [Google Scholar] [CrossRef] [Green Version]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak-Filipek, K.; Sawicka-Zugaj, W.; Litwińczuk, Z.; Chabuz, W.; Šveistienė, R.; Bulla, J. Assessment of the genetic structure of Central European cattle breeds based on functional gene polymorphism. Glob. Ecol. Conserv. 2019, 17, e00525. [Google Scholar] [CrossRef]
- Giovambattista, G.; Moe, K.K.; Polat, M.; Borjigin, L.; Hein, S.T.; Moe, H.H.; Takeshima, S.-N.; Aida, Y. Characterization of bovine MHC DRB3 diversity in global cattle breeds, with a focus on cattle in Myanmar. BMC Genet. 2020, 21, 95. [Google Scholar] [CrossRef]
- Besbes, B.; Tixier-Boichard, M.; Hoffmann, I.; Jain, G.L. Future trends for poultry genetic resources. In Poultry in the 21st Century Avian Inluenca and Beyond; FAO: Rome, Italy, 2007; pp. 299–323. [Google Scholar]
- Peripolli, E.; Munari, D.P.; Silva, M.V.G.B.; Lima, A.L.F.; Irgang, R.; Baldi, F. Runs of homozygosity: Current knowledge and applications in livestock. Anim. Genet. 2017, 48, 255–271. [Google Scholar] [CrossRef]
- Nait Saada, J.; Kalantzis, G.; Shyr, D.; Cooper, F.; Robinson, M.; Gusev, A.; Palamara, P.F. Identity-by-descent detection across 487,409 British samples reveals fine scale population structure and ultra-rare variant associations. Nat. Commun. 2020, 11, 6130. [Google Scholar] [CrossRef]
- Browning, S.R.; Browning, B.L. High-resolution detection of identity by descent in unrelated individuals. Am. J. Hum. Genet. 2010, 86, 526–539. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef]
- Groeneveld, L.F.; Lenstra, J.A.; Eding, H.; Toro, M.A.; Scherf, B.; Pilling, D.; Negrini, R.; Finlay, E.K.; Jianlin, H.; Groeneveld, E.; et al. Genetic diversity in farm animals—A review. Anim. Genet. 2010, 41 (Suppl. 1), 6–31. [Google Scholar] [CrossRef] [Green Version]
| Parameter | n | % | Software |
|---|---|---|---|
| Wright’s F-statistics | 61 | 90 | Arlequin, Cervus, FSTAT, GDA, GenAlEx, Genepop, Genetix, HP-Rare, MolKin, POPGENE, Populations, SAS |
| Observed Heterozygosity | 58 | 85 | Arlequin, Cervus, GenAlEx, Genetix, FSTAT, Microsatellite Toolkit, MolKin, PHYLIP, POPGENE |
| Expected Heterozygosity | 58 | 85 | Arlequin, Cervus, FSTAT, GenAlEx, Genetix, Microsatellite Toolkit, MolKin, POPGENE, PHYLIP |
| Population structure/Admixture | 51 | 75 | BAPS, CLUMPP, Distruct, Genetix, Leadmix, Structure |
| Genetic distances | 49 | 72 | Arlequin, Dispan, Genetix, MolKin, Phase, PHYLIP, POPGENE, Populations |
| Effective/mean number of alleles | 48 | 71 | Arlequin, FSTAT, GenAlEx, Genetix, Microsatellite Toolkit, MolKin, POPGENE |
| Hardy–Weinberg equilibrium test | 48 | 71 | Arlequin, Cervus, GenAlEx, Genepop, POPGENE, SAS |
| Neighbor-joining-/phylogenetic tree | 37 | 54 | Dispan, Mega, PHYLIP, r, SplitsTree |
| Allele frequencies | 36 | 53 | Cervus, FSTAT, GenAlEx, Genetix, Genepop, Microsatellite Toolkit, MolKin, Populations |
| Allelic richness | 28 | 41 | FSTAT, GenAlEx, HP-RARE, POPGENE |
| Polymorphic information content | 23 | 34 | Cervus, Excel, Microsatellite Toolkit, MolKin |
| Analysis of molecular variance | 16 | 24 | Arlequin, GenAlEx |
| Principal component analysis | 15 | 22 | Fortran, GenAlEx, MVSP, r, SAS, SPSS, XLSTAT |
| Private alleles | 12 | 18 | FSTAT, GenAlEx, GDA, HP-RARE, Microsatellite Toolkit, Populations |
| Linkage disequilibrium | 10 | 15 | Genepop, SAS |
| Null alleles | 8 | 12 | Cervus, FreeNA, Micro-Checker |
| Genetic relationships/coancestry | 8 | 12 | Admixture, Genetix, MolKin, r |
| Gene diversity | 5 | 7 | FSTAT, Genetix, Microsatellite Toolkit |
| Proportion of shared alleles | 5 | 7 | Microstat |
| Effective population size | 4 | 6 | Cervus, GenAlEx, POPGENE |
| Multidimensional scaling | 4 | 6 | r, DARwin, GenAlEx |
| Allelic diversity per locus | 3 | 4 | Microsatellite Toolkit, MolKin |
| Multiple co-inertia analysis | 2 | 3 | r |
| Percentage of polymorphic loci | 1 | 1 | POPGENE |
| * References | [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80] | ||
| Parameter | n | % | Software |
|---|---|---|---|
| Population structure/Admixture | 35 | 85 | Admixture, fastSTRUCTURE, Python, Structure, TreeMix |
| Wright’s F-statistics | 32 | 78 | Arlequin, Genepop, Golden Helix SNP Variation Suite, Power marker, Plink, r, VCFtools |
| Neighbor net/ neighbor-joining-/max. likelihood tree | 28 | 68 | Arlequin, hapFLK, Mega, PHYLIP, r, RAxML, SplitsTree, TreeMix |
| FROH/other inbreeding coefficients than FIS | 28 | 68 | Arlequin, Haploview, Plink, r |
| Principal component analysis | 26 | 63 | Eigensoft, Eigenstrat, GCTA, Golden Helix SNP variation Suite, Plink, r |
| Linkage disequilibrium | 26 | 63 | Haploview, Plink, r, SNeP |
| Expected heterozygosity | 26 | 63 | Arlequin, Plink, r |
| Observed heterozygosity | 23 | 56 | Arlequin, Plink, r |
| Effective population size | 21 | 51 | NeESTIMATOR, Plink, r, SNeP |
| Genetic distances | 20 | 49 | Arlequin, hapFLK, Genepop, PHYLIP, Plink, Power marker, r |
| Multidimensional scaling | 15 | 37 | Haploview, Plink, r, |
| relationship/ coancestry | 11 | 27 | Admixture, GCTA, Haploview, Plink, r, |
| Allelic richness | 10 | 24 | Adze, r |
| Analysis of molecular variance | 7 | 17 | Arlequin |
| Proportion of polymorphic markers/loci | 6 | 15 | Plink, r |
| Allele frequency | 5 | 12 | Plink, Golden Helix SNP variation Suite |
| Hardy–Weinberg equilibrium test | 4 | 10 | Plink |
| Proportion of shared alleles | 3 | 7 | Plink |
| Private alleles | 1 | 2 | |
| Total/mean number of alleles | 1 | 2 | |
| * References | [82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122] | ||
| Parameter | n | % | Software |
|---|---|---|---|
| Neighbor-joining-/phylogenetic/max. likelihood tree | 16 | 100 | Cipres, MEGA, MrBAYES, Network, Populations, SplitsTree |
| Haplotype diversity | 13 | 81 | Arlequin, DnaSP, SPSS, Vcftools |
| Nucleotide diversity | 13 | 81 | Arlequin, DnaSP, SPSS |
| Genetic distances | 6 | 38 | Arlequin, DnaSP, MEGA, Populations |
| Wrights or Fu’s F-statistics | 5 | 31 | Arlequin, Plink, r |
| Analysis of molecular variance | 4 | 25 | Arlequin, SAMOVA |
| Number of polymorphic sites | 3 | 19 | DnaSP |
| Principal component analysis | 3 | 19 | Plink, r, SAS, SPSS |
| Observed heterozygosity | 2 | 13 | Vcftools |
| Allele frequencies | 2 | 13 | Genetix, Vcftools |
| Relatedness | 2 | 13 | MEGA, Vcftools |
| Genetic diversity | 2 | 13 | DnaSP |
| Expected heterozygosity | 1 | 6 | Genetix |
| Linkage disequilibrium | 1 | 6 | Vcftools |
| Multidimensional scaling | 1 | 6 | XLSTAT |
| Multiple co-inertia analysis | 1 | 6 | r |
| * References | [25,32,40,67,69,75,123,124,125,126,127,128,129,130,131,132] | ||
| Parameter | n | % | Software |
|---|---|---|---|
| Principal component analysis | 7 | 100 | Eigensoft, GCTA, Plink |
| Neighbor-joining-/phylogenetic tree | 5 | 71 | MEGA, PHYLIP |
| Linkage disequilibrium | 5 | 71 | Plink, Vcftools |
| Population structure | 4 | 57 | Admixture, Structure |
| Expected heterozygosity | 3 | 43 | Arlequin, Plink |
| Observed heterozygosity | 3 | 43 | Arlequin, Plink |
| Genetic distances | 3 | 43 | FSTAT, MEGA, Plink |
| Wright’s F-statistics | 3 | 43 | Arlequin, BioPerl, FSTAT, Vcftools |
| Allelic richness | 3 | 38 | Adze |
| Nucleotide diversity | 2 | 29 | BioPerl, Vcftools |
| Proportion of polymorphic sites/marker | 2 | 29 | BioPerl, Plink |
| Effective population size | 2 | 29 | Plink |
| Allele frequencies | 1 | 14 | FSTAT |
| FROH/Inbreeding coefficients other than FIS | 1 | 14 | Plink |
| * References | [133,134,135,136,137,138,139] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olschewsky, A.; Hinrichs, D. An Overview of the Use of Genotyping Techniques for Assessing Genetic Diversity in Local Farm Animal Breeds. Animals 2021, 11, 2016. https://doi.org/10.3390/ani11072016
Olschewsky A, Hinrichs D. An Overview of the Use of Genotyping Techniques for Assessing Genetic Diversity in Local Farm Animal Breeds. Animals. 2021; 11(7):2016. https://doi.org/10.3390/ani11072016
Chicago/Turabian StyleOlschewsky, Anna, and Dirk Hinrichs. 2021. "An Overview of the Use of Genotyping Techniques for Assessing Genetic Diversity in Local Farm Animal Breeds" Animals 11, no. 7: 2016. https://doi.org/10.3390/ani11072016
APA StyleOlschewsky, A., & Hinrichs, D. (2021). An Overview of the Use of Genotyping Techniques for Assessing Genetic Diversity in Local Farm Animal Breeds. Animals, 11(7), 2016. https://doi.org/10.3390/ani11072016





