Unraveling the Genetic Diversity and Population Structure of Bangladeshi Indigenous Cattle Populations Using 50K SNP Markers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Animal Sampling and DNA Extraction
2.3. SNP Genotyping and Quality Control
2.4. Statistical Analysis
2.4.1. Genetic Diversity
2.4.2. Population Structure and Genetic Differentiation
2.4.3. Linkage Disequilibrium and Effective Population Size
3. Results
3.1. Intra-Population Genetic Diversity
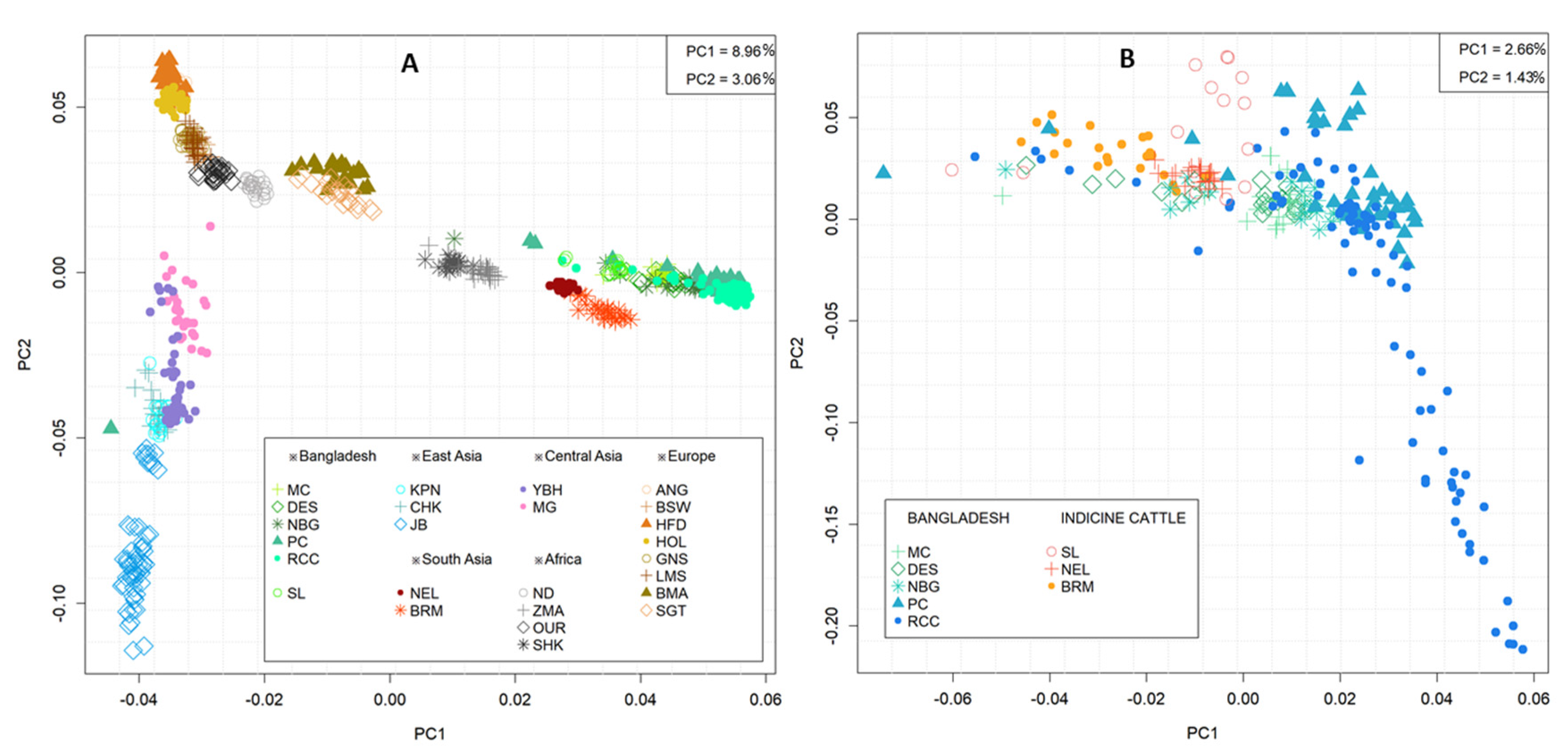
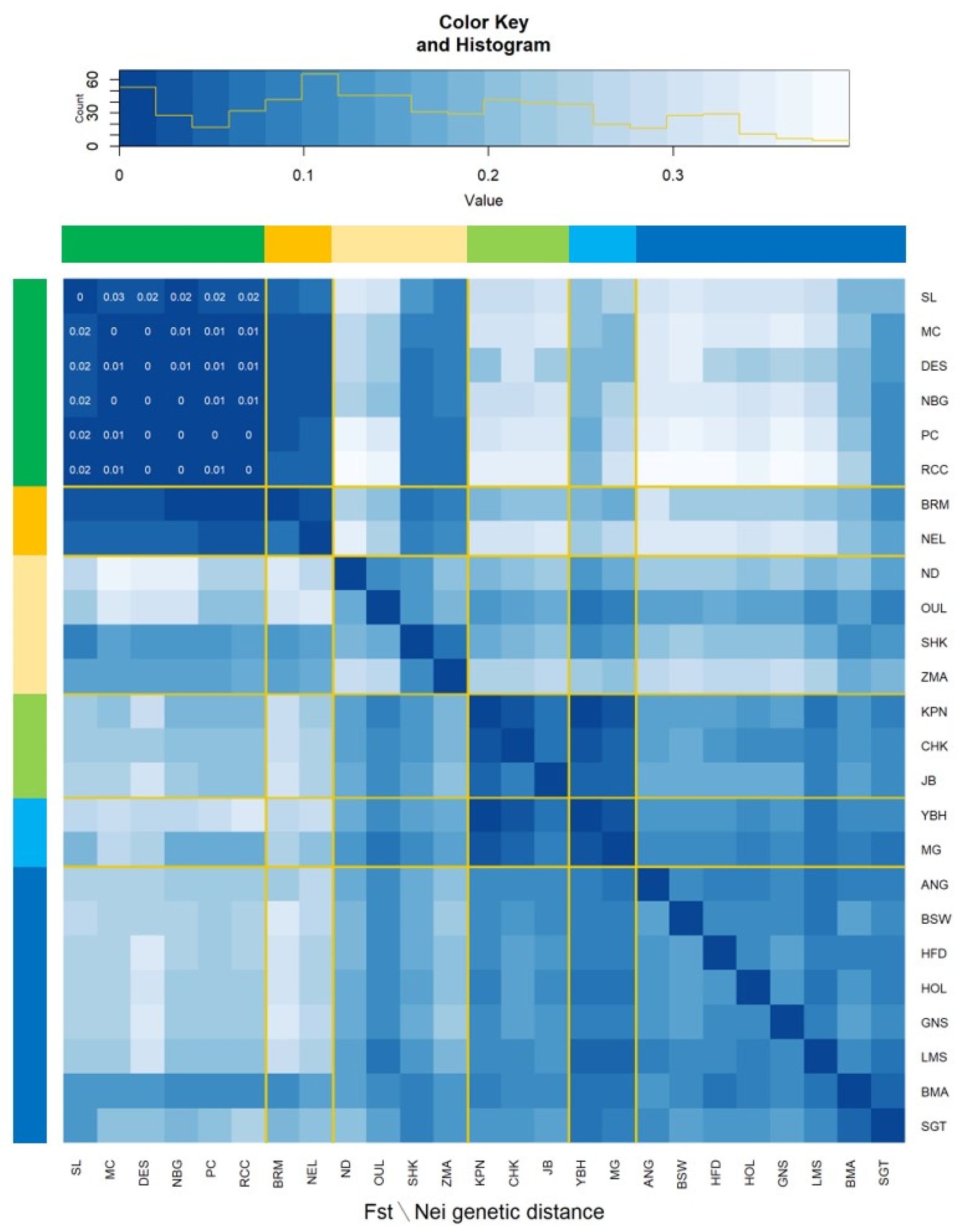
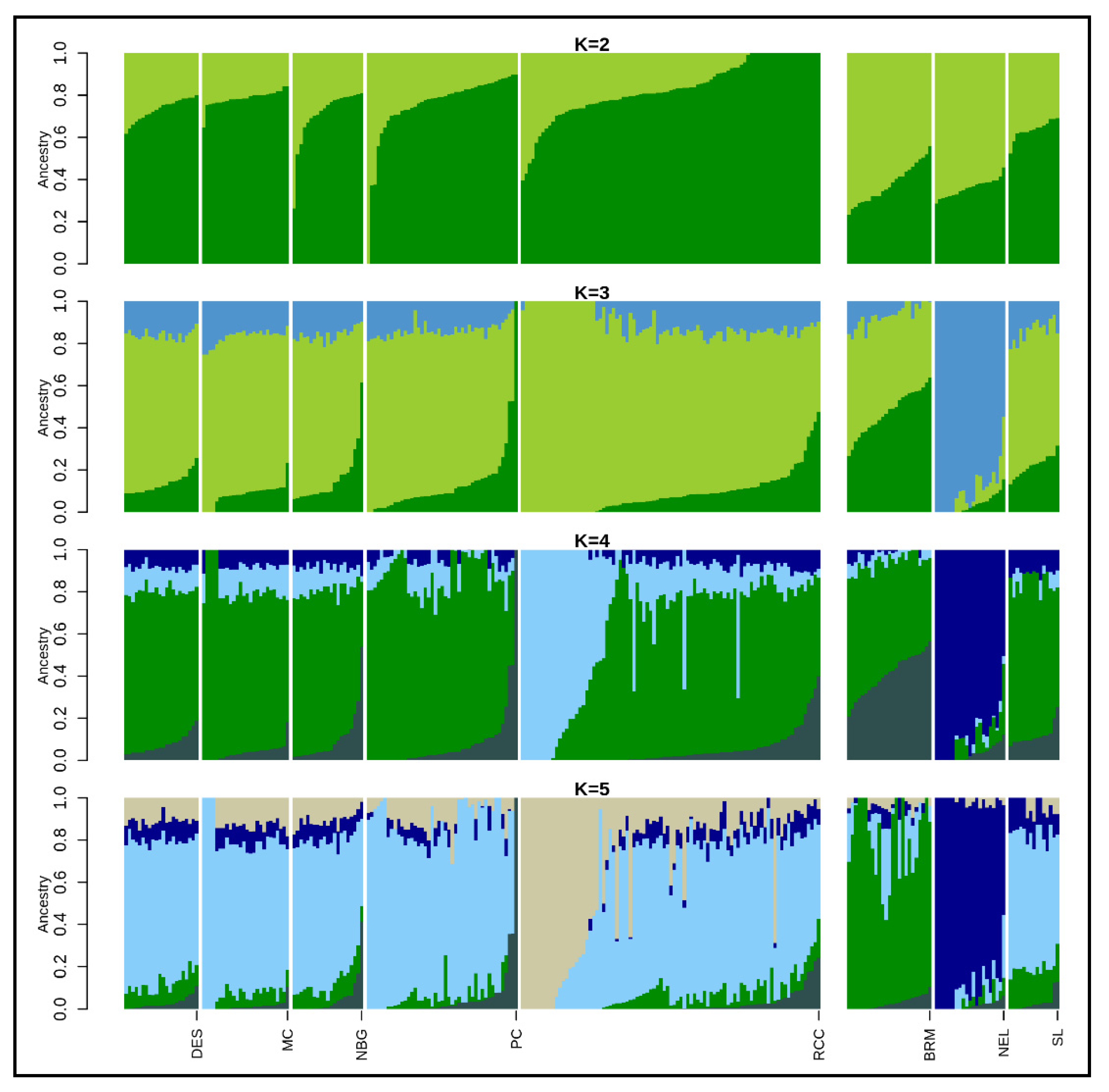
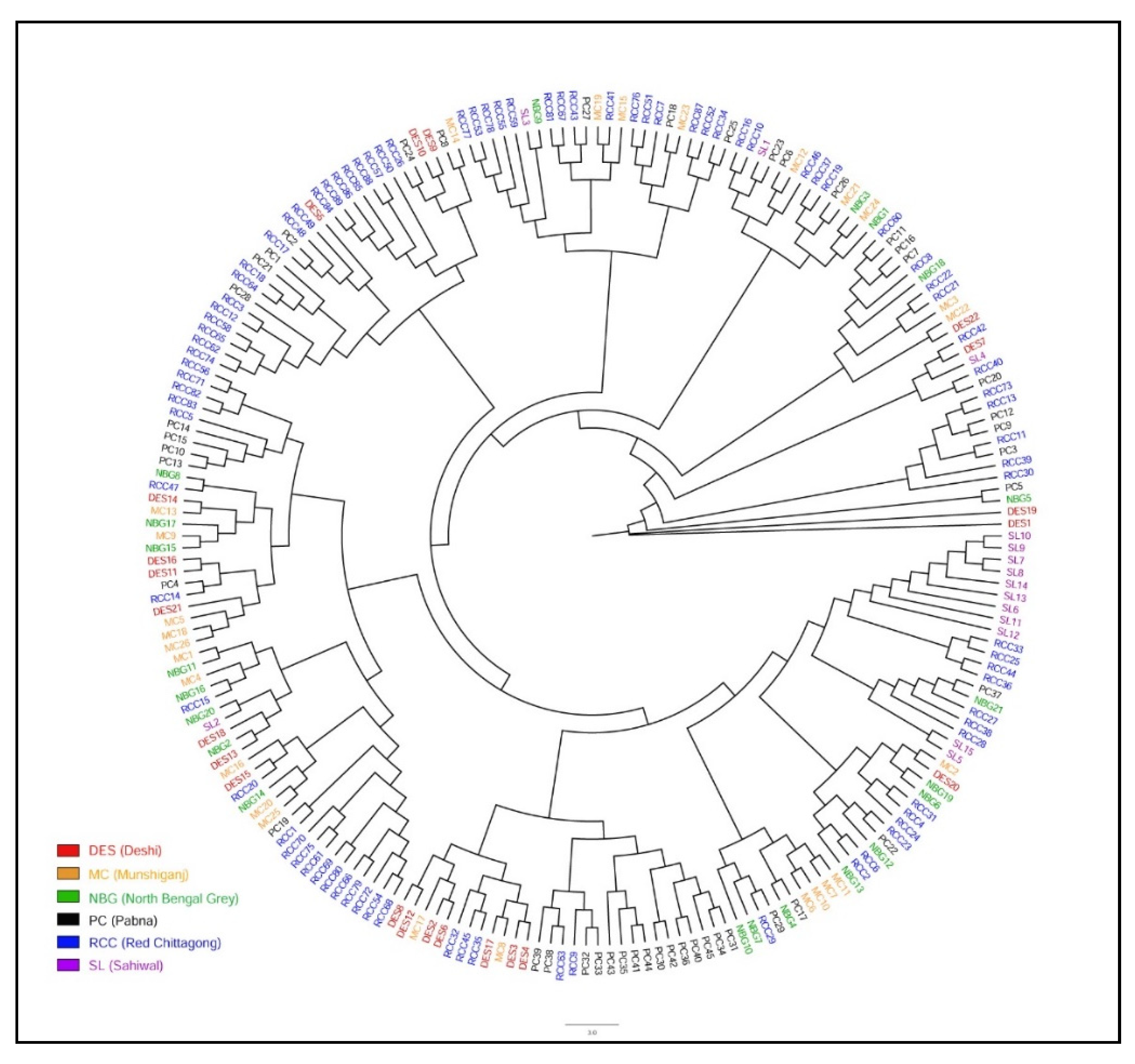
3.2. Population Structure and Genetic Differentiation
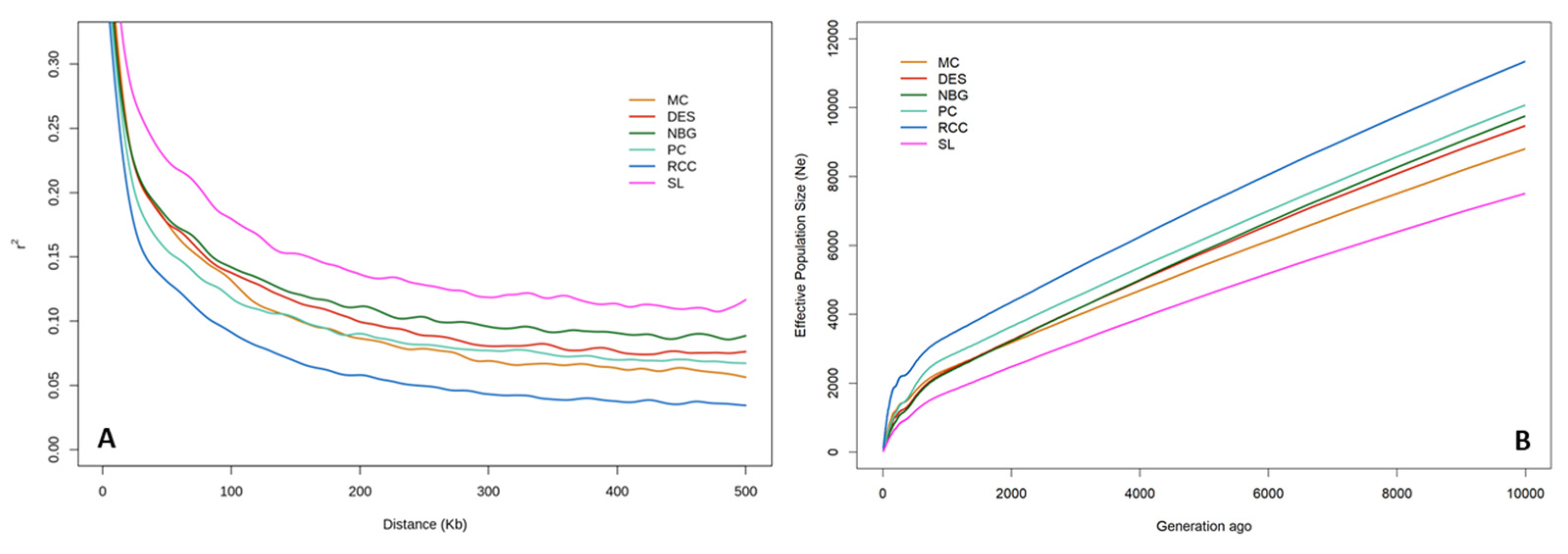
3.3. Analyses of Linkage Disequilibrium and Effective Population Size
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhuiyan, A.K.F.H. Farm Animal Genetic Resources in Bangladesh: Diversity, Conservation and Management. In Farm Animal Genetic Resources in SAARC Countries: Diversity, Conservation and Management; SAARC Agriculture Centre (SAC): Dhaka, Bangladesh, 2014; pp. 1–74. [Google Scholar]
- Hamid, M.; Rahman, A.; Zaman, M.; Hossain, K. Cattle Genetic Resources and their Conservation in Bangladesh. Asian J. Anim. Sci. 2017, 11, 54–64. [Google Scholar] [CrossRef] [Green Version]
- DLS. Livestock Economy at a Glance; Ministry of Fisheries and Livestock, Government of the People’s Republic of Bangladesh: Dhaka, Bangladesh, 2020; pp. 1–2.
- Al-Amin, M.; Nahar, A.; Bhuiyan, A.; Faruque, M. On-farm characterization and present status of North Bengal Grey (NBG) cattle in Bangladesh. Anim. Genet. Resour. Inf. 2007, 40, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Siddiquee, N.U.; Rahman, S.M.A.; Bhuiyan, M.S.A.; Rahman, A.K.M.A.; Bhuiyan, A.K.F.H. Practice, preference and per-formance of cattle under peri-urban dairy management system in Mymensingh. Bang. J. Anim. Sci. 2013, 42, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Bhuiyan, A.; Rashid, M.; Khan, R.; Habib, M.; Bhuiyan, M.; Faiz, M. Progeny tested bull production for dairy cattle development in Bangladesh. Bangladesh J. Anim. Sci. 2015, 44, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Woolliams, J.A.; Oldenbroek, J.K. Genetic diversity issues in animal populations in the genomic era. In Genomic Management of Animal Genetic Resources, 1st ed.; Oldenbroek, J.K., Ed.; Wageningen Academic Publisher: Gelderland, The Netherlands, 2018; pp. 13–47. [Google Scholar]
- Boettcher, P.J.; Hoffmann, I.; Baumung, R.; Drucker, A.G.; McManus, C.; Berg, P.; Stella, A.; Nilsen, L.B.; Moran, D.; Naves, M.; et al. Genetic resources and genomics for adaptation of livestock to climate change. Front. Genet. 2015, 5, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Lee, S.-H.; Lim, D.; Chai, H.-H.; Choi, B.-H.; Cho, Y. A genome-wide assessment of genetic diversity and population structure of Korean native cattle breeds. BMC Genet. 2016, 17, 139. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Cheong, H.S.; Shin, H.D.; Lee, S.-S.; Roh, H.-J.; Jeon, D.-Y.; Cho, C.-Y. Genetic diversity and divergence among Korean cattle breeds assessed using a BovineHD single-nucleotide polymorphism chip. Asian-Australas. J. Anim. Sci. 2018, 31, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Chagunda, M.G.G.; Mujibi, F.D.N.; Dusingizimana, T.; Kamana, O.; Cheruiyot, E.; Mwai, O.A. Use of High Density Single Nucleotide Polymorphism (SNP) Arrays to Assess Genetic Diversity and Population Structure of Dairy Cattle in Smallholder Dairy Systems: The Case of Girinka Programme in Rwanda. Front. Genet. 2018, 9, 438. [Google Scholar] [CrossRef]
- Xu, L.; Yang, L.; Zhu, B.; Zhang, W.; Wang, Z.; Chen, Y.; Zhang, L.; Gao, X.; Gao, H.; Liu, G.E.; et al. Genome-wide scan reveals genetic divergence and diverse adaptive selection in Chinese local cattle. BMC Genom. 2019, 20, 494. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, M.; Eriksson, S.; Mikko, S.; Strandberg, E.; Stålhammar, H.; Groenen, M.A.M.; Crooijmans, R.P.M.A.; Andersson, G.; Johansson, A.M. Genomic relatedness and diversity of Swedish native cattle breeds. Genet. Sel. Evol. 2019, 51, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bhuiyan, M.S.A.; Hanotte, O.; Bhuiyan, A.K.F.H. Genetic Diversity of Red Chittagong Cattle of Bangladesh Using Microsat-ellite Markers. Progress. Agric. 2005, 16, 141–148. [Google Scholar]
- Bhuiyan, M.S.A.; Bhuiyan, A.K.F.H.; Yoon, D.H.; Jeon, J.T.; Park, C.S.; Lee, J.H. Mitochondrial DNA Diversity and Origin of Red Chittagong Cattle. Asian-Australas. J. Anim. Sci. 2007, 20, 1478–1484. [Google Scholar] [CrossRef]
- Uzzaman, M.R.; Edea, Z.; Bhuiyan, M.S.A.; Walker, J.; Bhuiyan, A.K.F.H.; Kim., K.S. Genome-wide single nucleotide poly-morphism analyses reveal genetic diversity and structure of wild and domestic cattle in Bangladesh. Asian-Australas. J. Aim. Sci. 2014, 27, 1381–1386. [Google Scholar] [CrossRef] [Green Version]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [Green Version]
- Decker, J.E.; McKay, S.; Rolf, M.; Kim, J.; Alcalá, A.M.; Sonstegard, T.S.; Hanotte, O.; Gotherstrom, A.; Seabury, C.M.; Praharani, L.; et al. Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle. PLoS Genet. 2014, 10, e1004254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goudet, J. hierfstat, a package for r to compute and test hierarchical F-statistics. Mol. Ecol. Notes 2005, 5, 184–186. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Levine, D.; Shen, J.; Gogarten, S.M.; Laurie, C.; Weir, B.S. A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics 2012, 28, 3326–3328. [Google Scholar] [CrossRef] [Green Version]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pembleton, L.W.; Cogan, N.O.I.; Forster, J.W. StAMPP: An R package for calculation of genetic differentiation and structure of mixed-ploidy level populations. Mol. Ecol. Resour. 2013, 13, 946–952. [Google Scholar] [CrossRef]
- Lee, T.-H.; Guo, H.; Wang, X.; Kim, C.; Paterson, A.H. SNPhylo: A pipeline to construct a phylogenetic tree from huge SNP data. BMC Genom. 2014, 15, 162. [Google Scholar] [CrossRef] [Green Version]
- Sved, J. Linkage disequilibrium and homozygosity of chromosome segments in finite populations. Theor. Popul. Biol. 1971, 2, 125–141. [Google Scholar] [CrossRef]
- Barbato, M.; Orozco-Terwengel, P.; Tapio, M.; Bruford, M. SNeP: A tool to estimate trends in recent effective population size trajectories using genome-wide SNP data. Front. Genet. 2015, 6, 109. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, H.; Eiusoo, K.; Heather, H.; Ajmal, A.; Riley, D.; Pasha, N.; Ali, A.; Javed, K.; Sonstegard, T. Genome-wide SNPs analysis of indigenous zebu breeds in Pakistan. Biotechnol. Anim. Husb. 2017, 33, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Gao, X.; Zhang, Y.; Zhao, Y.; Zhang, J.; Jia, Y.; Zhu, B.; Xu, L.; Zhang, L.; Gao, H.; et al. Genome-wide assessment of genetic diversity and population structure insights into admixture and introgression in Chinese indigenous cat-tle. BMC Genet. 2018, 19, 114. [Google Scholar] [CrossRef] [Green Version]
- The Bovine HapMap Consortium; Gibbs, R.A.; Taylor, J.F.; Van Tassell, C.P.; Barendse, W.; Eversole, K.; Gill, C.; Green, R.D.; Hamernik, D.L.; Kappes, S.M.; et al. Genome-Wide Survey of SNP Variation Uncovers the Genetic Structure of Cattle Breeds. Science 2009, 324, 528–532. [Google Scholar] [CrossRef] [Green Version]
- Edea, Z.; Bhuiyan, M.S.A.; Dessie, T.; Rothschild, M.F.; Dadi, H.; Kim, K.S. Genome-wide genetic diversity, population structure and admixture analysis in African and Asian cattle breeds. Animals 2015, 9, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Pérez O’Brien, A.M.; Mészáros, G.; Utsunomiya, Y.T. Linkage disequilibrium levels in Bos indicus and Bos taurus cattle using medium and high-density SNP chip data and different minor allele frequency distributions. Livest. Sci. 2014, 166, 121–132. [Google Scholar] [CrossRef]
- Bejarano, D.; Martinez, R.; Manrique, C.; Parra, L.M.; Rocha, J.F.; Gómez, Y.; Abuabara, Y.; Gallego, J. Linkage disequilibrium levels and allele frequency distribution in Blanco Orejinegro and Romosinuano Creole cattle using medium density SNP chip data. Genet. Mol. Biol. 2018, 41, 426–433. [Google Scholar] [CrossRef]
- Murray, C.; Huerta-Sanchez, E.; Casey, F.; Bradley, D.G. Cattle demographic history modelled from autosomal sequence variation. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2531–2539. [Google Scholar] [CrossRef] [Green Version]
- Gautier, M.; Laloë, D.; Moazami-Goudarzi, K. Insights into the Genetic History of French Cattle from Dense SNP Data on 47 Worldwide Breeds. PLoS ONE 2010, 5, e13038. [Google Scholar] [CrossRef]
- Chen, S.; Lin, B.-Z.; Baig, M.; Mitra, B.; Lopes, R.J.; Santos, A.M.; Magee, D.A.; Azevedo, M.; Tarroso, P.; Sasazaki, S.; et al. Zebu Cattle Are an Exclusive Legacy of the South Asia Neolithic. Mol. Biol. Evol. 2009, 27, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Zerabruk, M.; Li, M.H.; Kantanen, J.; Olsaker, I.; Ibeagha-Awemu, E.M.; Erhardt, G.; Vangen, O. Genetic diversity and ad-mixture of indigenous cattle from North Ethiopia: Implications of historical introgressions in the gateway region to Africa. Anim. Genet. 2011, 43, 257–266. [Google Scholar] [CrossRef]
- Metta, M.; Kanginakudru, S.; Gudiseva, N.; Nagaraju, J. Genetic characterization of the Indian cattle breeds, Ongole and Deoni (Bos indicus) using microsatellite markers—A preliminary study. BMC Genet. 2004, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Shah, T.M.; Patel, J.S.; Bhong, C.D.; Doiphode, A.; Umrikar, U.D.; Parmar, S.S.; Rank, D.N.; Solanki, J.V.; Joshi, C.G. Evaluation of genetic diversity and population structure of west-central Indian cattle breeds. Anim. Genet. 2012, 44, 442–445. [Google Scholar] [CrossRef]
- Loftus, R.T.; MacHugh, D.; Bradley, D.G.; Sharp, P.; Cunningham, P. Evidence for two independent domestications of cattle. Proc. Natl. Acad. Sci. USA 1994, 91, 2757–2761. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.-H.; Cho, K.-H.; Park, K.-D.; Lee, H.-J.; Kim, H. Accurate Estimation of Effective Population Size in the Korean Dairy Cattle Based on Linkage Disequilibrium Corrected by Genomic Relationship Matrix. Asian-Australas. J. Anim. Sci. 2013, 26, 1672–1679. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Zhang, J.-J.; Niu, H.; Guan, L.; Guo, P.; Xu, L.-Y.; Chen, Y.; Zhang, L.-P.; Gao, H.-J.; Gao, X.; et al. Effects of marker density and minor allele frequency on genomic prediction for growth traits in Chinese Simmental beef cattle. J. Integr. Agric. 2017, 16, 911–920. [Google Scholar] [CrossRef]
- Bohmanova, J.; Sargolzaei, M.; Schenkel, F.S. Characteristics of linkage disequilibrium in North American Holsteins. BMC Genom. 2010, 11, 421. [Google Scholar] [CrossRef] [Green Version]
- Berihulay, H.; Islam, R.; Jiang, L.; Ma, Y. Genome-Wide Linkage Disequilibrium and the Extent of Effective Population Sizes in Six Chinese Goat Populations Using a 50K Single Nucleotide Polymorphism Panel. Animals 2019, 9, 350. [Google Scholar] [CrossRef] [Green Version]

| Breed/Population | N | PN | MAF ≤ 0.05 | Ho ± SD | He ± SD |
|---|---|---|---|---|---|
| Red Chittagong (RCC) | 89 | 0.905 | 0.272 | 0.250 ± 0.180 | 0.209 ± 0.129 |
| Pabna (PC) | 45 | 0.891 | 0.242 | 0.211 ± 0.166 | 0.176 ± 0.125 |
| Munshiganj (MC) | 26 | 0.734 | 0.189 | 0.215 ± 0.182 | 0.179 ± 0.136 |
| Non-descript Deshi (DES) | 22 | 0.770 | 0.154 | 0.221 ± 0.173 | 0.185 ± 0.129 |
| North Bengal Grey (NBG) | 21 | 0.818 | 0.205 | 0.209 ± 0.170 | 0.175 ± 0.127 |
| Sahiwal (SL) | 15 | 0.668 | 0.120 | 0.226 ± 0.177 | 0.188 ± 0.130 |
| Merged | 218 | 0.798 | 0.197 | 0.222 ± 0.175 | 0.185 ± 0.129 |
| Breed/ Population | n | Cluster 1 (Unspecified) | Cluster 2 (SL) | Cluster 3 (BRM) | Cluster 4 (NEL) | Cluster 5 (HOL) | Cluster 6 (JER) |
|---|---|---|---|---|---|---|---|
| RCC | 89 | 0.39 | 0.56 | 0.01 | 0.02 | 0.01 | 0.01 |
| PC | 45 | 0.11 | 0.83 | 0.01 | 0.01 | 0.03 | 0.01 |
| MC | 26 | 0.13 | 0.83 | 0.00 | 0.03 | 0.00 | 0.00 |
| NBG | 22 | 0.13 | 0.76 | 0.03 | 0.05 | 0.04 | 0.00 |
| DES | 21 | 0.13 | 0.78 | 0.02 | 0.05 | 0.01 | 0.01 |
| SL | 15 | 0.04 | 0.86 | 0.03 | 0.06 | 0.01 | 0.00 |
| BRM | 25 | 0.02 | 0.11 | 0.83 | 0.04 | 0.00 | 0.00 |
| NEL | 21 | 0.00 | 0.01 | 0.01 | 0.98 | 0.00 | 0.00 |
| HOL | 30 | 0.00 | 0.00 | 0.00 | 0.00 | 0.98 | 0.02 |
| JER | 24 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhuiyan, M.S.A.; Lee, S.-H.; Hossain, S.M.J.; Deb, G.K.; Afroz, M.F.; Lee, S.-H.; Bhuiyan, A.K.F.H. Unraveling the Genetic Diversity and Population Structure of Bangladeshi Indigenous Cattle Populations Using 50K SNP Markers. Animals 2021, 11, 2381. https://doi.org/10.3390/ani11082381
Bhuiyan MSA, Lee S-H, Hossain SMJ, Deb GK, Afroz MF, Lee S-H, Bhuiyan AKFH. Unraveling the Genetic Diversity and Population Structure of Bangladeshi Indigenous Cattle Populations Using 50K SNP Markers. Animals. 2021; 11(8):2381. https://doi.org/10.3390/ani11082381
Chicago/Turabian StyleBhuiyan, Mohammad Shamsul Alam, Soo-Hyun Lee, Sheikh Mohammad Jahangir Hossain, Gautam Kumar Deb, Most Farhana Afroz, Seung-Hwan Lee, and Abul Kashem Fazlul Haque Bhuiyan. 2021. "Unraveling the Genetic Diversity and Population Structure of Bangladeshi Indigenous Cattle Populations Using 50K SNP Markers" Animals 11, no. 8: 2381. https://doi.org/10.3390/ani11082381







