Effect of Fishmeal Content in the Diet on the Growth and Sexual Maturation of Olive Flounder (Paralichthys olivaceus) at a Typical Fish Farm
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish, Feeding Conditions, and Sample Collection
2.2. Experimental Diet Preparation
2.3. Male and Female Identification and Histology of Oocyte Development
2.4. Expression of Endocrine Hormones Related to Sexual Maturation
2.4.1. Total RNA Extraction
2.4.2. cDNA Synthesis
2.4.3. Oligonucleotide Primer Production
2.4.4. Real-Time qPCR (qPCR) Analysis
2.5. Statistical Analysis
3. Results
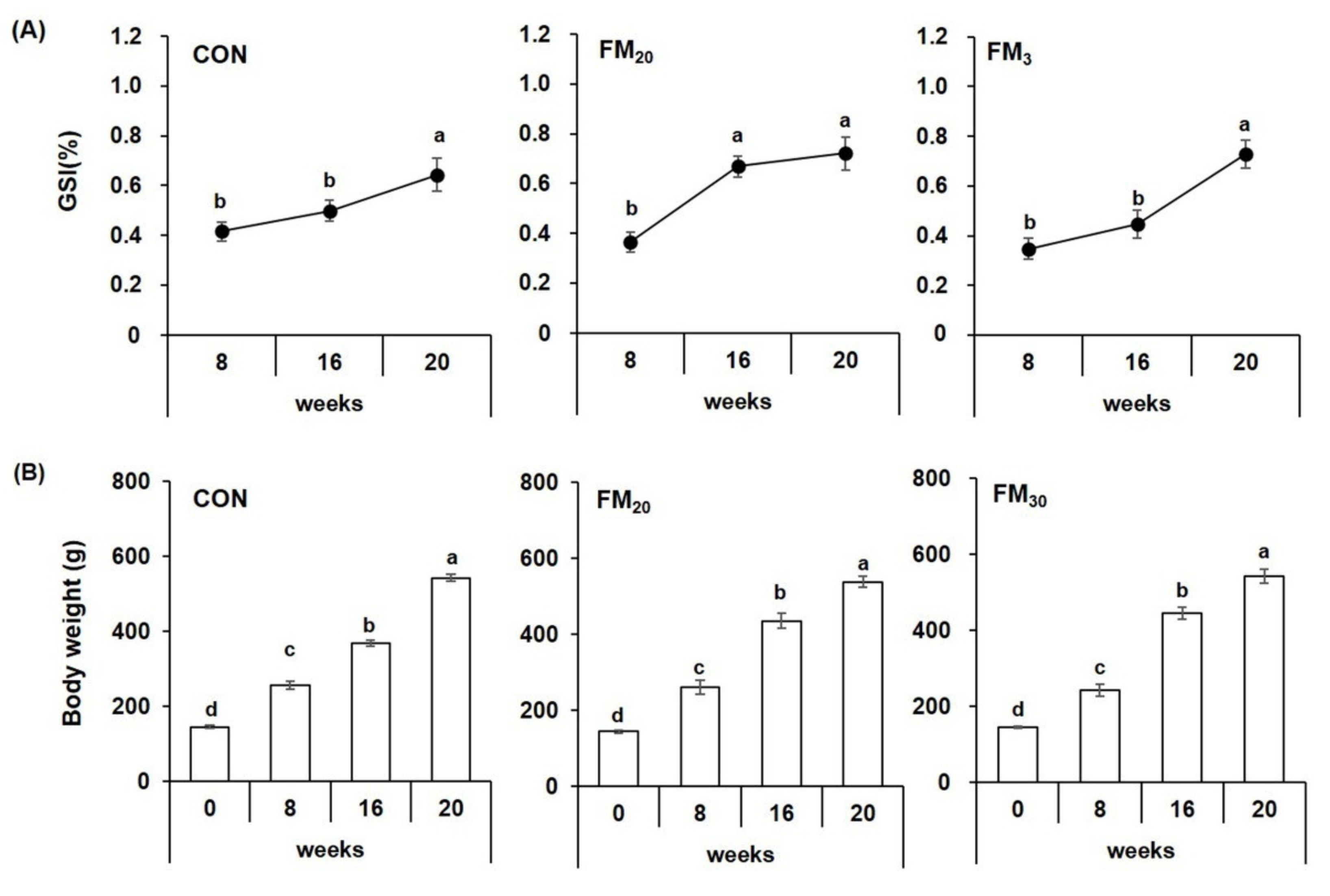
3.1. Growth Performance and Gonadosomatic Index (GSI) Analysis
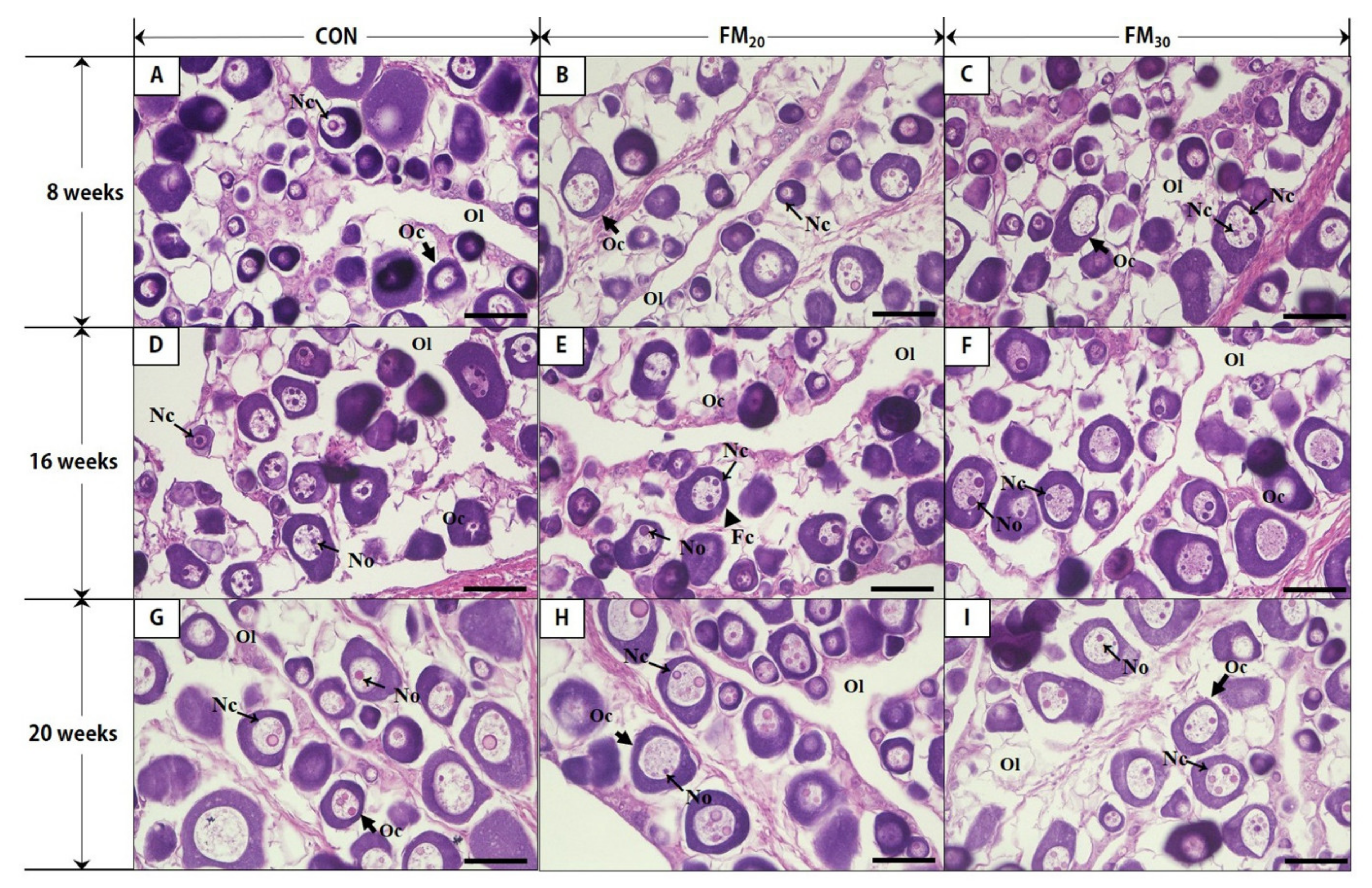
3.2. Male and Female Identification and the Histology of Oocyte Development
3.3. Expression of Endocrine Hormones Related to Sexual Maturation
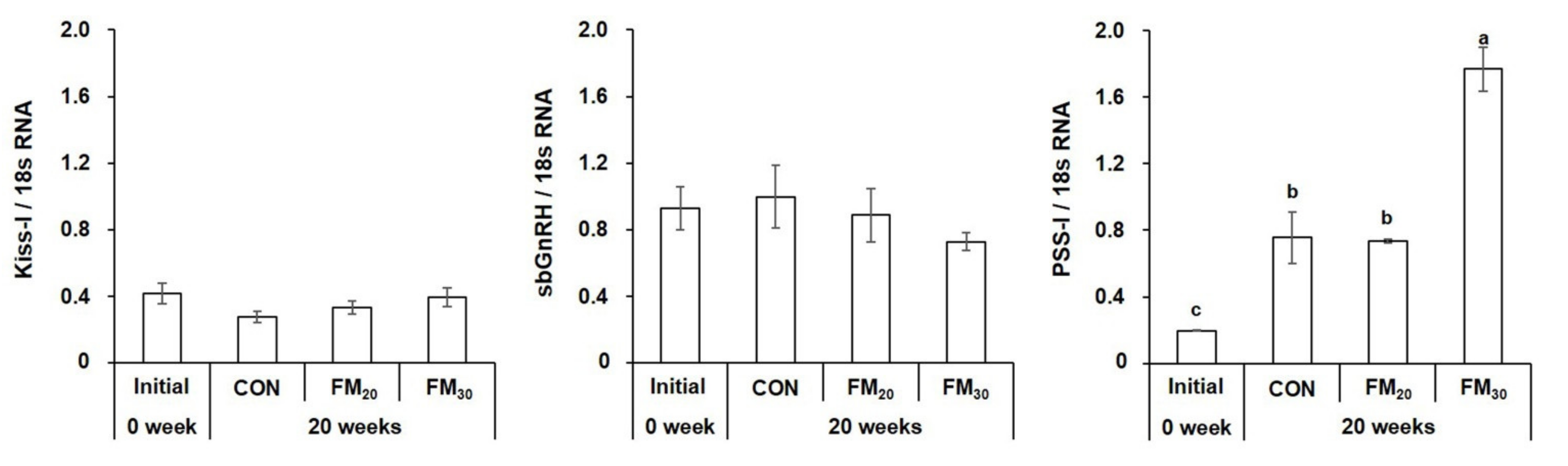
3.3.1. Hypothalamic Kiss-I, sbGnRH, and PSS-I mRNA Expression
3.3.2. Pituitary GH, FSH-β, and LH-β mRNA Expression
3.3.3. Liver ERα, ERβ, and Vtg mRNA Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Statistics Korea, 2019. Fishery Production Survey. Available online: http://kostat.go.kr/ (accessed on 22 May 2021).
- Kim, B.H.; Lee, C.H.; Hur, S.W.; Hur, S.P.; Kim, D.H.; Suh, H.L.; Kim, S.Y.; Lee, Y.D. Long photoperiod affects gonadal development in olive flounder Paralichthys olivaceus. Dev. Reprod. 2013, 17, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Pham, K.X.; Amando, M.; Amiya, N.; Kurita, Y.; Yamamori, K. Distribution of three GnRHs in the brain and pituitary of the wild Japanese flounder Paralichthys olivaceus. Fisheries Sci. 2006, 72, 89–94. [Google Scholar] [CrossRef]
- Kashiwagi, M. A periodic variation of spawning time of Japanese whiting Sillago japonica during the spawning season. Nip. Sui. Gak. 1984, 50, 2135. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T. Importance of the study of broodstock nutrition for further development of aquaculture. In Nutrition and Feeding in Fish; Cowey, C.B., Mackie, A.M., Bell, J.G., Eds.; Academic Press: London, UK, 1985; pp. 395–414. [Google Scholar]
- Wilson, R.P.; Halver, J.E. Protein and amino acid requirements of fishes. Ann. Rev. Nutr. 1986, 6, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Oliva-Teles, A.; Enes, P.; Peres, H. Replacing fishmeal and fish oil in industrial aquafeeds for carnivorous fish. Feed Feed. Pract. Aquac. 2015, 8, 203–233. [Google Scholar]
- McCoy, H.D. Fishmeal-the critical ingredient in aquaculture feeds. Aqua. Mag. 1990, 16, 43–50. [Google Scholar]
- Rodríguez-Serna, M.; Olvera-Novoa, M.A.; Carmona-Osalde, C. Nutritional value of animal by-product meal in practical diets for Nile tilapia Oreochromis niloticus (L.) fry. Aqua. Res. 1996, 27, 67–73. [Google Scholar] [CrossRef]
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.C.; Clay, J.; Folke, C.; Lubchenco, J.; Mooney, H.; Troell, M. Effect of aquaculture on world fish supplies. Nature 2000, 405, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Moon, J.S.; Seo, J.S.; Nam, T.J.; Lee, K.J.; Lim, S.G.; Kim, K.W.; Lee, B.J.; Hur, S.W.; Choi, Y.H. Effect of fish meal replacement on insulin-like growth factor-I expression in the liver and muscle and implications for the growth of olive flounder Paralichthys olivaceus. Korean J. Fish. Aquat. Sci. 2019, 52, 141–148. [Google Scholar]
- Kim, M.G.; Lim, H.; Lee, B.J.; Hur, S.W.; Lee, S.; Kim, K.W.; Lee, K.J. Replacing fish meal with a mixture of plant and animal protein sources in the diets of juvenile olive flounder Paralichthys olivaceus. Kor. J. Fish. Aquat. Sci. 2020, 53, 577–582. [Google Scholar]
- Cyr, D.G.; Eales, J.G. Interrelationships between thyroidal and reproductive endocrine systems in fish. Rev. Fish Biol. Fish. 1996, 6, 165–200. [Google Scholar] [CrossRef]
- Weltzien, F.A.; Andersson, E.; Andersen, Ø.; Shalchian-Tabrizi, K.; Norberg, B. The brain–pituitary–gonad axis in male teleosts, with special emphasis on flatfish (Pleuronectiformes). Comp. Biochem. Physiol. Mol. Integr. Physiol. 2004, 137, 447–477. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, A.S.; Gottsch, M.L.; Roa, J.; Byquist, A.C.; Crown, A.; Clifton, D.K.; Hoffman, G.E.; Steiner, R.A.; Tena-Sempere, M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 2007, 148, 1774–1783. [Google Scholar] [CrossRef]
- Xu, Y.J.; Liu, Z.H.; Liao, M.J.; Wang, H.P.; Wang, Q.Y. Molecular cloning and differential expression of three GnRH genes during ovarian maturation of spotted halibut, Verasper variegatus. J. Exp. Zool. 2012, 317, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, K.; Le Belle, N.; Pichavant, K.; Marchelidon, J.; Chow, B.K.; Boeuf, G.; Dufour, S. Pituitary growth hormone secretion in the turbot, a phylogenetically recent teleost, is regulated by a species-specific pattern of neuropeptides. Neuroendocrinology 2001, 74, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Very, N.M.; Sheridan, M.A. The role of somatostatins in the regulation of growth in fish. Fish Physiol. Biochem. 2002, 27, 217–226. [Google Scholar] [CrossRef]
- Pierce, J.G.; Parsons, T.F. Glycoprotein hormones: Structure and function. Ann. Rev. Biochem. 1981, 50, 465–495. [Google Scholar] [CrossRef]
- Tannenbaum, G.S.; Painson, J.C.; Lapointe, M.; Gurd, W.; McCarthy, G.F. Interplay of somatostatin and growth hormone-releasing hormone in genesis of episodic growth hormone secretion. Metabolism 1990, 39, 35–39. [Google Scholar] [CrossRef]
- Wiegand, M.D. Composition, accumulation and utilization of yolk lipids in teleost fish. Rev. Fish Biol. Fish. 1996, 6, 259–286. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed; Association of Official Analytical Chemist: Arlington, VA, USA, 1990. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, T.I.; Hur, Y.B.; Go, C.S.; Chang, Y.J. Gametogenic cycle and fine structure of ripe germ cells in the Pacific oyster, Crassostrea gigas on the south coast of Korea. J. Fish. Sci. Tech. 2003, 6, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Bateman, K.S.; Grant, D.S.; Stephen, W.F. A ranking system for the evaluation of intersex condition in European flounder (Platichthys flesus). Environ. Toxicol. Chem. 2004, 23, 2831–2836. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.M.; Lee, J.S. Changes of morphology and morphometric characteristics of the oocyte during oogenesis of the abalone Haliotis discus hannai. Korean J. Malacol. 2016, 32, 1–7. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Daskalopoulou, E.; Vogiatzis, I.; Rumbos, C.; Mente, E.; Athanassiou, C.G. Substitution of fishmeal by fly Hermetia illucens prepupae meal in the diet of gilthead seabream (Sparus aurata). In Proceedings of the HydroMedit 2014, Volos, Greece, 13–15 November 2014; pp. 110–114. [Google Scholar]
- Lim, H.; Kim, M.G.; Shin, J.; Shin, J.; Hur, S.W.; Lee, B.J.; Lee, K.J. Evaluation of three plant proteins for fish meal replacement in diet for growing olive flounder Paralichthys olivaceus. Korean J. Fish. Aquat. Sci. 2020, 53, 464–470. [Google Scholar]
- Shin, S.J.; Lee, T.K.; Lee, D.H.; Lim, S.R.; Yang, I.C.; Kim, S.S.; Choi, J.W.; Kim, J.S.; Kim, J.D. Effect of dietary fish meal replacement by a blend of plant and animal ingredients on the growth and blood chemistry of starry flounder Platichthys stellatus. Korean J. Fish Aquat. Sci. 2019, 52, 134–140. [Google Scholar]
- Chung, E.Y.; Chang, Y.J.; Lee, K.K. Activities of hepatocytes and changes of protein and total RNA contents in liver and muscle of Sebastes schlegeli with the gonadal maturation. Korean J. Fish. Aquat. Sci. 1995, 28, 338–346. [Google Scholar]
- Chung, E.Y.; Lee, K.K.; Oh, Y.N. Studies on changes of the activity of the hepatic cells, and variations of protein and nucleic acid contents of the liver tissue with the ovarian development phase of female Boleophthalmus pectinirostris (Linnaeus). Korean J. Ichthy. 1991, 3, 48–57. [Google Scholar]
- Wallace, R.A.; Selman, K. Cellular and dynamic aspects of oocyte growth in teleosts. Am. Zool. 1981, 21, 325–343. [Google Scholar] [CrossRef]
- Shimizu, A.; Hanyu, I. Factors involved in the development of and decline in photoperiodism as it relates to the gonadal activity of a spring-spawning bitterling, Acheilognathus tabira. J. Exp. Zool. 1993, 265, 134–143. [Google Scholar] [CrossRef]
- Kang, H.C.; Lee, C.H.; Song, Y.B.; Baek, H.J.; Kim, H.B.; Lee, Y.D. KiSS1, KiSS2, GPR54 mRNA expression of the blacktip grouper Epinephelus fasciatus. Dev. Reprod. 2012, 16, 121–128. [Google Scholar]
- Greives, T.J.; Mason, A.O.; Scotti, M.A.L.; Levine, J.; Ketterson, E.D.; Kriegsfeld, L.J.; Demas, G.E. Environmental control of kisspeptin: Implications for seasonal reproduction. Endocrinology 2007, 148, 1158–1166. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.E.; Imran, S.A.; Ur, E.; Wilkinson, M. KiSS-1 mRNA in adipose tissue is regulated by sex hormones and food intake. Mol. Cell. Endocrinol. 2008, 281, 64–72. [Google Scholar] [CrossRef]
- Luque, R.M.; Kineman, R.D.; Tena-Sempere, M. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: Analyses using mouse models and a cell line. Endocrinology 2007, 148, 4601–4611. [Google Scholar] [CrossRef]
- Wierman, M.E.; Rivier, J.E.; Wang, C. Gonadotropin-releasing hormone-dependent regulation of gonadotropin subunit messenger ribonucleic acid levels in the rat. Endocrinology 1989, 124, 272–278. [Google Scholar] [CrossRef]
- Du, J.L.; Lee, Y.H.; Yeuh, W.H.; Chang, C.F. Seasonal profiles of brain and pituitary gonadotropin-releasing hormone and plasma luteinizing hormone in relation to sex change of protandrous black porgy, Acanthopagrus schlegeli. Biol. Preprod. 2005, 72, 922–931. [Google Scholar] [CrossRef] [Green Version]
- Holland, M.C.H.; Meiri, Y.G.I.; King, J.A.; Okuzawa, K.; Elizur, A.; Zohar, Y. Levels of the native forms of GnRH in the pituitary of the gilthead Seabream, Sparus aurata, at several characteristic stages of the gonadal cycle. Gen. Comp. Endocrinol. 1998, 112, 394–405. [Google Scholar] [CrossRef]
- Prat, F.; Sumpter, J.P.; Tyler, C.R. Validation of radioimmunoassays for two salmon gonadotropins (GTH I and GTH II) and their plasma concentrations throughout the reproductive cycle in male and female rainbow trout (Oncorhynchus mykiss). Biol. Reprod. 1996, 54, 1375–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateos, J.; Mananos, E.; Carrillo, M.; Zanuy, S. Regulation of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) gene expression by gonadotropin-releasing hormone (GnRH) and sexual steroids in the Mediterranean Sea bass. Comp. Biochem. Physiol. Biochem. Mol. Biol. 2002, 132, 75–86. [Google Scholar] [CrossRef]
- Ando, H.; Swanson, P.; Kitani, T.; Koide, N.; Okada, H.; Ueda, H.; Urano, A. Synergistic effects of salmon gonadotropin-releasing hormone and estradiol-17β on gonadotropin subunit gene expression and release in masu salmon pituitary cells in vitro. Gen. Comp. Endocrinol. 2004, 137, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Glen, V.D.K. The GnRH system and the neuroendocrine regulation of reproduction. Fish. Physiol. 2009, 28, 115–149. [Google Scholar]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, A.; Sullivan, C.V.; Dickhoff, W.W. Isolation and some characterization of vitellogenin and its related egg yolk proteins from coho salmon (Oncorhynchus kisutch). Zool. Sci. 1993, 10, 245–256. [Google Scholar]
- Mommsen, T.P.; Walsh, P.J. 5 Vitellogenesis and oocyte assembly. Fish Physiol. 1988, 11, 347–406. [Google Scholar]
- Holloway, A.C.; Melroe, G.T.; Ehrman, M.M.; Reddy, P.K.; Leatherland, J.F.; Sheridan, M.A. Effect of 17β-estradiol on the expression of somatostatin genes in rainbow trout (Oncorhynchus mykiss). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R389–R393. [Google Scholar] [CrossRef] [PubMed]
- Marchant, T.A.; Peter, R.E. Seasonal variations in body growth rates and circulating levels of growth hormone in the goldfish, Carassius auratus. J. Exp. Zool. 1986, 237, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Melamed, P.; Eliahu, N.; Levavi-Sivan, B.; Ofir, M.; Farchi-Pisanty, O.; Rentier-Delrue, F.; Smal, J.; Yaron, Z.; Naor, Z. Hypothalamic and thyroidal regulation of growth hormone in tilapia. Gen. Comp. Endocrinol. 1995, 97, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.J.; Trudeau, V.L.; Cui, Z.; Brechin, J.; Mackenzie, K.; Zhu, Z.; Houlihan, D.F.; Peter, R.E. Estradiol stimulates growth hormone production in female goldfish. Gen. Comp. Endocrinol. 1997, 106, 102–112. [Google Scholar] [CrossRef]
- Filby, A.L.; Thorpe, K.L.; Tyler, C.R. Multiple molecular effect pathways of an environmental oestrogen in fish. J. Mol. Endocrinol. 2006, 37, 121–134. [Google Scholar] [CrossRef] [Green Version]




| Component | Diets | ||
|---|---|---|---|
| CON | FM20 | FM30 | |
| Sardine FM | 31.50 | 25.00 | 21.75 |
| Anchovy FM | 31.50 | 25.00 | 21.75 |
| Soybean meal | 12.00 | 12.00 | 12.00 |
| Wheat flour | 16.90 | 14.90 | 14.95 |
| Wheat gluten | 1.00 | 3.50 | 4.50 |
| SPC | - | 3.50 | 5.30 |
| Tankage meal | - | 3.50 | 6.25 |
| Poultry by-product meal | - | 3.50 | 4.00 |
| Fish oil | 3.40 | 4.00 | 4.30 |
| Lecithin | 0.50 | 0.50 | 0.50 |
| Betaine | - | 1.00 | 1.00 |
| Taurine | - | 0.40 | 0.50 |
| Mono calcium phosphate | 0.50 | 0.50 | 0.50 |
| Mineral mix | 1.00 | 1.00 | 1.00 |
| Vitamin mix | 0.80 | 0.80 | 0.80 |
| Vitamin C | 0.30 | 0.30 | 0.30 |
| Vitamin E | 0.30 | 0.30 | 0.30 |
| Choline | 0.30 | 0.30 | 0.30 |
| Total | 100 | 100 | 100 |
| Proximate composition (%, dry matter basis) | |||
| Crude protein | 56.80 ns | 57.20 | 56.80 |
| Crude lipid | 8.96 ns | 9.29 | 9.66 |
| Crude ash | 11.3 ns | 10.80 | 10.80 |
| Proximate composition (%, wet basis) | |||
| Moisture | 7.70 ns | 7.75 | 7.42 |
| Crude protein | 52.40 ns | 52.90 | 52.60 |
| Crude lipid | 8.27 ns | 8.59 | 8.94 |
| Crude ash | 10.40 ns | 10.00 | 9.97 |
| Ingredients | International Name | International Feed Number |
|---|---|---|
| Fish meal, sardine | Fish, sardine meal, mechanical, extruded | 5-02-015 |
| Fish meal, anchovy | Fish, anchovy meal, mechanical, extruded | 5-01-985 |
| Soybean meal | Soybean meal, solvent, extruded | 5-04-604 |
| Wheat gluten | Wheat gluten meal | 5-05-220 |
| Soy protein concentrate | Soybean protein concentrate | 5-08-008 |
| Tankage meal | Meat and bone meal | 5-00-388 |
| Poultry by-product meal | Poultry by-product, meal rendered | 5-03-798 |
| Primers | Sequence | Size | GenBank Accession |
|---|---|---|---|
| 18s rRNA | F: 5′-GGTCTGTGATGCCCTTAGATGTC-3′ | 107 bp | EF126037.1 |
| R: 5′-AGTGGGGTTCAGCGGGTTAC-3′ | |||
| Kiss-I | F: 5′-AGCCACTTGTATCACCCTGA-3′ | 240 bp | KP347690.1 |
| R: 5′-GCCCTCTCGTGTGTTTTAGA-3′ | |||
| sbGnRH | F: 5′-AAATGGCTGTGAAGACCTTG-3′ | 150 bp | DQ074693.1 |
| R: 5′-CCTCAACTACATTGCCCAGA-3′ | |||
| PSS-I | F: 5′-ATGAAGATGGTGTCCTCCTCG-3′ | 174 bp | AB693833.1 |
| R: 5′-CTCGTTCCAGATCCACATGG-3′ | |||
| GH | F: 5′-GGAGGATCAACGTCTTCTCAA-3′ | 183 bp | D29737.1 |
| R: 5′-ACTGCGGCTGTTACTTATTCA-3′ | |||
| FSH-β | F: 5′-AGCTTCGACTGTCGTCCAAC-3′ | 140 bp | AB042422.1 |
| R: 5′-TGTTTAGCCGGACCTGTTTC-3′ | |||
| LH-β | F: 5′-CCGACGTGTCTTCTCATCAA-3′ | 136 bp | AB042423.1 |
| R: 5′-TGTTGAGGAAGGGGATCTTG-3′ | |||
| Vtg-I | F: 5′-TGAGCTCTTTGAGTACAGCG-3′ | 181 bp | AB200267.1 |
| R: 5′-TCCTCTCTGGATGTTCAGCA-3′ | |||
| ERα | F: 5′-ATGCTGGAGACTATCACTGACG-3′ | 113 bp | AB070629.1 |
| R: 5′-TGTCTGATGTGGGAGAGCAG-3′ | |||
| ERβ | F: 5′-ACCATCCAGGGAAACTCATC-3′ | 189 bp | AB070630.1 |
| R: 5′-GGCACATGTTGGAGTTTAGG-3′ |
| Growth Performance | Experimental Groups | ||
|---|---|---|---|
| CON | FM20 | FM30 | |
| Weight gain (%) | 276.45 ± 11.5 ns | 273.94 ± 12.4 | 276.08 ± 12.4 |
| Specific growth rate (%/day) | 0.94 ± 0.02 ns | 0.93 ± 0.02 | 0.94 ± 0.02 |
| Condition factor | 1.11 ± 0.02 ns | 1.11 ± 0.02 | 1.12 ± 0.01 |
| Feed conversion ratio | 1.03 ± 0.04 ab | 1.11 ± 0.05 b | 0.95 ± 0.04 a |
| Protein efficiency ratio | 1.78 ± 0.07 ab | 1.64 ± 0.07 b | 1.92 ± 0.08 a |
| Feed intake (g) | 398 ± 54.7 ns | 422 ± 14.6 | 365 ± 10.1 |
| Survival (%) | 62.3 ± 2.32 ns | 62.5 ± 4.21 | 65.9 ± 1.37 |
| Measurement | Diets | ||
|---|---|---|---|
| CON | FM20 | FM30 | |
| Moisture (%) | 70.0 ± 0.01 ns | 68.3 ± 1.19 | 69.4 ± 0.52 |
| Protein (%) | 21.3 ± 0.73 ns | 21.5 ± 0.58 | 21.9 ± 0.70 |
| Lipid (%) | 4.69 ± 0.19 a | 5.44 ± 0.15 c | 5.01 ± 0.14 b |
| Ash (%) | 3.40 ± 0.01 b | 3.14 ± 0.17 ab | 3.05 ± 0.09 a |
| Weeks | Experimental Groups | ||
|---|---|---|---|
| CON (F:M) | FM20 (F:M) | FM30 (F:M) | |
| 8 | 9:1 | 8:2 | 7.5:2.5 |
| 16 | 7.5:2.5 | 8.5:1.5 | 5:5 |
| 20 | 7:3 | 9:1 | 8.5:1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-J.; Seo, B.S.; Park, H.S.; Lee, B.-J.; Hur, S.-W.; Nam, T.-J.; Lee, K.-J.; Lee, S.; Choi, Y.H. Effect of Fishmeal Content in the Diet on the Growth and Sexual Maturation of Olive Flounder (Paralichthys olivaceus) at a Typical Fish Farm. Animals 2021, 11, 2055. https://doi.org/10.3390/ani11072055
Park S-J, Seo BS, Park HS, Lee B-J, Hur S-W, Nam T-J, Lee K-J, Lee S, Choi YH. Effect of Fishmeal Content in the Diet on the Growth and Sexual Maturation of Olive Flounder (Paralichthys olivaceus) at a Typical Fish Farm. Animals. 2021; 11(7):2055. https://doi.org/10.3390/ani11072055
Chicago/Turabian StylePark, Su-Jin, Bong Seung Seo, Hung Sik Park, Bong-Joo Lee, Sang-Woo Hur, Taek-Jeong Nam, Kyeong-Jun Lee, Seunghyung Lee, and Youn Hee Choi. 2021. "Effect of Fishmeal Content in the Diet on the Growth and Sexual Maturation of Olive Flounder (Paralichthys olivaceus) at a Typical Fish Farm" Animals 11, no. 7: 2055. https://doi.org/10.3390/ani11072055
APA StylePark, S. -J., Seo, B. S., Park, H. S., Lee, B. -J., Hur, S. -W., Nam, T. -J., Lee, K. -J., Lee, S., & Choi, Y. H. (2021). Effect of Fishmeal Content in the Diet on the Growth and Sexual Maturation of Olive Flounder (Paralichthys olivaceus) at a Typical Fish Farm. Animals, 11(7), 2055. https://doi.org/10.3390/ani11072055







