Antibacterial Potential of Biosynthesized Zinc Oxide Nanoparticles against Poultry-Associated Foodborne Pathogens: An In Vitro Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Media
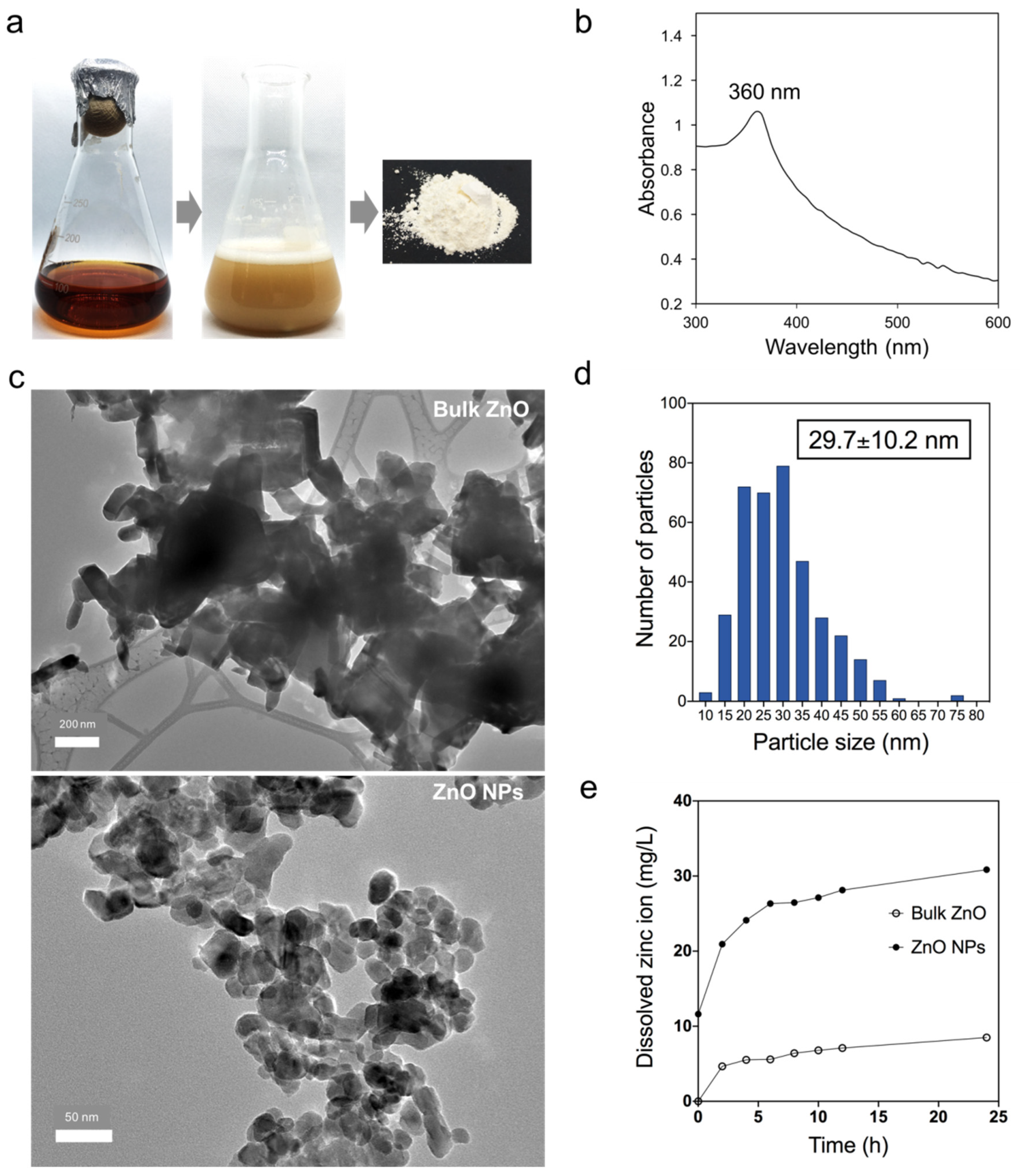
2.2. Zinc Oxide Nanoparticles Preparation and Characterization
2.3. Determination of Zn2+ Dissolution
2.4. In Vitro Antibacterial Activity of Biosynthesized Zinc Oxide Nanoparticles
2.4.1. Agar Well Diffusion Method
2.4.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Biosynthesized Zinc Oxide Nanoparticles
2.4.3. Antibiofilm Activities of Zinc Oxide Nanoparticles
Biofilm Inhibition Assay
Biofilm Eradication Assay
2.4.4. Time–Kill Assay of Zinc Oxide Nanoparticles
2.5. Assessment of Cell Membrane Integrity (Trypan Blue Exclusion Assay)
2.6. Quantification of Reactive Oxygen Species (ROS)
2.7. Assay for the Membrane Leakage of Protein and Reducing Sugar
2.8. Morphological Analysis of Bacterial Cell by Scanning Electron Microscope (SEM)
2.9. Data Analysis
3. Results and Discussion
3.1. Biosynthesized Zinc Oxide Nanoparticles Characterization
3.2. Agar Well Diffusion Assay
3.3. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Value
3.4. Antibiofilm Activity of Zinc Oxide Nanoparticles
3.5. Time–Kill Assay and Trypan Blue Exclusion Assay
3.6. Quantification of Reactive Oxygen Species (ROS) and Bacterial Cellular Leakage (Protein and Sugar)
3.7. Bacterial Surface Morphology Study Using Scanning Electron Microscope (SEM)
3.8. Overview of Risk Assessment for the Application of Zinc Oxide Nanoparticles in the Poultry Industry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, Q.; Chen, W.; Huang, H.; Tang, Y.; Wang, W.; Meng, F.; Wang, H.; Zheng, Y. Iron and zinc ions, potent weapons against multidrug-resistant bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 5213–5227. [Google Scholar] [CrossRef]
- Nie, L.; Deng, Y.; Zhang, Y.; Zhou, Q.; Shi, Q.; Zhong, S.; Sun, Y.; Yang, Z.; Sun, M.; Politis, C.; et al. Silver-doped biphasic calcium phosphate/alginate microclusters with antibacterial property and controlled doxorubicin delivery. J. Appl. Polym. Sci. 2020, 138, 50433. [Google Scholar] [CrossRef]
- Fiedot, M.; Maliszewska, I.; Rac-Rumijowska, O.; Suchorska-Wózniak, P.; Lewínska, A.; Teterycz, H. The relationship between the mechanism of zinc oxide crystallization and its antimicrobial properties for the surface modification of surgical meshes. Materials 2017, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Zeng, H.Y.; Ou-Yang, Y.S.; Chen, Y. Ben Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef]
- Ahmadi, A.; Ahmadi, P.; Ehsani, A. Development of an active packaging system containing zinc oxide nanoparticles for the extension of chicken fillet shelf life. Food Sci. Nutr. 2020, 8, 5461–5473. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Abdul Rahman, N. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: A review. J. Anim. Sci. Biotechnol. 2019, 10, 1–22. [Google Scholar] [CrossRef]
- Azad, S.K.; Shariatmadari, F.; Torshizi, M.A.K.; Chiba, L.I. Comparative Effect of Zinc Concentration and Sources on Growth Performance, Accumulation in Tissues, Tibia Status, Mineral Excretion and Immunity of Broiler Chickens. Braz. J. Poult. Sci. 2020, 22, 1245. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Poultry, 9th ed.; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Abedini, M.; Shariatmadari, F.; Torshizi, M.A.K.; Ahmadi, H. Effects of a dietary supplementation with zinc oxide nanoparticles, compared to zinc oxide and zinc methionine, on performance, egg quality, and zinc status of laying hens. Livest. Sci. 2017, 203, 30–36. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Tan, S.X.; Xiao, X.Y.; Qiu, X.S.; Pan, J.Q.; Tang, Z.X. Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol. Trace Elem. Res. 2014, 160, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Khah, M.M.; Ahmadi, F.; Amanlou, H. Influence of dietary different levels of zinc oxide nano particles on the yield and quality carcass of broiler chickens during starter stage. Indian J. Anim. Sci. 2015, 85, 287–290. [Google Scholar]
- Yausheva, E.; Miroshnikov, S.; Sizova, E. Intestinal microbiome of broiler chickens after use of nanoparticles and metal salts. Environ. Sci. Pollut. Res. 2018, 25, 18109–18120. [Google Scholar] [CrossRef]
- Feng, Y.; Gong, J.; Yu, H.; Jin, Y.; Zhu, J.; Han, Y. Identification of changes in the composition of ileal bacterial microbiota of broiler chickens infected with Clostridium perfringens. Vet. Microbiol. 2010, 140, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Min, L.; Zhang, W.; Liu, J.; Hou, Z.; Chu, M.; Li, L.; Shen, W.; Zhao, Y.; Zhang, H. Zinc oxide nanoparticles influence microflora in ileal digesta and correlate well with blood metabolites. Front. Microbiol. 2017, 8, 992. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Lai, W.; Han, M.; Han, M.; Ma, X.; Zhang, L. Dietary ZnO nanoparticles alters intestinal microbiota and inflammation response in weaned piglets. Oncotarget 2017, 8, 64878–64891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehdi, Y.; Létourneau-Montminy, M.-P.; Gaucher, M.-L.; Chorfi, Y.; Suresh, G.; Rouissi, T.; Brar, S.K.; Côté, C.; Ramirez, A.A.; Godbout, S. Use of antibiotics in broiler production: Global impacts and alternatives. Anim. Nutr. 2018, 4, 170–178. [Google Scholar] [CrossRef]
- Thames, H.T.; Theradiyil Sukumaran, A. A Review of Salmonella and Campylobacter in Broiler Meat: Emerging Challenges and Food Safety Measures. Foods 2020, 9, 776. [Google Scholar] [CrossRef]
- Heredia, N.; García, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef]
- King, T.; Osmond-McLeod, M.J.; Du, L.L. Trends in Food Science & Technology Nanotechnology in the food sector and potential applications for the poultry industry. Trends Food Sci. Technol. 2018, 72, 62–73. [Google Scholar]
- Wang, C.; Zhang, L.; Su, W.; Ying, Z.; He, J.; Zhang, L.; Zhong, X.; Wang, T. Zinc oxide nanoparticles as a substitute for zinc oxide or colistin sulfate: Effects on growth, serum enzymes, zinc deposition, intestinal morphology and epithelial barrier in weaned piglets. PLoS ONE 2017, 12, e0181136. [Google Scholar] [CrossRef]
- Duffy, L.L.; Osmond-McLeod, M.J.; Judy, J.; King, T. Investigation into the antibacterial activity of silver, zinc oxide and copper oxide nanoparticles against poultry-relevant isolates of Salmonella and Campylobacter. Food Control 2018, 92, 293–300. [Google Scholar] [CrossRef]
- Kadiyala, U.; Turali-Emre, E.S.; Bahng, J.H.; Kotov, N.A.; VanEpps, J.S. Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus (MRSA). Nanoscale 2018, 10, 4927–4939. [Google Scholar] [CrossRef] [PubMed]
- Ajitha, B.; Kumar Reddy, Y.A.; Reddy, P.S.; Jeon, H.J.; Ahn, C.W. Role of capping agents in controlling silver nanoparticles size, antibacterial activity and potential application as optical hydrogen peroxide sensor. RSC Adv. 2016, 6, 36171–36179. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Abdul Rahman, N.; Mohamad, R.; Zaidan, U.H. Microbial Mediated Synthesis of Silver Nanoparticles by Lactobacillus plantarum TA4 and its Antibacterial and Antioxidant Activity. Appl. Sci. 2020, 10, 6973. [Google Scholar] [CrossRef]
- Darvishi, E.; Kahrizi, D.; Arkan, E. Comparison of different properties of zinc oxide nanoparticles synthesized by the green (using Juglans regia L. leaf extract) and chemical methods. J. Mol. Liq. 2019, 286, 110831. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Abdul Rahman, N.; Mohamad, R.; Zaidan, U.H.; Samsudin, A.A. Biosynthesis of zinc oxide nanoparticles by cell-biomass and supernatant of Lactobacillus plantarum TA4 and its antibacterial and biocompatibility properties. Sci. Rep. 2020, 10, 19996. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Rahman, N.A. Sustainable microbial cell nanofactory for zinc oxide nanoparticles production by zinc-tolerant probiotic Lactobacillus plantarum strain TA4. Microb. Cell Fact. 2020, 19, 10. [Google Scholar] [CrossRef]
- Haque, M.A.; Imamura, R.; Brown, G.A.; Krishnamurthi, V.R.; Niyonshuti, I.I.; Marcelle, T.; Mathurin, L.E.; Chen, J.; Wang, Y. An experiment-based model quantifying antimicrobial activity of silver nanoparticles on Escherichia coli. RSC Adv. 2017, 7, 56173–56182. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Seventh Informational Supplement; CLSI Document M100-S27.; CLSI: Wayne, PA, USA, 2017; ISBN 1562387855. [Google Scholar]
- Ashengroph, M.; Khaledi, A.; Bolbanabad, E.M. Extracellular biosynthesis of cadmium sulphide quantum dot using cell-free extract of Pseudomonas chlororaphis CHR05 and its antibacterial activity. Process Biochem. 2020, 89, 63–70. [Google Scholar] [CrossRef]
- Famuyide, I.M.; Aro, A.O.; Fasina, F.O.; Eloff, J.N.; McGaw, L.J. Antibacterial and antibiofilm activity of acetone leaf extracts of nine under-investigated south African Eugenia and Syzygium (Myrtaceae) species and their selectivity indices. BMC Complement. Altern. Med. 2019, 19, 141. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, D.S.; El-Baky, R.M.A.; Sandle, T.; Mandour, S.A.; Ahmed, E.F. Antimicrobial activity of silver-treated bacteria against other multi-drug resistant pathogens in their environment. Antibiotics 2020, 9, 181. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.M.; Polash, S.A.; Takikawa, M.; Shubhra, R.D.; Saha, T.; Islam, Z.; Hossain, S.; Hasan, M.A.; Takeoka, S.; Sarker, S.R. Investigation of the Antibacterial Activity and in vivo Cytotoxicity of Biogenic Silver Nanoparticles as Potent Therapeutics. Front. Bioeng. Biotechnol. 2019, 7, 239. [Google Scholar] [CrossRef]
- Tiwari, V.; Mishra, N.; Gadani, K.; Solanki, P.S.; Shah, N.A.; Tiwari, M. Mechanism of Anti-bacterial Activity of Zinc Oxide Nanoparticle against Carbapenem-Resistant Acinetobacter baumannii. Front. Microbiol. 2018, 9, 1218. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Ezealisiji, K.M.; Siwe-Noundou, X.; Maduelosi, B.; Nwachukwu, N.; Krause, R.W.M. Green synthesis of zinc oxide nanoparticles using Solanum torvum (L.) leaf extract and evaluation of the toxicological profile of the ZnO nanoparticles–hydrogel composite in Wistar albino rats. Int. Nano Lett. 2019, 9, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Hanif, M.; Lee, I.; Akter, J.; Islam, M.; Zahid, A.; Sapkota, K.; Hahn, J. Enhanced Photocatalytic and Antibacterial Performance of ZnO Nanoparticles Prepared by an Efficient Thermolysis Method. Catalysts 2019, 9, 608. [Google Scholar] [CrossRef] [Green Version]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef] [PubMed]
- Swain, P.S.; Rao, S.B.N.; Rajendran, D.; Dominic, G.; Selvaraju, S. Nano zinc, an alternative to conventional zinc as animal feed supplement: A review. Anim. Nutr. 2016, 2, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.K.; Li, J. Current and future prospects for nanotechnology in animal production. J. Anim. Sci. Biotechnol. 2017, 8, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umar, H.; Kavaz, D.; Rizaner, N. Biosynthesis of zinc oxide nanoparticles using Albizia lebbeck stem bark, and evaluation of its antimicrobial, antioxidant, and cytotoxic activities on human breast cancer cell lines. Int. J. Nanomed. 2018, 14, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.; Das, D.; Andler, R.; Bandopadhyay, R. Green Synthesis of Silver Nanoparticles Using Exopolysaccharides Produced by Bacillus anthracis PFAB2 and Its Biocidal Property. J. Polym. Environ. 2021, 3, 02051. [Google Scholar]
- Sobhanifar, S.; Worrall, L.J.; Gruninger, R.J.; Wasney, G.A.; Blaukopf, M.; Baumann, L.; Lameignere, E.; Solomonson, M.; Brown, E.D.; Withers, S.G.; et al. Structure and mechanism of Staphylococcus aureus TarM, the wall teichoic acid α-glycosyltransferase. Proc. Natl. Acad. Sci. USA 2015, 112, E576–E585. [Google Scholar] [CrossRef] [Green Version]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Rossi, D.A.; Melo, R.T.; Mendonça, E.P.; Monteiro, G.P. Biofilms of Salmonella and Campylobacter in the Poultry Industry. In Poultry Science; InTechOpen: London, UK, 2017; pp. 93–113. [Google Scholar]
- Bhattacharyya, P.; Agarwal, B.; Goswami, M.; Maiti, D.; Baruah, S.; Tribedi, P. Zinc oxide nanoparticle inhibits the biofilm formation of Streptococcus pneumoniae. Antonie Van Leeuwenhoek 2018, 111, 89–99. [Google Scholar] [CrossRef]
- Khan, S.T.; Ahamed, M.; Musarrat, J.; Al-Khedhairy, A.A. Anti-biofilm and antibacterial activities of zinc oxide nanoparticles against the oral opportunistic pathogens Rothia dentocariosa and Rothia mucilaginosa. Eur. J. Oral Sci. 2014, 122, 397–403. [Google Scholar] [CrossRef]
- Shkodenko, L.; Kassirov, I.; Koshel, E. Metal Oxide Nanoparticles Against Bacterial Biofilms: Perspectives and Limitations. Microorganisms 2020, 8, 1545. [Google Scholar] [CrossRef] [PubMed]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Thomsen, T.R.; Winkler, H.; Xu, Y. Influence of biofilm growth age, media, antibiotic concentration and exposure time on Staphylococcus aureus and Pseudomonas aeruginosa biofilm removal in vitro. BMC Microbiol. 2020, 20, 264. [Google Scholar] [CrossRef]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial Activity and Mechanism of Action of Zinc Oxide Nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [Green Version]
- Hoseinzadeh, E.; Alikhani, M.-Y.; Samarghandi, M.-R.; Shirzad-Siboni, M. Antimicrobial potential of synthesized zinc oxide nanoparticles against gram positive and gram negative bacteria. Desalin. Water Treat. 2014, 52, 4969–4976. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, H.; Shen, Y.; Zhang, W.; Zhang, L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio Natriegens. PLoS ONE 2019, 14, e0222322. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Chaudhary, A. One Pot Synthesis of Gentamicin Conjugated Gold Nanoparticles as an Efficient Antibacterial Agent. J. Clust. Sci. 2020, 20, 1864. [Google Scholar] [CrossRef]
- Griendling, K.K.; Touyz, R.M.; Zweier, J.L.; Dikalov, S.; Chilian, W.; Chen, Y.R.; Harrison, D.G.; Bhatnagar, A. Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System: A Scientific Statement from the American Heart Association. Circ. Res. 2016, 119, e39–e75. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Cheng, Z.; Dai, G.; Wu, G.; Wang, L.; et al. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2019, 14, 1469–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Lee, H.S.; Ryu, D.S.; Choi, S.J.; Lee, D.S. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011, 39, 77–85. [Google Scholar]
- Jiang, Y.; Zhang, L.; Wen, D.; Ding, Y. Role of physical and chemical interactions in the antibacterial behavior of ZnO nanoparticles against E. coli. Mater. Sci. Eng. C 2016, 69, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Wu, C. Antimicrobial activity and the mechanism of silver nanoparticle thermosensitive gel. Int. J. Nanomed. 2011, 6, 2873–2877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravinayagam, V.; Rehman, S. Zeolitic imidazolate framework-8 (ZIF-8) doped TiZSM-5 and Mesoporous carbon for antibacterial characterization. Saudi J. Biol. Sci. 2020, 27, 1726–1736. [Google Scholar] [CrossRef]
- Alavi, M.; Karimi, N.; Salimikia, I. phytosynthesis of zinc oxide nanoparticles and its antibacterial, antiquorum sensing, antimotility, and antioxidant capacities against multidrug resistant bacteria. J. Ind. Eng. Chem. 2019, 72, 457–473. [Google Scholar] [CrossRef]
- Gad El-Rab, S.M.F.; Abo-Amer, A.E.; Asiri, A.M. Biogenic Synthesis of ZnO Nanoparticles and Its Potential Use as Antimicrobial Agent Against Multidrug-Resistant Pathogens. Curr. Microbiol. 2020, 77, 1767–1779. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; De Conti, R.; Alves, O.L.; Costa, F.T.M.; Brocchi, M. Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. J. Braz. Chem. Soc. 2010, 21, 949–959. [Google Scholar] [CrossRef]
- Wong, K.K.Y.; Liu, X. Silver nanoparticles—the real “silver bullet” in clinical medicine? Medchemcomm 2010, 1, 125. [Google Scholar] [CrossRef]
- Holt, K.B.; Bard, A.J. Interaction of silver(I) ions with the respiratory chain of Escherichia coli: An electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag. Biochemistry 2005, 44, 13214–13223. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2013, 5, 108–119. [Google Scholar] [CrossRef]
- Reda, F.M.; El-Saadony, M.T.; El-Rayes, T.K.; Attia, A.I.; El-Sayed, S.A.A.; Ahmed, S.Y.A.; Madkour, M.; Alagawany, M. Use of biological nano zinc as a feed additive in quail nutrition: Biosynthesis, antimicrobial activity and its effect on growth, feed utilisation, blood metabolites and intestinal microbiota. Ital. J. Anim. Sci. 2021, 20, 324–335. [Google Scholar] [CrossRef]
- Pei, X.; Xiao, Z.; Liu, L.; Wang, G.; Tao, W.; Wang, M.; Zou, J.; Leng, D. Effects of dietary zinc oxide nanoparticles supplementation on growth performance, zinc status, intestinal morphology, microflora population, and immune response in weaned pigs. J. Sci. Food Agric. 2019, 99, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Masood, S.; Zaneb, H.; Faseh-ur-Rehman, H.; Masood, S.; Khan, M.-R.; Khan Tahir, S.; ur Rehman, H. Supplementation of zinc oxide nanoparticles has beneficial effects on intestinal morphology in broiler chicken. Pak. Vet. J. 2017, 37, 335. [Google Scholar]
- Shao, Y.; Lei, Z.; Yuan, J.; Yang, Y.; Guo, Y.; Zhang, B. Effect of Zinc on Growth Performance, Gut Morphometry, and Cecal Microbial Community in Broilers Challenged with Salmonella enterica serovar typhimurium. J. Microbiol. 2014, 52, 1002–1011. [Google Scholar] [CrossRef]
- Khajeh Bami, M.; Afsharmanesh, M.; Salarmoini, M.; Tavakoli, H. Effect of zinc oxide nanoparticles and Bacillus coagulans as probiotic on growth, histomorphology of intestine, and immune parameters in broiler chickens. Comp. Clin. Path. 2018, 27, 399–406. [Google Scholar] [CrossRef]
- Deng, X.Y.; Luan, Q.X.; Chen, W.T.; Wang, Y.L.; Wu, M.H.; Zhang, H.J.; Jiao, Z. Nanosized zinc oxide particles induce neural stem cell apoptosis. Nanotechnology 2009, 20, 115101. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.; Layne, J.; Punnoose, A.; Reddy, K.M.; Coombs, I.; Coombs, A.; Feris, K.; Wingett, D. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 2008, 19, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pujalte, I.; Passagne, I.; Brouillaud, B.; Treguer, M.; Durand, E.; Ohayon-Courtes, C.; L’Azou, B. Cytotoxicity and oxidative stress induced by different metallic nanoparticles on human kidney cells. Part. Fibre Toxicol. 2011, 8, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Lu, J.; Zhou, L.; Li, J.; Xu, J.; Li, W.; Zhang, L.; Zhong, X.; Wang, T. Effects of long-term exposure to zinc oxide nanoparticles on development, zinc metabolism and biodistribution of minerals (Zn, Fe, Cu, Mn) in mice. PLoS ONE 2016, 11, e0164434. [Google Scholar] [CrossRef]
- Bisht, G.; Rayamajhi, S. ZnO Nanoparticles: A Promising Anticancer Agent. Nanobiomedicine 2016, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Niño-Martínez, N.; Salas Orozco, M.F.; Martínez-Castañón, G.-A.; Torres Méndez, F.; Ruiz, F. Molecular Mechanisms of Bacterial Resistance to Metal and Metal Oxide Nanoparticles. Int. J. Mol. Sci. 2019, 20, 2808. [Google Scholar] [CrossRef] [Green Version]
- Barreto, M.S.R.; Andrade, C.T.; da Silva, L.C.R.P.; Cabral, L.M.; Flosi Paschoalin, V.M.; Del Aguila, E.M. In vitro physiological and antibacterial characterization of ZnO nanoparticle composites in simulated porcine gastric and enteric fluids. BMC Vet. Res. 2017, 13, 181. [Google Scholar] [CrossRef] [Green Version]

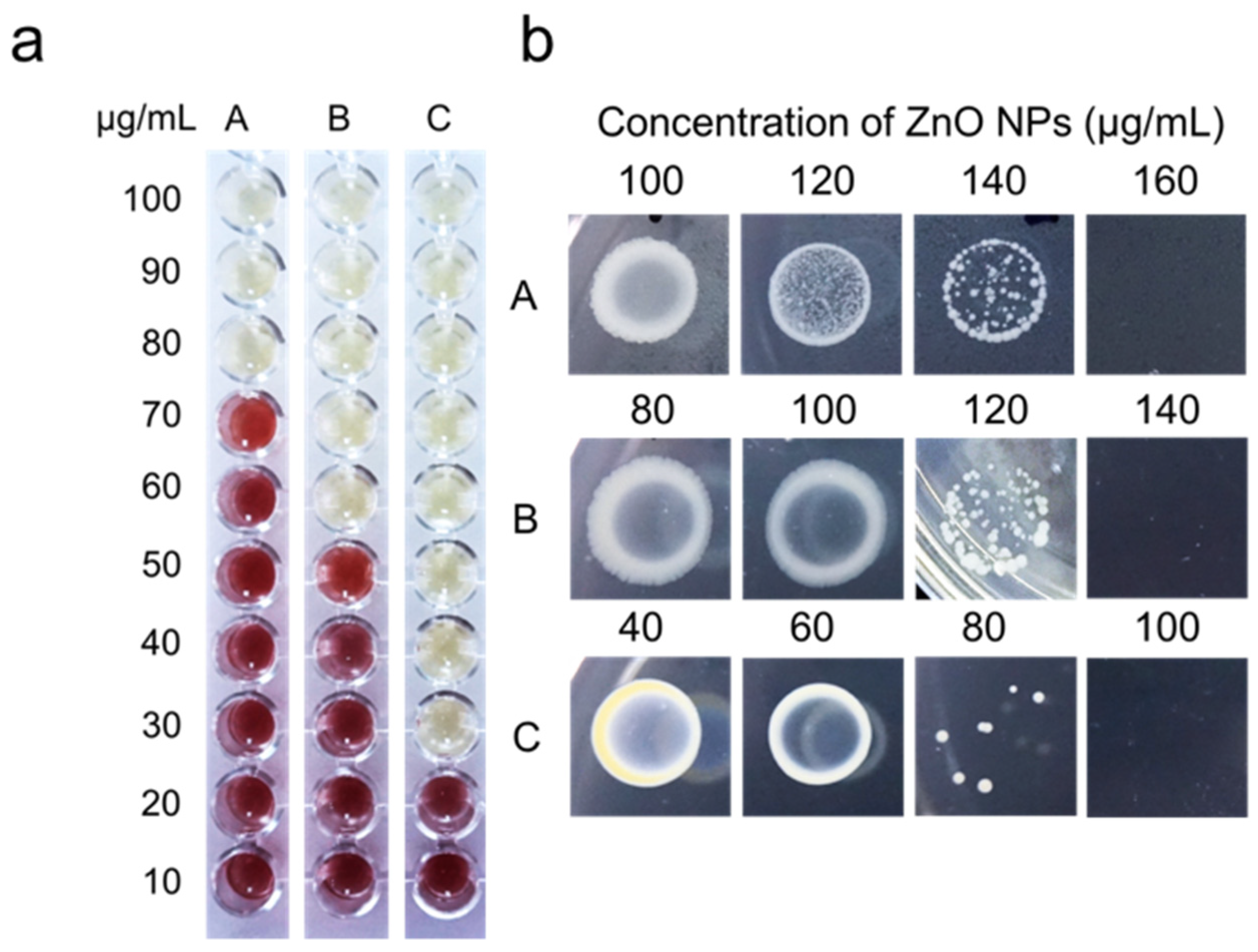





| Concentration (µg/mL) | Zone of Inhibition (mm) | |||||
|---|---|---|---|---|---|---|
| Salmonella spp. | E. coli | S. aureus | ||||
| Bulk ZnO | ZnO NPs | Bulk ZnO | ZnO NPs | Bulk ZnO | ZnO NPs | |
| 1000 | ND | 8.00 ± 0.00 d | ND | 8.00 ± 0.00 c | 7.33 ± 0.58 c,y | 11.33 ± 1.15 c,x |
| 2000 | ND | 9.33 ± 0.58 c | ND | 9.00 ± 1.00 b | 7.67 ± 0.58 c,y | 12.00 ± 1.00 c,x |
| 3000 | ND | 10.67 ± 0.58 b | ND | 10.33 ± 0.58 a,b | 8.67 ± 0.58 b,y | 15.00 ± 1.00 b,x |
| 4000 | ND | 12.00 ± 0.00 a | 7.00 ± 1.00 a,y | 11.00 ± 1.00 a,x | 9.67 ± 0.58 a,y | 16.00 ± 1.00 b,x |
| 5000 | ND | 12.33 ± 1.53 a | 8.00 ± 0.00 a,y | 12.00 ± 1.00 a,x | 10.00 ± 1.00 a,y | 19.67 ± 0.58 a,x |
| Bacteria | Bulk ZnO | ZnO NPs | ||
|---|---|---|---|---|
| MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | |
| Salmonella spp. | 200 | 800 | 80 | 160 |
| E. coli | 200 | 1000 | 60 | 140 |
| S. aureus | 100 | 800 | 30 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Yusof, H.; Abdul Rahman, N.; Mohamad, R.; Hasanah Zaidan, U.; Samsudin, A.A. Antibacterial Potential of Biosynthesized Zinc Oxide Nanoparticles against Poultry-Associated Foodborne Pathogens: An In Vitro Study. Animals 2021, 11, 2093. https://doi.org/10.3390/ani11072093
Mohd Yusof H, Abdul Rahman N, Mohamad R, Hasanah Zaidan U, Samsudin AA. Antibacterial Potential of Biosynthesized Zinc Oxide Nanoparticles against Poultry-Associated Foodborne Pathogens: An In Vitro Study. Animals. 2021; 11(7):2093. https://doi.org/10.3390/ani11072093
Chicago/Turabian StyleMohd Yusof, Hidayat, Nor’Aini Abdul Rahman, Rosfarizan Mohamad, Uswatun Hasanah Zaidan, and Anjas Asmara Samsudin. 2021. "Antibacterial Potential of Biosynthesized Zinc Oxide Nanoparticles against Poultry-Associated Foodborne Pathogens: An In Vitro Study" Animals 11, no. 7: 2093. https://doi.org/10.3390/ani11072093
APA StyleMohd Yusof, H., Abdul Rahman, N., Mohamad, R., Hasanah Zaidan, U., & Samsudin, A. A. (2021). Antibacterial Potential of Biosynthesized Zinc Oxide Nanoparticles against Poultry-Associated Foodborne Pathogens: An In Vitro Study. Animals, 11(7), 2093. https://doi.org/10.3390/ani11072093







