Discriminant Canonical Tool for Differential Biometric Characterization of Multivariety Endangered Hen Breeds
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
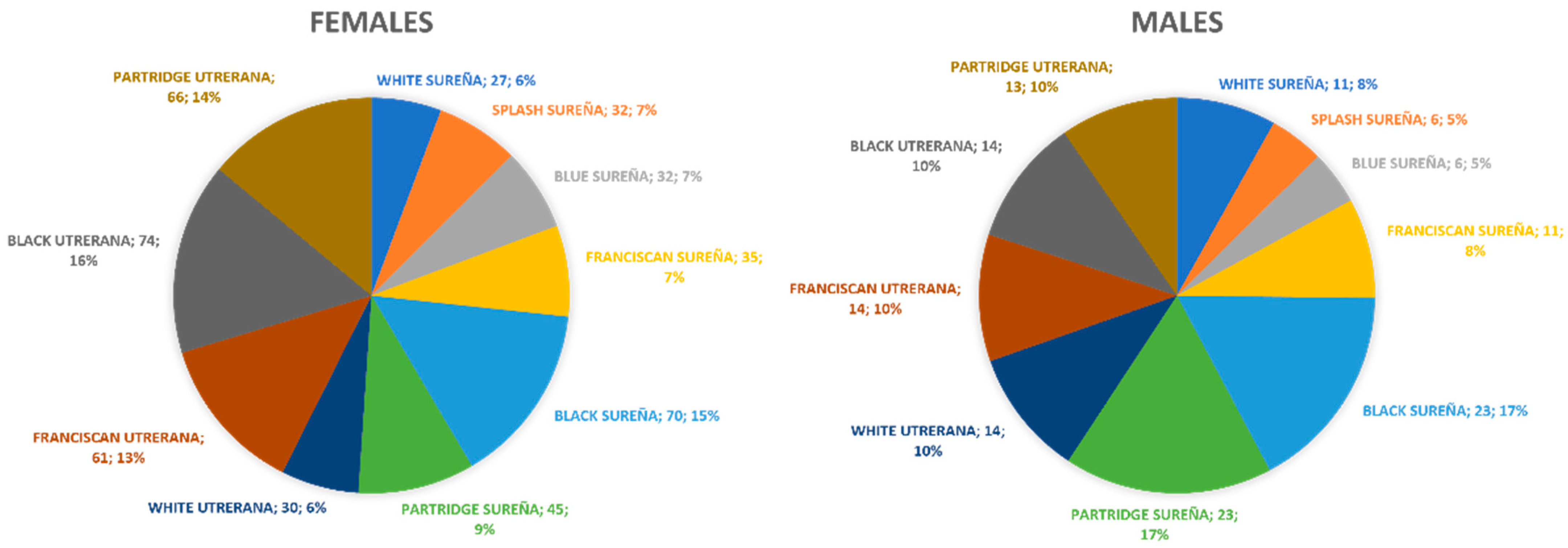
2.1. Animals, Sample Size, and Distribution
2.2. Biometric Measurement Collection
2.3. Normality and Kruskall–Wallis Tests
2.4. Discriminant Canonical Analysis (DCA)
2.4.1. Multicollinearity Preliminary Testing
2.4.2. Canonical Correlation Dimension Determination
2.4.3. Discriminant Canonical Analysis Efficiency
2.4.4. Discriminant Canonical Analysis Model Reliability
2.4.5. Variable Dimensionality Reduction
2.4.6. Canonical Coefficient and Loading Interpretation and Spatial Representation
2.4.7. Discriminant Function Cross-Validation
2.5. Data Mining CHAID Decision Tree
3. Results
3.1. Discriminant Canonical Analysis Reliability
3.2. Canonical Coefficients, Loading Interpretation, and Spatial Representation
3.3. Data Mining CHAID Decision Tree
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orozco, F. Razas de Gallinas Españolas; S.A. MUNDI-PRENSA LIBROS: Madrid, Spain, 1989; pp. 111–123. [Google Scholar]
- Dávila, S.; Gil, M.; Resino-Talaván, P.; Campo, J. Evaluation of diversity between different Spanish chicken breeds, a tester line, and a White Leghorn population based on microsatellite markers. Poult. Sci. 2009, 88, 2518–2525. [Google Scholar] [CrossRef] [PubMed]
- Sreenivas, D.; Prakash, M.; Mahender, M.; Chatterjee, R. Molecular genotyping of some poultry populations using microsatellite markers. Indian J. Poult. Sci. 2018, 53, 251–255. [Google Scholar] [CrossRef]
- Liverpool-Tasie, L.S.O.; Sanou, A.; Tambo, J.A. Climate change adaptation among poultry farmers: Evidence from Nigeria. Clim. Change 2019, 157, 527–544. [Google Scholar] [CrossRef] [Green Version]
- Hill, H. Raising chickens with altitude. Barnyards Backyards 2012, 1, 16–17. [Google Scholar]
- Macrì, M.; Martínez, A.; Landi, V.; Canales, A.; Arando, A.; Delgado, J.; Camacho, M. Genetic diversity of Utrerana chicken breed. Actas Iberoam. Conserv. Anim. 2019, 13, 52–59. [Google Scholar]
- Araújo de Carvalho, D.; Martínez Martínez, A.; Carolino, I.; Barros, M.C.; Camacho Vallejo, M.E.; Santos-Silva, F.; de Oliveira Almeida, M.J.; Carolino, N.; Delgado Bermejo, J.V.; Sarmento, J.L.R. Diversity and Genetic Relationship of Free-Range Chickens from the Northeast Region of Brazil. Animals 2020, 10, 1857. [Google Scholar] [CrossRef]
- Ocaña, J.; Morales, F.; Morilla, M.; Liñán, A.; Cabello, A.; León, J.M. Aproximación al patrón racial de la gallina sureña. Feagas 2008, 31, 38–41. [Google Scholar]
- González Ariza, A.; Navas González, F.J.; Arando Arbulu, A.; León Jurado, J.M.; Barba Capote, C.J.; Camacho Vallejo, M.E. Non-Parametrical Canonical Analysis of Quality-Related Characteristics of Eggs of Different Varieties of Native Hens Compared to Laying Lineage. Animals 2019, 9, 153. [Google Scholar] [CrossRef] [Green Version]
- Bettridge, J.M.; Psifidi, A.; Terfa, Z.G.; Desta, T.T.; Lozano-Jaramillo, M.; Dessie, T.; Kaiser, P.; Wigley, P.; Hanotte, O.; Christley, R.M. The role of local adaptation in sustainable production of village chickens. Nat. Sustain. 2018, 1, 574–582. [Google Scholar] [CrossRef]
- Canales, A.; Landi, V.; Martínez, A.; Macri, M.; Pizarro, G.; Delgado, J.; Cervantes, P.; Hernández, A.; Camacho, E. Genetic characterization of the domestic turkey of Mexican backyard. Arch. Zootec. 2019, 68, 480–487. [Google Scholar] [CrossRef] [Green Version]
- Dalle Zotte, A.; Gleeson, E.; Franco, D.; Cullere, M.; Lorenzo, J.M. Proximate Composition, Amino Acid Profile, and Oxidative Stability of Slow-Growing Indigenous Chickens Compared with Commercial Broiler Chickens. Foods 2020, 9, 546. [Google Scholar] [CrossRef] [PubMed]
- González Ariza, A.; Arando Arbulu, A.; Navas González, F.J.; Ruíz Morales, F.d.A.; León Jurado, J.M.; Barba Capote, C.J.; Camacho Vallejo, M.E. Sensory preference and professional profile affinity definition of endangered native breed eggs compared to commercial laying lineages’ eggs. Animals 2019, 9, 920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toalombo, P.; Camacho, C.; Buenaño, R.; Jiménez, S.; Navas-González, F.; Landi, V.; Delgado, J. Socioeconomic effect on morphological traits of Ecuador autochthonous hens. Arch. Zootec. 2019, 68, 416–421. [Google Scholar] [CrossRef] [Green Version]
- Torres, A.; Muth, P.C.; Capote, J.; Rodríguez, C.; Fresno, M.; Valle Zárate, A. Suitability of dual-purpose cockerels of 3 different genetic origins for fattening under free-range conditions. Poult. Sci. 2019, 98, 6564–6571. [Google Scholar] [CrossRef]
- Brito, N.V.; Lopes, J.C.; Ribeiro, V.; Dantas, R.; Leite, J.V. Biometric Characterization of the Portuguese Autochthonous Hens Breeds. Animals 2021, 11, 498. [Google Scholar] [CrossRef]
- Otecko, N.O.; Ogali, I.; Mauki, D.H.; Ogada, S.; Moraa, G.K.; Lichoti, J.; Agwanda, B.; Peng, M.S.; Ommeh, S.C.; Zhang, Y.P. Phenotypic and morphometric differentiation of indigenous chickens from Kenya and other tropical countries augments perspectives for genetic resource improvement and conservation. Poult. Sci. 2019, 98, 2747–2755. [Google Scholar] [CrossRef]
- Dorji, N.; Sunar, S. Short communication Morphometric variations among five Bhutanese indigenous chickens (Gallus domesticus). J. Anim. Poult. Sci. 2014, 3, 76–85. [Google Scholar]
- Stevens, J.P. Applied Multivariate Statistics for the Social Sciences; Routledge: New York, NY, USA, 2012. [Google Scholar]
- Toalombo Vargas, P.A.; Navas González, F.J.; Landi, V.; León Jurado, J.M.; Delgado Bermejo, J.V. Sexual dimorphism and breed characterization of Creole hens through biometric canonical discriminant analysis across Ecuadorian agroecological areas. Animals 2020, 10, 32. [Google Scholar] [CrossRef] [Green Version]
- Francesch, A.; Villalba, I.; Cartañà, M. Methodology for morphological characterization of chicken and its application to compare Penedesenca and Empordanesa breeds. Anim. Genet. Resour. 2011, 48, 79–84. [Google Scholar] [CrossRef] [Green Version]
- González Ariza, A.; Arando Arbulu, A.; Navas González, F.J.; Delgado Bermejo, J.V.; Camacho Vallejo, M.E. Discriminant Canonical Analysis as a Validation Tool for Multivariety Native Breed Egg Commercial Quality Classification. Foods 2021, 10, 632. [Google Scholar] [CrossRef]
- Marín Navas, C.; Delgado Bermejo, J.V.; McLean, A.K.; León Jurado, J.M.; Navas González, F.J. Discriminant Canonical Analysis of the Contribution of Spanish and Arabian Purebred Horses to the Genetic Diversity and Population Structure of Hispano-Arabian Horses. Animals 2021, 11, 269. [Google Scholar] [CrossRef]
- Tai, F.; Pan, W. Incorporating prior knowledge of gene functional groups into regularized discriminant analysis of microarray data. Bioinformatics 2007, 23, 3170–3177. [Google Scholar] [CrossRef] [Green Version]
- Rogerson, P.A. Data reduction: Factor analysis and cluster analysis. In Statistical Methods for Geography; SAGE Publishing: New York, NY, USA, 2001; pp. 192–197. [Google Scholar]
- Nanda, M.A.; Seminar, K.B.; Nandika, D.; Maddu, A. Discriminant analysis as a tool for detecting the acoustic signals of termites Coptotermes curvignathus (Isoptera: Rhinotermitidae). Int. J. Technol. 2018, 4, 840–851. [Google Scholar] [CrossRef] [Green Version]
- Anuthama, K.; Shankar, S.; Ilayaraja, V.; Kumar, G.S.; Rajmohan, M.; Vignesh. Determining dental sex dimorphism in South Indians using discriminant function analysis. Forensic Sci. Int. 2011, 212, 86–89. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Bai, Z. Modified Pillai’s trace statistics for two high-dimensional sample covariance matrices. J. Stat. Plan. Inference 2020, 207, 255–275. [Google Scholar] [CrossRef] [Green Version]
- Manly, B.F.; Alberto, J.A.N. Multivariate Statistical Methods: A Primer; CRC Press: Boca Ratón, FL, USA, 2016. [Google Scholar]
- Breiman, L.; Friedman, J.; Stone, C.J.; Olshen, R.A. Classification and Regression Trees; CRC Press: Boca Ratón, FL, USA, 1984. [Google Scholar]
- Ceylan, Z.; Gürsev, S.; Bulkan, S. An application of data mining in individual pension savings and investment system. EJOSAT 2018, 7–11. [Google Scholar]
- Baykara, B. Impact of Evaluation Methods on Decision Tree Accuracy. Master’s Thesis, University of Tampere, Tampere, Finland, April 2015. [Google Scholar]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Canonical correlation: A supplement to multivariate data analysis. In Multivariate Data Analysis: A Global Perspective, 7th ed.; Pearson Prentice Hall Publishing: Upper Saddle River, NY, USA, 2010. [Google Scholar]
- Chan, Y. Biostatistics 303. Discriminant analysis. SMJ 2005, 46, 54. [Google Scholar]
- Yakubu, A.; Salako, A. Path coefficient analysis of body weight and morphological traits of Nigerian indigenous chickens. Egypt. Poult. Sci. J. 2009, 29, 837–850. [Google Scholar]
- Coyne, J.A.; Kay, E.H.; Pruett-Jones, S. The genetic basis of sexual dimorphism in birds. Evolution 2008, 62, 214–219. [Google Scholar] [CrossRef]
- Desta, T. Phenotypic characteristic of junglefowl and chicken. Worlds Poult. Sci. J. 2019, 75, 69–82. [Google Scholar] [CrossRef]
- Ning, T.; Zhou, S.; Chang, F.; Shen, H.; Li, Z.; Liu, W. Interaction of vegetation, climate and topography on evapotranspiration modelling at different time scales within the Budyko framework. Agric. For. Meteorol. 2019, 275, 59–68. [Google Scholar] [CrossRef]
- Assefa, H.; Melesse, A. Morphological and morphometric characterization of indigenous chicken populations in Sheka Zone, South Western Ethiopia. Poultry Fish. Wildl. Sci. 2018, 6, 1–9. [Google Scholar] [CrossRef]
- Yaemkong, S.; Ngoc, T.N. Diversity of phenotypic characteristics of White Tailed-Yellow Chicken populations reared under free range system in Phitsanulok Province, Thailand. Biodiversitas 2019, 1, 20. [Google Scholar] [CrossRef] [Green Version]
- Patbandha, T.; Garg, D.; Vaghamashi, D.; Marandi, S.; Ravikala, K.; Patil, S.; Dash, S. Prediction of Market Weight in Caribro-Dhanraja Broilers with Different Plumage Colour Using Growth Traits. IJCMAS 2018, 7, 2018. [Google Scholar] [CrossRef]
- Wideman, N.; O’Bryan, C.A.; Crandall, P.G. Factors affecting poultry meat colour and consumer preferences—A review. Worlds Poult. Sci. J. 2016, 72, 353–366. [Google Scholar] [CrossRef]
- Dohner, J.V. The Encyclopedia of Historic and Endangered Livestock and Poultry Breeds; Yale University Press: New Haven, CT, USA, 2008. [Google Scholar]
- Wiener, P.; Wilkinson, S. Deciphering the genetic basis of animal domestication. Proc. Royal Soc. B 2011, 278, 3161–3170. [Google Scholar] [CrossRef] [Green Version]
- Keeling, L.; Andersson, L.; Schütz, K.; Kerje, S.; Fredriksson, R.; Carlborg, Ö.; Cornwallis, C.; Pizzari, T.; Jensen, P. Chicken genomics: Feather-pecking and victim pigmentation. Nature 2004, 431, 645–646. [Google Scholar] [CrossRef] [PubMed]
- Nätt, D.; Kerje, S.; Andersson, L.; Jensen, P. Plumage Color and Feather Pecking—Behavioral Differences Associated with PMEL17 Genotypes in Chicken (Gallus gallus). Behav. Genet. 2007, 37, 399–407. [Google Scholar] [CrossRef]
- Tickell, W.N. White plumage. Waterbirds 2003, 26, 1–12. [Google Scholar] [CrossRef]
- White, C.M.; Weeden, R.B. Hunting Methods of Gyrfalcons and Behavior of Their Prey (Ptarmigan). The Condor 1966, 68, 517–519. [Google Scholar] [CrossRef]
- Wisely, C.E.; Sayed, J.A.; Tamez, H.; Zelinka, C.; Abdel-Rahman, M.H.; Fischer, A.J.; Cebulla, C.M. The chick eye in vision research: An excellent model for the study of ocular disease. Prog. Retin. Eye Res. 2017, 61, 72–97. [Google Scholar] [CrossRef]
- Jones, M.P.; Pierce, K.E.; Ward, D. Avian Vision: A Review of Form and Function with Special Consideration to Birds of Prey. J. Exot. Pet Med. 2007, 16, 69–87. [Google Scholar] [CrossRef]
- Brooke, M.d.L.; Hanley, S.; Laughlin, S. The scaling of eye size with body mass in birds. Proc. Royal Soc. B Biol. Sci. 1999, 266, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Hall, M.; Ross, C. Eye shape and activity pattern in birds. J. Zool. 2007, 271, 437–444. [Google Scholar] [CrossRef]
- Podkowa, P.; Surmacki, A. The importance of illumination in nest site choice and nest characteristics of cavity nesting birds. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Møller, A.P.; Erritzøe, J. Flight distance and eye size in birds. Ethology 2010, 116, 458–465. [Google Scholar] [CrossRef]
- Banks, M.S.; Sprague, W.W.; Schmoll, J.; Parnell, J.A.Q.; Love, G.D. Why do animal eyes have pupils of different shapes? Sci. Adv. 2015, 1, 1500391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Juricic, E.; Gall, M.D.; Dolan, T.; Tisdale, V.; Martin, G.R. The visual fields of two ground-foraging birds, House Finches and House Sparrows, allow for simultaneous foraging and anti-predator vigilance. Ibis 2008, 150, 779–787. [Google Scholar] [CrossRef]
- Tätte, K.; Møller, A.P.; Mänd, R. Towards an integrated view of escape decisions in birds: Relation between flight initiation distance and distance fled. Anim. Behav. 2018, 136, 75–86. [Google Scholar] [CrossRef]
- De la Cruz Blanco, M.; Ocaña, J.; Rodríguez, M.; Cabello, A.; León, J.M.; Doctor, J. Estudio de la curva de crecimiento en la gallina Sureña. Feagas 2011, 34, 158–162. [Google Scholar]
- Tyasi, T.; Makgowo, K.; Mokoena, K.; Rashijane, L.; Mathapo, M.; Danguru, L.; Molabe, K.; Bopape, P.; Mathye, N.; Maluleke, D. Classification and regression tree (CRT) analysis to predict body weight of Potchefstroom koekoek laying hens. Adv. Anim. Vet. Sci 2020, 8, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Ukwu, H.O.; Okoro, V.M.O.; Nosike, R. Statistical modelling of body weight and linear body measurements in Nigerian indigenous chicken. IOSR-JAVS 2014, 7, 27–30. [Google Scholar]
- Lacin, E.; Yildiz, A.; Esenbuga, N.; Macit, M. Effects of differences in the initial body weight of groups on laying performance and egg quality parameters of Lohmann laying hens. Czech J. Anim. Sci 2008, 53, 466–471. [Google Scholar] [CrossRef] [Green Version]
- Lambertz, C.; Wuthijaree, K.; Gauly, M. Performance, behavior, and health of male broilers and laying hens of 2 dual-purpose chicken genotypes. Poult. Sci. 2018, 97, 3564–3576. [Google Scholar] [CrossRef] [PubMed]
- Jasouri, M.; Zamani, P.; Alijani, S. Dominance genetic and maternal effects for genetic evaluation of egg production traits in dual-purpose chickens. Br. Poult. Sci. 2017, 58, 498–505. [Google Scholar] [CrossRef]
- Wang, Y.; Bennewitz, J.; Wellmann, R. Novel optimum contribution selection methods accounting for conflicting objectives in breeding programs for livestock breeds with historical migration. Genet. Sel. Evol. 2017, 49, 45. [Google Scholar] [CrossRef] [Green Version]
- Delgado Bermejo, J.V.; Martínez Martínez, M.A.; Rodríguez Galván, G.; Stemmer, A.; Navas González, F.J.; Camacho Vallejo, M.E. Organization and management of conservation programs and research in domestic animal genetic resources. Diversity 2019, 11, 235. [Google Scholar] [CrossRef] [Green Version]
- Campo, J.L. Las Razas Ganaderas de Andalucía; Consejería de Agricultura y Pesca: Sevilla, Spain, 2007; Volume II, pp. 433–439. [Google Scholar]
- González Ariza, A.; Navas González, F.J.; Arando Arbulu, A.; Delgado Bermejo, J.V.; Camacho Vallejo, M.E. Hen breed and variety factors as a source of variability for the chemical composition of eggs. J. Food Compos. Anal. 2021, 95, 103673. [Google Scholar] [CrossRef]





| Corporal Region | Variable | Units | Measuring Procedure |
|---|---|---|---|
| General characteristics | Bodyweight | kg | With an electronic scale |
| Ornithological measurement | cm | Leaning the bird on its back, the distance between the tip of the beak and the tip of a central rectrix, in a straight line | |
| Wingspan | cm | Distance between the ends of the longest primaries with outstretched wings | |
| Head | Skull length | mm | Taken between the most protruding point of the occipital and the tip of the beak |
| Skull width | mm | Taken at eye level | |
| Comb length | mm | Measured between the insertion of the comb in the beak and the end of the comb’s lobe | |
| Comb width | mm | Measured from the tip of the central spike until the insertion of the comb in the skull; when the number of spikes was even, the highest was chosen | |
| Number of spikes in the comb | n | By manual counting | |
| Ocular length | mm | Measured between eyelid corners | |
| Ocular width | mm | Measured including the folds of the eyelid, perpendicular to the ocular length | |
| Beak length | mm | Measured from the tip of the beak until the insertion of the beak in the head | |
| Beak width | mm | Measured at level of insertion of the beak in the head | |
| Earlobe length | mm | Maximum length, keeping the bird’s head perpendicular to the neck | |
| Earlobe width | mm | As in the previous measure, measured the second-largest dimension | |
| Wattle length | mm | Measured from the insertion of wattle in the beak until the end of the wattle, in a straight line | |
| Wattle width | mm | As in the previous measure, measured the second-largest dimension | |
| Neck | Neck length | cm | Distance from the base of the neck to the chest |
| Body | Back length | cm | Distance from the insertion of the neck into the body to the tail insertion |
| Keel of sternum length | cm | Leaning the bird on its back, the distance between the two vertices of the sternum | |
| Breast circumference | cm | Measured at the level of the tip of the keel, passing the tape measure through the back of the wing insert | |
| Longitudinal diameter | cm | Measured from the cranial end of the coracoid to the most caudal portion of the pubis | |
| Tail length | cm | Distance from the tip of a central rectrix to the insertion of the tail | |
| Extremities | Folding wing length | cm | Distance from the carpal joint until the end of the longest primary |
| Thigh length | cm | Distance from the middle region of the coxal bone to the knee joint | |
| Tarsus length | cm | Distance from the notch of the shinbone tarsus until the tip of the nail of the middle finger | |
| Anteroposterior tarsus diameter | mm | Diameter of the tarsus in an anteroposterior direction in the middle part of the metatarsus bone | |
| Lateromedial tarsus diameter | mm | Diameter of the tarsus in a lateromedial direction in the middle part of the metatarsus bone |
| Trait | Mathematical Expression | |
|---|---|---|
| Skull ratio | SI: skull ratio; SL: skull length; SW: skull width | |
| Ocular ratio | OI: ocular ratio; OL: ocular length; OW: ocular width | |
| Beak ratio | BI: beak ratio; BL: beak length; BW: beak width | |
| Tarsus ratio | TI: tarsus ratio; APTD: anteroposterior tarsus diameter; LMTD: lateromedial tarsus diameter | |
| Females | Pillai’s trace criterion | 2.8664 |
| F (Observed value) | 7.1227 | |
| F (Critical value) | 1.1540 | |
| df1 | 261 | |
| df2 | 3978 | |
| p-value | <0.0001 | |
| alpha | 0.05 | |
| Males | Pillai’s trace criterion | 3.8256 |
| F (Observed value) | 2.7989 | |
| F (Critical value) | 1.1740 | |
| df1 | 252 | |
| df2 | 954 | |
| p-value | <0.0001 | |
| alpha | 0.05 |
| Test of Function(s) | Wilks’ Lambda | Chi-Square | df | Sig. | |
|---|---|---|---|---|---|
| Females | 1 through 7 | 0.045 | 1436.63 | 63 | 0 |
| 2 through 7 | 0.411 | 410.85 | 48 | 0 | |
| 3 through 7 | 0.814 | 95.218 | 35 | 0 | |
| Males | 1 through 4 | 0.017 | 515.527 | 36 | 0 |
| 2 through 4 | 0.242 | 180.18 | 24 | 0 | |
| 3 through 4 | 0.813 | 26.252 | 14 | 0.024 |
| Sex | Function | Eigenvalue | Discrimination (%) | Cumulative % |
|---|---|---|---|---|
| Females | F1 | 9.6611 | 58.8681 | 58.8681 |
| F2 | 5.1701 | 31.5034 | 90.3716 | |
| F3 | 0.7705 | 4.6950 | 95.0665 | |
| Males | F1 | 26.9110 | 69.5353 | 69.5353 |
| F2 | 7.3362 | 18.9561 | 88.4914 | |
| F3 | 2.7997 | 7.2342 | 95.7256 |
| Variables | Lambda | F | df1 | df2 | ρ-Value | Rank |
|---|---|---|---|---|---|---|
| Nail color (white) | 0.1911 | 217.2864 | 9 | 462 | <0.0001 | 1 |
| Ocular ratio | 0.3571 | 92.3999 | 9 | 462 | <0.0001 | 2 |
| Back length | 0.4291 | 68.3067 | 9 | 462 | <0.0001 | 3 |
| Body weight | 0.4318 | 67.5522 | 9 | 462 | <0.0001 | 4 |
| Ocular length | 0.4982 | 51.6983 | 9 | 462 | <0.0001 | 5 |
| Longitudinal diameter | 0.5184 | 47.6874 | 9 | 462 | <0.0001 | 6 |
| Keel of esternum length | 0.5262 | 46.2222 | 9 | 462 | <0.0001 | 7 |
| Wattle length | 0.5381 | 44.0615 | 9 | 462 | <0.0001 | 8 |
| Folding wing length | 0.5691 | 38.8630 | 9 | 462 | <0.0001 | 9 |
| Comb length | 0.5828 | 36.7513 | 9 | 462 | <0.0001 | 10 |
| Wattle width | 0.5986 | 34.4272 | 9 | 462 | <0.0001 | 11 |
| Breast circumference | 0.6052 | 33.4926 | 9 | 462 | <0.0001 | 12 |
| Thigh length | 0.6358 | 29.4067 | 9 | 462 | <0.0001 | 13 |
| Nail color (black/corneous) | 0.6736 | 24.8741 | 9 | 462 | <0.0001 | 14 |
| Ornithological measurement | 0.6831 | 23.8125 | 9 | 462 | <0.0001 | 15 |
| Comb width | 0.6868 | 23.4102 | 9 | 462 | <0.0001 | 16 |
| Beak width | 0.6935 | 22.6921 | 9 | 462 | <0.0001 | 17 |
| Earlobe width | 0.7001 | 21.9939 | 9 | 462 | <0.0001 | 18 |
| Tail length | 0.7660 | 15.6822 | 9 | 462 | <0.0001 | 19 |
| Beak length | 0.7855 | 14.0167 | 9 | 462 | <0.0001 | 20 |
| Earlobe length | 0.8005 | 12.7947 | 9 | 462 | <0.0001 | 21 |
| Nail color (slate/corneous) | 0.8156 | 11.6036 | 9 | 462 | <0.0001 | 22 |
| Nail color (slate) | 0.8426 | 9.5928 | 9 | 462 | <0.0001 | 23 |
| Skull length | 0.8629 | 8.1568 | 9 | 462 | <0.0001 | 24 |
| Number of beaks in comb | 0.9095 | 5.1094 | 9 | 462 | <0.0001 | 25 |
| Tarsus ratio | 0.9416 | 3.1857 | 9 | 462 | 0.0009 | 26 |
| Skull ratio | 0.9703 | 1.5692 | 9 | 462 | 0.1217 | 27 |
| Nail color (black/white) | 0.9869 | 0.6793 | 9 | 462 | 0.7279 | 28 |
| Presence or absence of spurs | 0.9903 | 0.5005 | 9 | 462 | 0.8743 | 29 |
| Variables | Lambda | F | df1 | df2 | ρ-Value | Rank |
|---|---|---|---|---|---|---|
| Ocular ratio | 0.1797 | 63.4040 | 9 | 125 | <0.0001 | 1 |
| Beak color (black/corneous) | 0.2102 | 52.1922 | 9 | 125 | <0.0001 | 2 |
| Beak color (white) | 0.3489 | 25.9192 | 9 | 125 | <0.0001 | 3 |
| Wingspan | 0.3765 | 22.9996 | 9 | 125 | <0.0001 | 4 |
| Beak color (black) | 0.4526 | 16.7993 | 9 | 125 | <0.0001 | 5 |
| Back length | 0.4547 | 16.6534 | 9 | 125 | <0.0001 | 6 |
| Ocular length | 0.5279 | 12.4222 | 9 | 125 | <0.0001 | 7 |
| Longitudinal diameter | 0.5536 | 11.1984 | 9 | 125 | <0.0001 | 8 |
| Anteroposterior tarsus diameter | 0.5576 | 11.0173 | 9 | 125 | <0.0001 | 9 |
| Body weight | 0.6399 | 7.8142 | 9 | 125 | <0.0001 | 10 |
| Breast circumference | 0.6511 | 7.4427 | 9 | 125 | <0.0001 | 11 |
| Folding wing length | 0.6653 | 6.9859 | 9 | 125 | <0.0001 | 12 |
| Earlobe width | 0.7245 | 5.2821 | 9 | 125 | <0.0001 | 13 |
| Beak color (corneous) | 0.7272 | 5.2092 | 9 | 125 | <0.0001 | 14 |
| Keel of sternum length | 0.7424 | 4.8184 | 9 | 125 | <0.0001 | 15 |
| Wattle length | 0.7819 | 3.8731 | 9 | 125 | 0.0002 | 16 |
| Comb length | 0.7899 | 3.6936 | 9 | 125 | 0.0004 | 17 |
| Beak width | 0.7903 | 3.6848 | 9 | 125 | 0.0004 | 18 |
| Beak length | 0.8000 | 3.4712 | 9 | 125 | 0.0007 | 19 |
| Earlobe length | 0.8194 | 3.0609 | 9 | 125 | 0.0024 | 20 |
| Number of beaks in comb | 0.8225 | 2.9981 | 9 | 125 | 0.0029 | 21 |
| Thigh length | 0.8296 | 2.8519 | 9 | 125 | 0.0043 | 22 |
| Neck length | 0.8707 | 2.0623 | 9 | 125 | 0.0378 | 23 |
| Ornithological measurement | 0.8798 | 1.8980 | 9 | 125 | 0.0580 | 24 |
| Comb width | 0.9029 | 1.4932 | 9 | 125 | 0.1574 | 25 |
| Tarsus ratio | 0.9072 | 1.4215 | 9 | 125 | 0.1858 | 26 |
| Skull ratio | 0.9254 | 1.1189 | 9 | 125 | 0.3544 | 27 |
| Beak color (caramel/corneous) | 0.9300 | 1.0460 | 9 | 125 | 0.4077 | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González Ariza, A.; Arando Arbulu, A.; León Jurado, J.M.; Navas González, F.J.; Delgado Bermejo, J.V.; Camacho Vallejo, M.E. Discriminant Canonical Tool for Differential Biometric Characterization of Multivariety Endangered Hen Breeds. Animals 2021, 11, 2211. https://doi.org/10.3390/ani11082211
González Ariza A, Arando Arbulu A, León Jurado JM, Navas González FJ, Delgado Bermejo JV, Camacho Vallejo ME. Discriminant Canonical Tool for Differential Biometric Characterization of Multivariety Endangered Hen Breeds. Animals. 2021; 11(8):2211. https://doi.org/10.3390/ani11082211
Chicago/Turabian StyleGonzález Ariza, Antonio, Ander Arando Arbulu, José Manuel León Jurado, Francisco Javier Navas González, Juan Vicente Delgado Bermejo, and María Esperanza Camacho Vallejo. 2021. "Discriminant Canonical Tool for Differential Biometric Characterization of Multivariety Endangered Hen Breeds" Animals 11, no. 8: 2211. https://doi.org/10.3390/ani11082211
APA StyleGonzález Ariza, A., Arando Arbulu, A., León Jurado, J. M., Navas González, F. J., Delgado Bermejo, J. V., & Camacho Vallejo, M. E. (2021). Discriminant Canonical Tool for Differential Biometric Characterization of Multivariety Endangered Hen Breeds. Animals, 11(8), 2211. https://doi.org/10.3390/ani11082211






