A Retrospective Study on Bacteriology, Clinicopathologic and Radiographic Features in 28 Cats Diagnosed with Pyothorax
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Data
2.2. Radiographic Findings
2.3. Pleural Fluid Analysis and Cytological Evaluation
2.4. Bacterial Culture and Antimicrobial Susceptibility
2.5. Therapy and Outcomes
3. Results
3.1. Signalment and History
3.2. Clinical Findings
3.3. Haematology and Biochemical Results
3.4. Radiographic Findings
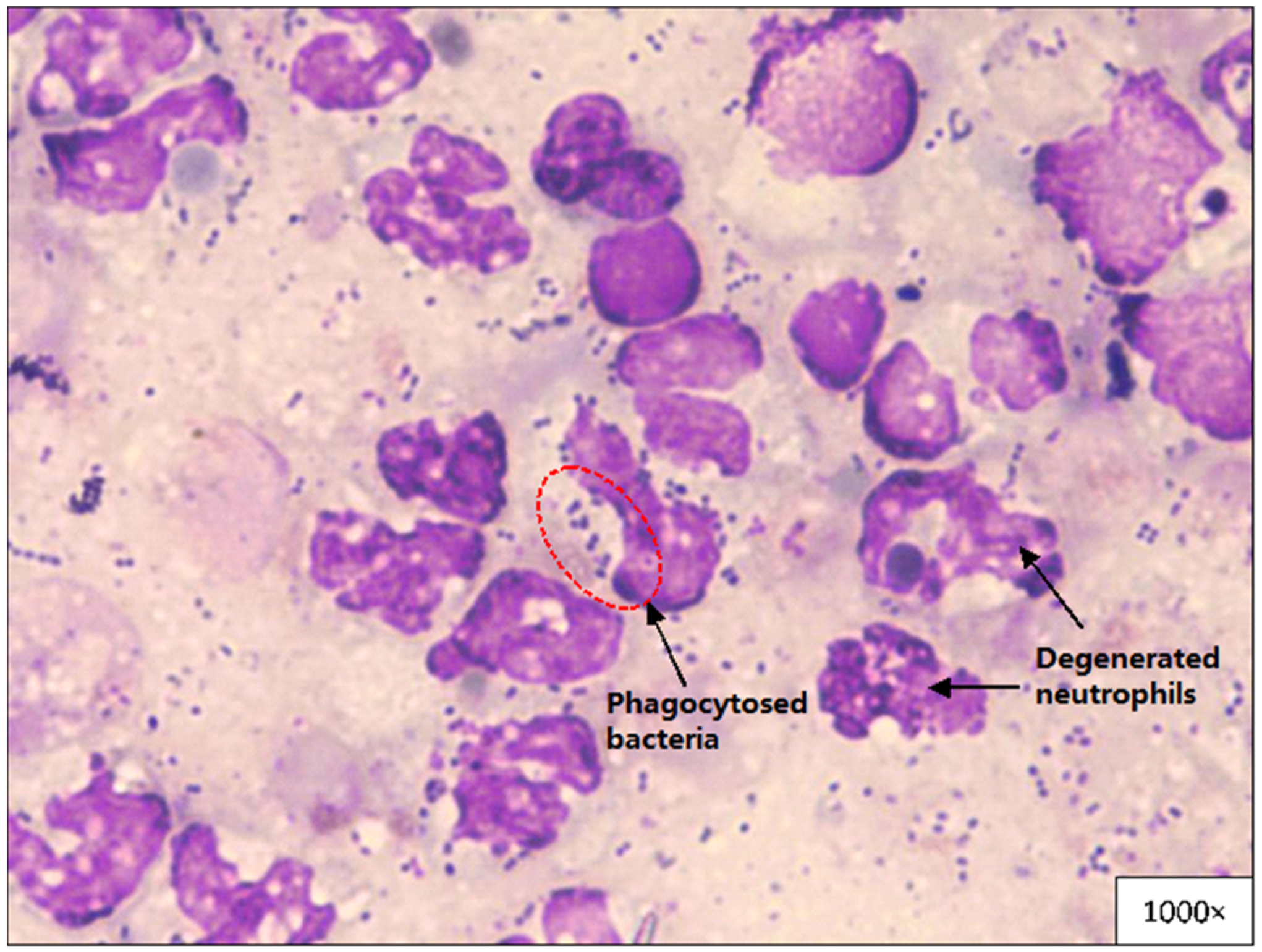
3.5. Pleural Fluid Analysis and Cytological Evaluation
3.6. Bacterial Identification and Antimicrobial Susceptibility
3.7. Therapy and Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| spp. | Species (plural) |
| BCS | Body condition score |
| RL | Reference limit |
| RBC | Red blood cell |
| PCV | Packed cell volume |
| CWCC | Complete white cell count |
| WBC | White blood cell |
| ALT | Alanine transaminase |
| ALP | Alkaline phosphatase |
| GGT | Gamma glutamyl transferase |
| AST | Aspartate transaminase |
| TP | Total protein |
| A:G | Albumin:globulin ratio |
| SE | Standard error |
| g | Gram |
| L | Litre |
| mmol | Millimoles |
| IV | Intravenous |
| PO | Per oral |
| SQ | Subcutaneous |
| n | Sample size |
| FeLV | Feline leukaemia virus |
| FIV | Feline immunodeficiency virus |
| UK | United Kingdom |
| PCR | Polymerase chain reaction |
References
- Alashraf, A.R.; Lau, S.F.; Khor, K.H.; Khairani-Bejo, S.; Bahaman, A.R.; Roslan, M.A.; Rahman, M.S.A.; Goh, S.H.; Radzi, R. Serological Detection of Anti-Leptospira Antibodies in Shelter Cats in Malaysia. Top. Companion Anim. Med. 2019, 34, 10–13. [Google Scholar] [CrossRef]
- Sivagurunathan, A.; Atwa, A.M.; Lobetti, R. Prevalence of feline immunodeficiency virus and feline leukaemia virus infection in Malaysia: A retrospective study. J. Feline Med. Surg. Open Rep. 2018, 4, 2055116917752587. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.M.L.; Stellato, A.C.; Niel, L. Uncontrolled outdoor access for cats: An assessment of risks and benefits. Animals 2020, 10, 258. [Google Scholar] [CrossRef] [Green Version]
- Debbra, M.; Matsura, Y.; Shariffah, N.; Muhammad Nazri, K.; Azjeemah Bee, S.H.; Sharil Azwan, M.Z.; Fakhrulisham, R. Assessing the status of pet ownership in the community of Putrajaya. Malays. J. Vet. Res. 2019, 10, 61–71. [Google Scholar]
- Stillion, J.R.; Letendre, J.A. A clinical review of the pathophysiology, diagnosis, and treatment of pyothorax in dogs and cats. J. Vet. Emerg. Crit. Care 2015, 25, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Randolph, M.B. The Thoracic Cavity. In The Cat: Clinical Medicine and Management; Little, S., Ed.; W.B. Saunders: Saint Louis, MO, USA, 2012; pp. 892–913. ISBN 978-1-4377-0660-4. [Google Scholar]
- Barrs, V.R.; Allan, G.S.; Martin, P.; Beatty, J.A.; Malik, R. Feline pyothorax: A retrospective study of 27 cases in Australia. J. Feline Med. Surg. 2005, 7, 211–222. [Google Scholar] [CrossRef]
- Waddell, L.S.; Brady, C.A.; Drobatz, K.J. Risk factors, prognostic indicators, and outcome of pyothorax in cats: 80 cases (1986–1999). J. Am. Vet. Med Assoc. 2002, 221, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Gorris, F.; Faut, S.; Daminet, S.; De Rooster, H.; Saunders, J.H.; Paepe, D. Pyothorax in cats and dogs. Vlaams Diergeneeskd. Tijdschr. 2017, 86, 183–197. [Google Scholar] [CrossRef]
- Odunayo, A. Pyothorax. In August’s Consultations in Feline Internal Medicine; Little, S., Ed.; W.B. Saunders: Saint Louis, MO, USA, 2016; Volume 7, ISBN 9780323226530. [Google Scholar]
- Gulbahar, M.Y.; Gurturk, K. Pyothorax associated with a Mycoplasma sp and Arcanobacterium pyogenes in a kitten. Aust. Vet. J. 2002, 80, 344–345. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.F.; Martin, P.; Allan, G.S.; Barrs, V.R.; Malik, R. Lower respiratory tract infections in cats: 21 cases (1995–2000). J. Feline Med. Surg. 2004, 6, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.E.; Balsa, I.M. Canine and Feline Exudative Pleural Diseases. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 467–487. [Google Scholar] [CrossRef]
- Demetriou, J.L.; Foale, R.D.; Ladlow, J.; McGrotty, Y.; Faulkner, J.; Kirby, B.M. Canine and feline pyothorax: A retrospective study of 50 cases in the UK and Ireland. J. Small Anim. Pract. 2002, 43, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.E. Exudative pleural diseases in small animals. Vet. Clin. N. Am. Small Anim. Pract. 2014, 44, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Holloway, S.; Trott, D.; Shipstone, M.; Barrs, V.; Malik, R.; Burrows, M. Antibiotic Prescribing Detailed Guideline; Australasian Infectious Disease Advisory Panel: St. Lucia, Australia, 2013. [Google Scholar]
- Gaskell, R.M.; Radford, A.D.; Dawson, S. Feline Infectious Respiratory Disease. In Feline Medicine and Therapeutics; Chandler, E.A., Gaskell, C.J., Gaskell, R.M., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; pp. 577–595. ISBN 9780470690727. [Google Scholar]
- Crowe, J.E. Viral pneumonia. In Kendig Chernick’s Disord. Respir. Tract. Child.; Chernick, V., Boat, T.F., Wilmott, R.W., Bush, A., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2012; pp. 453–460. ISBN 978-0-7216-3695-5. [Google Scholar]
- Racklyeft, D.J.; Raidal, S.; Love, D.N. Towards an understanding of equine pleuropneumonia: Factors relevant for control. Aust. Vet. J. 2000, 78, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.R. Interpreting small animal thoracic radiographs. Proced. Pro/NAVC Clin. Br. 2010, 65–70. Available online: https://www.cliniciansbrief.com/article/interpreting-small-animal-thoracic-radiographs (accessed on 1 August 2021).
- Watts, J.L. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 3rd ed.; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2008; ISBN 9781562386597. [Google Scholar]
- Barrs, V.R.; Beatty, J.A. Feline pyothorax—New insights into an old problem: Part 1. Aetiopathogenesis and diagnostic investigation. Vet. J. 2009, 179, 163–170. [Google Scholar] [CrossRef]
- Kuhajda, I.; Zarogoulidis, K.; Tsirgogianni, K.; Tsavlis, D.; Kioumis, I.; Kosmidis, C.; Tsakiridis, K.; Mpakas, A.; Zarogoulidis, P.; Zissimopoulos, A.; et al. Lung abscess-etiology, diagnostic and treatment options. Ann. Transl. Med. 2015, 3. [Google Scholar] [CrossRef]
- Arroyo, M.G.; Slovis, N.M.; Moore, G.E.; Taylor, S.D. Factors Associated with Survival in 97 Horses with Septic Pleuropneumonia. J. Vet. Intern. Med. 2017, 31, 894–900. [Google Scholar] [CrossRef] [Green Version]
- Wohleb, E.S.; McKim, D.B.; Sheridan, J.F.; Godbout, J.P. Monocyte trafficking to the brain with stress and inflammation: A novel axis of immune-to-brain communication that influences mood and behavior. Front. Neurosci. 2015, 8, 447. [Google Scholar] [CrossRef]
- Chiu, S.; Bharat, A. Role of monocytes and macrophages in regulating immune response following lung transplantation. Curr. Opin. Organ. Transplant. 2016, 21, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Savigny, M.; Macintire, D.K. Human Albumin therapy in Hypoalbuminemic Dogs. Stand. Care Emerg. Crit. Care Med. 2006, 8, 5. [Google Scholar]
- Ottenjann, M.; Weingart, C.; Arndt, G.; Kohn, B. Characterization of the anemia of inflammatory disease in cats with abscesses, pyothorax, or fat necrosis. J. Vet. Intern. Med. 2006, 20, 1143–1150. [Google Scholar] [CrossRef]
- Gorris, F.; Faut, S.; De Rooster, H.; Vandervekens, E.; Bosmans, T.; Daminet, S.; Smets, P.; Paepe, D. Two cases of feline pyothorax: Medical versus surgical treatment and associated challenges. Vlaams Diergeneeskd. Tijdschr. 2017, 86, 162–172. [Google Scholar] [CrossRef]
- Thrall, D.E. Textbook of Veterinary Diagnostic Radiology-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Beatty, J.; Barrs, V. Pleural effusion in the cat. A practical approach to determining aetiology. J. Feline Med. Surg. 2010, 12, 693–707. [Google Scholar] [CrossRef]
- Barrs, V.R.; Beatty, J.A. Feline pyothorax—New insights into an old problem: Part 2. Treatment recommendations and prophylaxis. Vet. J. 2009, 179, 171–178. [Google Scholar] [CrossRef]
- Woods, S.J.; Spriet, M.; Safra, N.; Cissell, D.D.; Borjesson, D.L. Hounsfield units are a useful predictor of pleural effusion cytological type in dogs but not in cats. Vet. Radiol. Ultrasound 2018, 59, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Briola, C.; Zoia, A.; Rocchi, P.; Caldin, M.; Bertolini, G. Computed tomography attenuation value for the characterization of pleural effusions in dogs: A cross-sectional study in 58 dogs. Res. Vet. Sci. 2019, 124, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Schultz, R.M.; Zwingenberger, A. Radiographic, computed tomographic, and ultrasonographic findings with migrating intrathoracic grass awns in dogs and cats. Vet. Radiol. Ultrasound 2008, 49, 249–255. [Google Scholar] [CrossRef]
- Walker, A.L.; Jang, S.S.; Hirsh, D.C. Bacteria associated with pyothorax of dogs and cats: 98 cases (1989–1998). J. Am. Vet. Med. Assoc. 2000, 216, 359–363. [Google Scholar] [CrossRef]
- Ahmed, A.E.H.; Yacoub, T.E. Empyema thoracis. Clin. Med. Insights Circ. Respir. Pulm. Med. 2010, 4, 75–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razali, K.; Kaidi, R.; Abdelli, A.; Menoueri, M.N.; Ait-Oudhia, K. Oral flora of stray dogs and cats in Algeria: Pasteurella and other zoonotic bacteria. Vet. World 2020, 13, 2806–2814. [Google Scholar] [CrossRef]
- Lloret, A.; Egberink, H.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lutz, H.; et al. Pasteurella Multocida Infection in Cats: ABCD guidelines on prevention and management. J. Feline Med. Surg. 2013, 15, 570–572. [Google Scholar] [CrossRef]
- Frymus, T.; Addie, D.D.; Boucraut-Baralon, C.; Egberink, H.; Gruffydd-Jones, T.; Hartmann, K.; Horzinek, M.C.; Hosie, M.J.; Lloret, A.; Lutz, H.; et al. Streptococcal infections in cats: ABCD guidelines on prevention and management. J. Feline Med. Surg. 2015, 17, 620–625. [Google Scholar] [CrossRef]
- Sykes, J.E. Bacterial Bronchopneumonia and Pyothorax. In Canine and Feline Infectious Diseases; Sykes, J.E., Ed.; W.B. Saunders: Saint Louis, MO, USA, 2014; pp. 847–858. ISBN 978-1-4377-0795-3. [Google Scholar]
- Rzewuska, M.; Czopowicz, M.; Kizerwetter-świda, M.; Chrobak, D.; Błaszczak, B.; Binek, M. Multidrug resistance in Escherichia coli strains isolated from infections in dogs and cats in Poland (2007–2013). Sci. World J. 2015, 2015, 408205. [Google Scholar] [CrossRef] [Green Version]
- Valat, C.; Drapeau, A.; Beurlet, S.; Bachy, V.; Boulouis, H.J.; Pin, R.; Cazeau, G.; Madec, J.Y.; Haenni, M. Pathogenic Escherichia coli in Dogs Reveals the Predominance of ST372 and the Human-Associated ST73 Extra-Intestinal Lineages. Front. Microbiol. 2020, 11, 580. [Google Scholar] [CrossRef]
- Brooks, J.W.; Roberts, E.L.; Kocher, K.; Kariyawasam, S.; DebRoy, C. Fatal pneumonia caused by Extraintestinal Pathogenic Escherichia coli (ExPEC) in a juvenile cat recovered from an animal hoarding incident. Vet. Microbiol. 2013, 167, 704–707. [Google Scholar] [CrossRef]
- Highland, M.A.; Byrne, B.A.; DebRoy, C.; Samitz, E.M.; Peterson, T.S.; Oslund, K.L. Extraintestinal pathogenic Escherichia coli-induced pneumonia in three kittens and fecal prevalence in a clinically healthy cohort population. J. Vet. Diagn. Investig. 2009, 21, 609–615. [Google Scholar] [CrossRef] [Green Version]
- Kimura, R.; Hayashi, Y.; Takeuchi, T.; Shimizu, M.; Iwata, M.; Tanahashi, J.; Ito, M. Pasteurella multocida septicemia caused by close contact with a domestic cat: Case report and literature review. J. Infect. Chemother. 2004, 10, 250–252. [Google Scholar] [CrossRef]
- Liu, W.; Chemaly, R.F.; Tuohy, M.J.; LaSalvia, M.M.; Procop, G.W. Pasteurella multocida Urinary Tract Infection with Molecular Evidence of Zoonotic Transmission. Clin. Infect. Dis. 2003, 36, e58–e60. [Google Scholar] [CrossRef] [Green Version]
- Miyoshi, S.; Hamada, H.; Miyoshi, A.; Ito, R.; Hamaguchi, N.; Murakami, S.; Miyamoto, H.; Takeuchi, T.; Okura, T.; Higaki, J. Pasteurella multocida pneumonia: Zoonotic transmission confirmed by molecular epidemiological. Geriatr. Gerontol. Int. 2012, 12, 159–163. [Google Scholar] [CrossRef]
- Hariharan, H.; Hariharan, S.H. Zoonotic bacteria associated with cats. Vet. Med. Open J. 2017, 2, 68–75. [Google Scholar] [CrossRef]
- Bélanger, L.; Garenaux, A.; Harel, J.; Boulianne, M.; Nadeau, E.; Dozois, C.M. Escherichia coli from animal reservoirs as a potential source of human extraintestinal pathogenic E. coli. FEMS Immunol. Med. Microbiol. 2011, 62, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Davies, H.E.; Davies, R.J.O.; Davies, C.W.H. Management of pleural infection in adults: British Thoracic Society pleural disease guideline 2010. Thorax 2010, 65, ii41–ii53. [Google Scholar] [CrossRef] [Green Version]
- Chapman, S.J.; Davies, R.J.O. The management of pleural space infections. Respirology 2004, 9, 4–11. [Google Scholar] [CrossRef]
- Moyaert, H.; de Jong, A.; Simjee, S.; Rose, M.; Youala, M.; El Garch, F.; Vila, T.; Klein, U.; Rzewuska, M.; Morrissey, I. Survey of antimicrobial susceptibility of bacterial pathogens isolated from dogs and cats with respiratory tract infections in Europe: ComPath results. J. Appl. Microbiol. 2019, 127, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Aarestrup, F.M.; Bager, F.; Jensen, N.E.; Madsen, M.; Meyling, A.; Wegener, H.C. Surveillance of antimicrobial resistance in bacteria isolated from food animals to antimicrobial growth promoters and related therapeutic agents in Denmark. Apmis 1998, 106, 606–622. [Google Scholar] [CrossRef]
- Hwang, I.Y.; Ku, H.O.; Lim, S.K.; Park, C.K.; Jung, G.S.; Jung, S.C.; Nam, H.M. Species distribution and resistance patterns to growth-promoting antimicrobials of enterococci isolated from pigs and chickens in Korea. J. Vet. Diagn. Investig. 2009, 21, 858–862. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Zhou, Z.C.; Zhu, L.; Wei, Y.Y.; Feng, W.Q.; Xu, L.; Liu, Y.; Lin, Z.J.; Shuai, X.Y.; Zhang, Z.J.; et al. The impact and mechanism of quaternary ammonium compounds on the transmission of antibiotic resistance genes. Environ. Sci. Pollut. Res. 2019, 26, 28352–28360. [Google Scholar] [CrossRef] [PubMed]
- Guardabassi, L.; Schwarz, S.; Lloyd, D.H. Pet animals as reservoirs of antimicrobial-resistant bacteria. J. Antimicrob. Chemother. 2004, 54, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Valiakos, G.; Pavlidou, E.; Zafeiridis, C.; Tsokana, C.N.; Del Rio Vilas, V.J. Antimicrobial practices among small animal veterinarians in Greece: A survey. One Health Outlook 2020, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lappin, M.R.; Blondeau, J.; Boothe, D.; Breitschwerdt, E.B.; Guardabassi, L.; Lloyd, D.H.; Papich, M.G.; Rankin, S.C.; Sykes, J.E.; Turnidge, J.; et al. Antimicrobial use Guidelines for Treatment of Respiratory Tract Disease in Dogs and Cats: Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases. J. Vet. Intern. Med. 2017, 31, 279–294. [Google Scholar] [CrossRef]
- Hawkins, E.C.; Fossum, T.W. Medical and surgical management of pleural effusion. Kirk’s Curr. Vet. Ther. Small Anim. Pract. XIII 2000, 13, 819–824. [Google Scholar]
- Boyle, T.E.; Hawkins, E.C. Feline pyothorax. Stand. Care Emerg. Crit. Care Med. 2005, 7, 7–12. [Google Scholar]
- DeBiasi, E.M.; Pisani, M.A.; Murphy, T.E.; Araujo, K.; Kookoolis, A.; Argento, A.C.; Puchalski, J. Mortality among patients with pleural effusion undergoing thoracentesis. Eur. Respir. J. 2015, 46, 495–502. [Google Scholar] [CrossRef] [Green Version]
- MacPhail, C.M. Medical and Surgical Management of Pyothorax. Vet. Clin. North. Am. Small Anim. Pract. 2007, 37, 975–988. [Google Scholar] [CrossRef] [PubMed]
- Moores, A.L. Lung. In Feline Soft Tissue and General Surgery; Langley-Hobbs, S.J., Demetriou, J.L., Ladlow, J., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2014; pp. 541–556. ISBN 978-0-7020-4336-9. [Google Scholar]
- Ringvold, C.H.O.; Weinreich, U.M. A case report of tardive subcutaneous emphysema in relation to iatrogenic pneumothorax. SAGE Open Med. Case Rep. 2019, 7, 2050313X19870970. [Google Scholar] [CrossRef]
- Sherman, A.; Holt, D.; Drobatz, K.; Mison, M. Evaluation of Jackson-Pratt Thoracostomy Drains Compared with Traditional Trocar Type and Guidewire-Inserted Thoracostomy Drains. J. Am. Anim. Hosp. Assoc. 2020, 56, 92–97. [Google Scholar] [CrossRef]
- Rehbein, S.; Manchi, G.; Gruber, A.D.; Kohn, B. Successful Treatment of Pneumothorax in a Dog with Sterile Pleural Fibrosis Caused by Chylothorax. Front. Vet. Sci. 2019, 6, 278. [Google Scholar] [CrossRef]
- Doyle, R.S.; Bellenger, C.R.; Campoy, L.; McAllister, H. Pyothorax in a cat managed by intrathoracic debridement and postoperative ventilatory support. Ir. Vet. J. 2005, 58, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Reed, N. Chronic Rhinitis in the Cat: An Update. Vet. Clin. Small Anim. Pract. 2020, 50, 311–329. [Google Scholar] [CrossRef]
- Di Cesare, A.; Di Francesco, G.; Frangipane di Regalbono, A.; Eleni, C.; De Liberato, C.; Marruchella, G.; Iorio, R.; Malatesta, D.; Romanucci, M.R.; Bongiovanni, L.; et al. Retrospective study on the occurrence of the feline lungworms Aelurostrongylus abstrusus and Troglostrongylus spp. in endemic areas of Italy. Vet. J. 2015, 203, 233–238. [Google Scholar] [CrossRef]

| Compartments | Checklist of Abnormalities |
|---|---|
| Extrathoracic structures | Discontinuity of diaphragm |
| Loss of diaphragm outline | |
| Bite wound at cervical area | |
| Evidence of tumour/trauma | |
| Presence of subcutaneous emphysema | |
| Abnormal size of liver | |
| Pleural space | Pleural effusion (bilateral or unilateral) |
| Pleural thickening | |
| Presence of fluid or air or mass | |
| Pneumothorax | |
| Extra-pleural sign | |
| Pulmonary parenchyma | Presence of atelectasis |
| Presence of consolidation | |
| Presence of cavitary/mass opacity | |
| Presence of bullae | |
| Presence of pneumothorax | |
| Mediastinum | |
| Cranial mediastinum | Presence of mediastinal widening |
| Displacement/changes in size and contour of trachea | |
| Oesophageal abnormalities | |
| Pneumomediastinum | |
| Middle mediastinum | Enlargement of tracheobronchial lymph nodes |
| Cardiac size and shape (inclusive of vertebral heart size) | |
| Caudal mediastinum | Displacement of aorta and oesophagus |
| Border effacement of accessory lung lobe |
| Patient Signalment | Number of Cases (n) | Percentage (%) |
|---|---|---|
| Male | ||
| Intact Male | 8 | 28.6 |
| Castrated Male | 4 | 14.3 |
| Female | ||
| Intact Female | 16 | 57.1 |
| Spayed Female | 0 | 0 |
| Vaccination | ||
| Yes | 6 | 21.4 |
| No | 22 | 78.6 |
| Access to outdoors | ||
| Yes | 18 | 64.3 |
| No | 6 | 21.4 |
| Not sure | 4 | 14.3 |
| Number of cats in household | ||
| Single cat household | 6 | 21.4 |
| Multi-cat household | 17 | 60.7 |
| Not sure | 5 | 17.9 |
| Concurrent Conditions | Number of Cases (n) | Percentage (%) |
|---|---|---|
| Concurrent feline upper respiratory disease * | 7 | 25.0 |
| Thoracic puncture wounds | 4 | 14.3 |
| Diaphragmatic hernia | 1 | 3.6 |
| Other concurrent condition/disease * | 4 | 14.3 |
| Unknown | 13 | 46.4 |
| Clinical Signs | Number of Cases (n) | Percentage (%) |
|---|---|---|
| Dyspnoea | 21 | 75.0 |
| Harsh/dull lung sound | 21 | 75.0 |
| Open-mouth breathing | 18 | 64.3 |
| Abdominal breathing | 16 | 57.1 |
| Tachypnoea | 11 | 39.3 |
| Lethargy | 11 | 39.3 |
| Hyporexia/anorexia | 10 | 35.7 |
| Dehydration | 10 | 35.7 |
| Ocular/nasal discharge | 8 | 28.6 |
| Pale mucous membrane | 7 | 25.0 |
| Crackle lung sound | 6 | 21.4 |
| Pyrexia | 5 | 17.9 |
| Tachycardia | 5 | 17.9 |
| Cyanotic | 5 | 17.9 |
| Weight loss | 4 | 14.3 |
| Recumbent | 3 | 10.7 |
| Lymph node enlargement | 3 | 10.7 |
| Cough | 2 | 7.1 |
| Parameters (Number of Samples) | Normal Reference Range | Mean ± SE | Median | n (%) with Value > Upper RL | n (%) with Value < Lower RL |
|---|---|---|---|---|---|
| Erythrocytes (RBC) (×1012/L) (n = 26) | 5–10 | 7.01 ± 0.33 | 7.07 | 0 (0) | 2 (7.7) |
| Haemoglobin (g/L) (n = 27) | 80–150 g/L | 102.05 ± 4.41 | 101.85 | 0 (0) | 4 (14.8) |
| PCV (L/L) (n = 26) | 0.24–0.45 | 0.28 ± 0.01 | 0.29 | 0 (0) | 5 (19.2) |
| CWCC (×109/L) (n = 26) | 5.5–19.5 | 26.79 ± 3.43 | 24.05 | 16 (61.5) | 3 (11.5) |
| Band neutrophils (×109/L) (n = 25) | <0.3 | 1.40 ± 0.37 | 0.79 | 17 (68) | 0 (0) |
| Segmented neutrophils (×109/L) (n = 25) | 2.5–12.5 | 20.31 ± 2.74 | 22.14 | 16 (64) | 3 (12) |
| Lymphocytes (×109/L) (n = 25) | 1.5–7.0 | 3.46 ± 0.52 | 2.23 | 3 (12) | 7 (28) |
| Monocytes (×109/L) (n = 25) | 0.2–0.8 | 1.62 ± 0.23 | 1.5 | 17 (68) | 2 (8) |
| Eosinophils (×109/L) (n = 25) | 0.1–1.5 | 0.66 ± 0.19 | 0.22 | 3 (12) | 7 (28) |
| Basophils (×109/L) (n = 7) | Rare | 0.0025 ± 0.0025 | 0 | - | - |
| Platelets (×109/L) (n = 24) | 300–700 | 228.20 ± 34.92 | 206 | 0 (0) | 17 (70.8) |
| Nucleated erythrocytes/100WBC (n = 8) | Rare | 5.5 ± 1.83 | 3.5 | - | - |
| Reticulocytes/100RBC (n = 16) | 0.5–1.5 | 2.91 ± 1.16 | 0.5 | 6 (37.5) | 7 (43.8) |
| Plasma Protein (g/L) (n = 26) | 60–80 | 86.85 ± 2.67 | 85 | 15 (57.7) | 0 (0) |
| Icterus Index (Unit) (n = 22) | <10 | 4.30 ± 1.18 | 2 | 2 (9.1) | 0 (0) |
| Sodium (mmol/L) (n = 21) | 146–156 | 149.40 ± 1.66 | 148.4 | 3 (14.3) | 6 (28.6) |
| Potassium (mmol/L) (n = 20) | 3.9–5.5 | 4.89 ± 0.17 | 4.95 | 4 (20) | 2 (10) |
| Chloride (mmol/L) (n = 21) | 110–132 | 112.62 ± 1.49 | 112.3 | 0 (0) | 5 (23.8) |
| Calcium (mmol/L) (n = 2) | 2.2–2.9 | 2.15 ± 0.05 | 2.15 | 0 (0) | 1 (50) |
| Inorganic phosphate (mmol/L) (n = 5) | 1.1–2.8 | 2.07 ± 0.23 | 2.4 | 0 (0) | 0 (0) |
| Urea (mmol/L) (n = 27) | 3.0–10.0 | 10.62 ± 1.11 | 8.8 | 13 (48.1) | 0 (0) |
| Creatinine (mmol/L) (n = 27) | 60–193 | 79.04 ± 5.48 | 80 | 0 (0) | 8 (29.6) |
| ALT (U/L) (n = 27) | 10–90 | 64.62 ± 16.89 | 41.6 | 2 (7.4) | 0 (0) |
| GGT (U/L) (n = 3) | <6.0 | 3.33 ± 2.03 | 3 | 1 (33.3) | - |
| Total Protein (g/L) (n = 27) | 55–75 | 78.74 ± 3.06 | 76 | 15 (55.6) | 1 (3.7) |
| Albumin (g/L) (n = 27) | 25–40 | 25.64 ± 0.82 | 25.4 | 0 (0) | 11 (40.7) |
| Globulin (g/L) (n = 26) | 25–45 | 53.14 ± 3.22 | 48.6 | 17 (65.4) | 0 (0) |
| A:G (n = 27) | 0.5–1.4 | 0.52 ± 0.03 | 0.5 | 0 (0) | 9 (33.3) |
| Radiographic Findings | Number of Cases (n) | Percentage (%) |
|---|---|---|
| Pleural effusion | 28 | 100 |
| Unilateral | 9 | 32.1 |
| Bilateral | 19 | 67.9 |
| Lung consolidation | 21 | 75.0 |
| Right | ||
| -Cranial | 14 | 50 |
| -Medial | 12 | 42.9 |
| -Caudal | 7 | 25 |
| Left | ||
| -Cranial | 12 | 42.9 |
| -Caudal | 5 | 17.9 |
| Obscured cardiac silhouette | 20 | 71.4 |
| Hepatomegaly | 9 | 32.1 |
| Peritoneal effusion | 3 | 10.7 |
| Vascular pattern | 2 | 7.1 |
| Cranial mediastinal lymph node enlargement | 1 | 3.6 |
| Sternal lymph node enlargement | 2 | 7.1 |
| Tracheobronchial lymph node enlargement | 0 | 0 |
| Bacteria Cultured | Number of Cases (n) | Percentage (%) |
|---|---|---|
| Pasteurella multocida | 9 | 28.12 |
| Streptococcus canis | 5 | 15.63 |
| Escherichia coli | 5 | 15.63 |
| Streptococcus viridans | 2 | 6.25 |
| Staphylococcus pseudintermedius | 2 | 6.25 |
| Streptococcus equi | 1 | 3.13 |
| Staphylococcus intermedius | 1 | 3.13 |
| Methylobacterium mesophilicum | 1 | 3.13 |
| Aggregatibacter actinomycetemcomitans | 1 | 3.13 |
| Neisseria zoodegmatis | 1 | 3.13 |
| Enterobacter cloacae | 1 | 3.13 |
| Acinetobacter lwoffii | 1 | 3.13 |
| Pseudmonas aeruginosa | 1 | 3.13 |
| Fungus-like bacteria | 1 | 3.13 |
| Total number of bacteria identified | 32 |
| Antimicrobials (Number of Samples) | Disk Content (µg) | Resistance (R + I) | Susceptibility (S) |
|---|---|---|---|
| Amoxicillin (n = 4) | 10 | 3 | 1 |
| Amoxicillin–clavulanate (n = 17) | 30 | 4 | 13 |
| Ampicillin (n = 1) | 10 | 1 | 0 |
| Azithromycin (n = 2) | 15 | 1 | 1 |
| Cephalexin (n = 13) | 30 | 8 | 5 |
| Ceftriaxone (n = 8) | 30 | 3 | 5 |
| Clindamycin (n = 1) | 2 | 1 | 0 |
| Enrofloxacin (n = 14) | 5 | 8 | 6 |
| Erythromycin (n = 2) | 15 | 1 | 1 |
| Gentamycin (n = 5) | 10 | 3 | 2 |
| Marbofloxacin (n = 14) | 5 | 10 | 4 |
| Norfloxacin (n = 1) | 10 | 0 | 1 |
| Sulfamethoxazole/trimethoprim (n = 3) | 25 | 1 | 2 |
| Tetracycline (n = 9) | 30 | 4 | 5 |
| Antimicrobial | Number of Cases | Route Administration | Dose (mg/kg) |
|---|---|---|---|
| Metronidazole | 21 | IV/PO | 10 |
| Amoxicillin–clavulanate | 14 | SQ/PO | 12.5 |
| Marbofloxacin | 13 | IV/PO | 2 |
| Cephalexin | 4 | PO | 20 |
| Ceftriaxone | 3 | IV | 25 |
| Enrofloxacin | 2 | IV | 5 |
| Number | Pre-Diagnosis (Antimicrobials) | Post-diagnosis (Antimicrobials) | Number of Cats (n = 28) | Outcome (n = 28) | Remarks |
|---|---|---|---|---|---|
| 1 | Amoxicillin–clavulanate + metronidazole | - | 10 | 6 died, 2 euthanised, 2 recovered | Six cats died in pre-diagnosed treatment. Two cats were euthanised due poor response to treatment. Two cats recovered in pre-diagnosed treatment. |
| 2 | Amoxicillin–clavulanate + metronidazole | Remained amoxicillin–clavulanate + metronidazole | 1 | 1 recovered | Bacteria were susceptible to amoxicillin–clavulanate. |
| 3 | Amoxicillin–clavulanate + marbofloxacin | - | 2 | 2 died | Two cats died in pre-diagnosed treatment. |
| 4 | Amoxicillin–clavulanate + marbofloxacin + metronidazole | - | 3 | 3 recovered | Three cats recovered in pre-diagnosed treatment. |
| 5 | Cephalexin + metronidazole | - | 1 | 1 died | One cat died in pre-diagnosed treatment. |
| 6 | Cephalexin + metronidazole | Switched to amoxicillin–clavulanate | 1 | 1 died | Infection relapsed after an apparently full recovery, the owner decided to stop the treatment. |
| 7 | Cephalexin + marbofloxacin + metronidazole | - | 1 | 1 recovered | One cat recovered in pre-diagnosed treatment. |
| 8 | Ceftriaxone + enrofloxacin + metronidazole | - | 1 | 1 recovered | Cat recovered in pre-diagnosed treatment. |
| 9 | Enrofloxacin + metronidazole | - | 1 | 1 died | The cat died on the day of presentation. |
| 10 | Marbofloxacin + metronidazole | - | 6 | 2 died, 4 recovered | Two cats died in pre-diagnosed treatment. Four cats recovered in pre-diagnosed treatment. |
| 11 | Marbofloxacin + metronidazole | Remained marbofloxacin + metronidazole | 1 | 1 recovered | Bacteria were susceptible to marbofloxacin |
| Status (n = 28) | Number of Cases (n) | Percentage n (%) |
|---|---|---|
| Survived | 13 | 46.4 |
| Dead | 15 | 53.6 |
| Cats with chest tube | 18 | 64.3 |
| Survived | 12 | 66.7 |
| Dead | 6 | 33.3 |
| Cats without chest tube | 10 | 35.7 |
| Survived | 1 | 10.0 |
| Dead | 9 | 90.0 |
| Cats with unilateral pleural effusion | 9 | 32.1 |
| Survived | 4 | 44.4 |
| Dead | 5 | 55.6 |
| Cats with bilateral pleural effusion | 19 | 67.9 |
| Survived | 9 | 47.4 |
| Dead | 10 | 52.6 |
| Complication (n = 11) | Number of Cases (n) | Percentage (%) |
|---|---|---|
| Lung bullae | 3 | 27.3 |
| Subcutaneous emphysema | 3 | 27.3 |
| Infection at site of chest tube placement | 3 | 27.3 |
| Pneumothorax | 2 | 18.2 |
| Failure of recovery of instilled lavage | 2 | 18.2 |
| Leakage of pleural fluid | 1 | 9.1 |
| Displaced chest tube | 1 | 9.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, J.J.; Lau, S.F.; Omar, S.; Watanabe, M.; Aslam, M.W. A Retrospective Study on Bacteriology, Clinicopathologic and Radiographic Features in 28 Cats Diagnosed with Pyothorax. Animals 2021, 11, 2286. https://doi.org/10.3390/ani11082286
Sim JJ, Lau SF, Omar S, Watanabe M, Aslam MW. A Retrospective Study on Bacteriology, Clinicopathologic and Radiographic Features in 28 Cats Diagnosed with Pyothorax. Animals. 2021; 11(8):2286. https://doi.org/10.3390/ani11082286
Chicago/Turabian StyleSim, Juin Jia, Seng Fong Lau, Sharina Omar, Malaika Watanabe, and Muhammad Waseem Aslam. 2021. "A Retrospective Study on Bacteriology, Clinicopathologic and Radiographic Features in 28 Cats Diagnosed with Pyothorax" Animals 11, no. 8: 2286. https://doi.org/10.3390/ani11082286
APA StyleSim, J. J., Lau, S. F., Omar, S., Watanabe, M., & Aslam, M. W. (2021). A Retrospective Study on Bacteriology, Clinicopathologic and Radiographic Features in 28 Cats Diagnosed with Pyothorax. Animals, 11(8), 2286. https://doi.org/10.3390/ani11082286






