Does PRGF Work? A Prospective Clinical Study in Dogs with A Novel Polylactic Acid Scaffold Injected with PRGF Using the Modified Maquet Technique
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Trial
2.2. PRGF Fabrication
2.3. Data Collection
2.4. Radiographic Assessment
2.5. Lameness Assessment
2.6. Statistical Method
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Montavon, P.M.; Damur, D.M. Advancement of the tibial tuberosity for the treatment of cranial cruciate deficient canine stifle. In Proceedings of the 1st World Orthopeadic Veterinary Congress, ESVOT-VOS Munich, Germany, 5–8 September 2002; p. 152. [Google Scholar]
- Montavon, P.M. Tibial tuberosity advancement (TTA) for the treatment of cranial cruciate disease in dogs: Evidences, technique and initial clinical results. In Proceedings of the 12th ESVOT Congress, Munich, Germany, 10–12 September 2004; pp. 254–255. [Google Scholar]
- Lafaver, S.; Miller, N.A.; Stubbs, W.P.; Taylor, R.A.; Boudrieau, R.J. Tibial tuberosity advancement for stabilization of the canine cranial cruciate ligament-deficient stifle joint: Surgical technique, early results, and complications in 101 dogs. Vet. Surg. 2007, 36, 573–586. [Google Scholar] [CrossRef]
- Bander, N.B.; Barnhart, M.D.; Watson, A.T.; Naber, S.J. Short-term prospective clinical evaluation of a polyglycolic acid tibial tuberosity advancement cage implant. J. Am. Anim. Hosp. Assoc. 2018, 54, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, D.; de Bruijn, J.D.; Luo, X.; Farè, S.; Grijpma, D.W.; Yuan, H. Controlling dynamic mechanical properties and degradation of composites for bone regeneration by means of filler content. J. Mech. Behav. Biomed. Mater. 2013, 20, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.; Dias, M.; Vorndran, E.; Gbureck, U.; Fernandes, P.; Pires, I.; Gouveia, B.; Armes, H.; Pires, E.; Rodrigues, J. Application of a 3D printed customized implant for canine cruciate ligament treatment by tibial tuberosity advancement. Biofabrication 2014, 6, 025005. [Google Scholar] [CrossRef] [PubMed]
- Etchepareborde, S.; Barthelemy, N.; Brunel, L.; Claeys, S.; Balligand, M. Biomechanical testing of a β-tricalcium phosphate wedge for advancement of the tibial tuberosity. Vet. Comp. Orthop. Traumatol. 2014, 27, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Danielson, B.; Barnhart, M.; Watson, A.; Kennedy, S.; Naber, S. Short-term radiographic complications and healing assessment of single-session bilateral tibial tuberosity advancements. J. Am. Anim. Hosp. Assoc. 2016, 52, 109–114. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, X.; Du, Z.; Jiang, W.; Han, X.; Zhao, D.; Han, D.; Li, Q. Three dimensional printed macroporous polylactic acid/hydroxyapatite composite scaffolds for promoting bone formation in a critical-size rat calvarial defect model. Sci. Technol. Adv. Mater. 2016, 17, 136–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castilho, M.; Rodrigues, J.; Vorndran, E.; Gbureck, U.; Quental, C.; Folgado, J.; Fernandes, P.R. Computational design and fabrication of a novel bioresorbable cage for tibial tuberosity advancement application. J. Mech. Behav. Biomed. Mater. 2017, 65, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Müller-Färber, J. Die metallentfernung nach osteosynthesen. Indikationen und risiken. Orthopade 2003, 32, 1039–1058. [Google Scholar]
- Onche, I.I.; Osagie, O.E.; INuhu, S. Removal of orthopaedic implants: Indications, outcome and economic implications enlèvement des implants orthopédiques: Indications, résultat et implications économiques. J. West African Coll. Surg. 2011, 1, 101–112. [Google Scholar]
- McCartney, W.; Ober, C.; Benito, M.; MacDonald, B. Comparison of tension band wiring and other tibial tuberosity advancement techniques for cranial cruciate ligament repair: An experimental study. Acta Vet. Scand. 2019, 61, 44. [Google Scholar] [CrossRef]
- Senatov, F.S.; Niaza, K.V.; Zadorozhnyy, M.Y.; Maksimkin, A.V.; Kaloshkin, S.D.; Estrin, Y.Z. Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. J. Mech. Behav. Biomed. Mater. 2016, 57, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Grémare, A.; Guduric, V.; Bareille, R.; Heroguez, V.; Latour, S.; L’heureux, N.; Fricain, J.C.; Catros, S.; Le Nihouannen, D. Characterization of printed PLA scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2018, 106, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Le, A.D.K.; Enweze, L.; DeBaun, M.R.; Dragoo, J.L. Platelet-Rich Plasma. Clin. Sports Med. 2019, 38, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Tsiridis, E.; Upadhyay, N.; Giannoudis, P. Molecular aspects of fracture healing: Which are the important molecules? Injury 2007, 38, S11–S25. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.H.R.W.; Mills, L.; Noble, B. The role of growth factors and related agents in accelerating fracture healing. J. Bone Jt. Surg.-Ser. B 2006, 88, 701–705. [Google Scholar] [CrossRef] [Green Version]
- Veillette, C.J.H.; McKee, M.D. Growth factors-BMPs, DBMs, and buffy coat products: Are there any proven differences amongst them? Injury 2007, 38, S38–S48. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Sommeling, C.E.; Heyneman, A.; Hoeksema, H.; Verbelen, J.; Stillaert, F.B.; Monstrey, S. The use of platelet-rich plasma in plastic surgery: A systematic review. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 301–311. [Google Scholar] [CrossRef]
- Marcazzan, S.; Weinstein, R.L.; Del Fabbro, M. Efficacy of platelets in bone healing: A systematic review on animal studies. Platelets 2018, 29, 326–337. [Google Scholar] [CrossRef]
- Nami, N.; Feci, L.; Napoliello, L.; Giordano, A.; Lorenzini, S.; Galeazzi, M.; Rubegni, P.; Fimiani, M. Crosstalk between platelets and PBMC: New evidence in wound healing. Platelets 2016, 27, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Intini, G. The use of platelet-rich plasma in bone reconstruction therapy. Biomaterials 2009, 30, 4956–4966. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, M.; Bortolin, M.; Taschieri, S.; Ceci, C.; Weinstein, R.L. Antimicrobial properties of platelet-rich preparations. A systematic review of the current pre-clinical evidence. Platelets 2016, 27, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.B.; Rahbek, O.; Overgaard, S.; Søballe, K. Platelet rich plasma and fresh frozen bone allograft as enhancement of implant fixation: An experimental study in dogs. J. Orthop. Res. 2004, 22, 653–658. [Google Scholar] [CrossRef]
- Roldán, J.C.; Jepsen, S.; Miller, J.; Freitag, S.; Rueger, D.C.; Açil, Y.; Terheyden, H. Bone formation in the presence of platelet-rich plasma vs. bone morphogenetic protein-7. Bone 2004, 34, 80–90. [Google Scholar] [CrossRef]
- Schlegel, K.A.; Donath, K.; Rupprecht, S.; Falk, S.; Zimmermann, R.; Felszeghy, E.; Wiltfang, J. De novo bone formation using bovine collagen and platelet-rich plasma. Biomaterials 2004, 25, 5387–5393. [Google Scholar] [CrossRef]
- Han, B.; Woodell-May, J.; Ponticiello, M.; Yang, Z.; Nimni, M. The effect of thrombin activation of platelet-rich plasma on demineralized bone matrix osteoinductivity. J. Bone Jt. Surg. Ser. A 2009, 91, 1459–1470. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Mao, T.; Chen, F. Influence of platelet-rich plasma on ectopic bone formation of bone marrow stromal cells in porous coral. Int. J. Oral Maxillofac. Surg. 2011, 40, 961–965. [Google Scholar] [CrossRef]
- Metzler, P.; Von Wilmowsky, C.; Zimmermann, R.; Wiltfang, J.; Schlegel, K.A. The effect of current used bone substitution materials and platelet-rich plasma on periosteal cells by ectopic site implantation: An in-vivo pilot study. J. Cranio-Maxillofac. Surg. 2012, 40, 409–415. [Google Scholar] [CrossRef]
- Anitua, E.; Sánchez, M.; Zalduendo, M.M.; De La Fuente, M.; Prado, R.; Orive, G.; Andía, I. Fibroblastic response to treatment with different preparations rich in growth factors. Cell Prolif. 2009, 42, 162–170. [Google Scholar] [CrossRef]
- Anitua, E.; Sánchez, M.; Orive, G.; Andía, I. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials 2007, 28, 4551–4560. [Google Scholar] [CrossRef] [PubMed]
- Solakoglu, Ö.; Heydecke, G.; Amiri, N.; Anitua, E. The use of plasma rich in growth factors (PRGF) in guided tissue regeneration and guided bone regeneration. A review of histological, immunohistochemical, histomorphometrical, radiological and clinical results in humans. Ann. Anat. 2020, 231, 151528. [Google Scholar] [CrossRef]
- López, S.; Vilar, J.M.; Sopena, J.J.; Damià, E.; Chicharro, D.; Carrillo, J.M.; Cuervo, B.; Rubio, M. Assessment of the efficacy of platelet-rich plasma in the treatment of traumatic canine fractures. Int. J. Mol. Sci. 2019, 20, 1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio, M.; Sopena, J.; Carrillo, J.M.; Cugat, R.; Dominguez, J.M.; Vilar, J.; Morales, M.; Cuervo, B. Hip osteoarthritis in dogs: A randomized study using mesenchymal stem cells from adipose tissue and plasma rich in growth factors. Int. J. Mol. Sci. 2014, 15, 13437–13460. [Google Scholar] [CrossRef]
- Valiño-Cultelli, V.; Varela-López, Ó.; González-Cantalapiedra, A. Preliminary Clinical and Radiographic Evaluation of a Novel Resorbable Implant of Polylactic Acid (PLA) for Tibial Tuberosity Advancement (TTA) by Modified Maquet Technique (MMT). Animals 2021, 11, 1271. [Google Scholar] [CrossRef]
- Millet, M.; Bismuth, C.; Labrunie, A.; Marin, B.; Filleur, A.; Pillard, P.; Sonet, J.; Cachon, T.; Etchepareborde, S. Measurement of the patellar tendon-tibial plateau angle and tuberosity advancement in dogs with cranial cruciate ligament rupture: Reliability of the common tangent and tibial plateau methods of measurement. Vet. Comp. Orthop. Traumatol. 2013, 26, 469–478. [Google Scholar] [CrossRef]
- Anitua, E.; Andía, I.; Sanchez, M.; Azofra, J.; del Mar Zalduendo, M.; de la Fuente, M.; Nurden, P.; Nurden, A.T. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J. Orthop. Res. 2005, 23, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Evans, R.; Conzemius, M.G.; Lascelles, B.D.X.; McIlwraith, C.W.; Pozzi, A.; Clegg, P.; Innes, J.; Schulz, K.; Houlton, J.; et al. Proposed Definitions and Criteria for Reporting Time Frame, Outcome, and Complications For Clinical Orthopedic Studies in Veterinary Medicine. Vet. Surg. 2010, 39, 905–908. [Google Scholar] [CrossRef]
- Hoffmann, D.E.; Miller, J.M.; Ober, C.P.; Lanz, O.I.; Martin, R.A.; Shires, P.K. Tibial tuberosity advancement in 65 canine stifles. Vet. Comp. Orthop. Traumatol. 2006, 19, 219–227. [Google Scholar]
- Etchepareborde, S.; Brunel, L.; Bollen, G.; Balligand, M. Preliminary experience of a modified maquet technique for repair of cranial cruciate ligament rupture in dogs. Vet. Comp. Orthop. Traumatol. 2011, 24, 223–227. [Google Scholar] [CrossRef]
- Steinberg, E.J.; Prata, R.G.; Palazzini, K.; Brown, D.C. Tibial tuberosity advancement for treatment of CrCL injury: Complications and owner satisfaction. J. Am. Anim. Hosp. Assoc. 2011, 47, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, M.D.; Watson, A.T.; Thatcher, L.G.; Wotton, H.; Naber, S.J. Prospective Randomized Clinical and Radiographic Evaluation of a Novel Bioabsorbable Biocomposite Tibial Tuberosity Advancement Cage Implant. Vet. Surg. 2016, 45, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, T.G.; Makara, M.A.; Katiofsky, K.; Fluckiger, M.A.; Morgan, J.P.; Haessig, M.; Montavon, P.M. Comparison of Healing of the Osteotomy Gap after Tibial Tuberosity Advancement with and without Use of an Autogenous Cancellous Bone Graft. Vet. Surg. 2011, 40, 27–33. [Google Scholar] [CrossRef]
- Panda, S.; Doraiswamy, J.; Malaiappan, S.; Varghese, S.S.; Del Fabbro, M. Additive effect of autologous platelet concentrates in treatment of intrabony defects: A systematic review and meta-analysis. J. Investig. Clin. Dent. 2016, 7, 13–26. [Google Scholar] [CrossRef]
- Griffin, X.L.; Smith, C.M.; Costa, M.L. The clinical use of platelet-rich plasma in the promotion of bone healing: A systematic review. Injury 2009, 40, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.A.; Growney Kalaf, E.A.; Bowlin, G.L.; Sell, S.A. Platelet-rich plasma in bone regeneration: Engineering the delivery for improved clinical efficacy. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Jovani-Sancho, M.D.M.; Sheth, C.C.; Marqués-Mateo, M.; Puche-Torres, M. Platelet-Rich Plasma: A Study of the Variables that May Influence Its Effect on Bone Regeneration. Clin. Implant Dent. Relat. Res. 2016, 18, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Plachokova, A.S.; van den Dolder, J.; van den Beucken, J.J.J.P.; Jansen, J.A. Bone regenerative properties of rat, goat and human platelet-rich plasma. Int. J. Oral Maxillofac. Surg. 2009, 38, 861–869. [Google Scholar] [CrossRef]
- Ferreira, A.J.A.; Bom, R.M.; Tavares, S.O. Tibial tuberosity advancement technique in small breed dogs: Study of 30 consecutive dogs (35 stifles). J. Small Anim. Pract. 2019, 60, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Bernardi-Villavicencio, C.; Jimenez-Socorro, A.N.; Rojo-Salvador, C.; Robles-Sanmartin, J.; Rodriguez-Quiros, J. Short-term outcomes and complications of 65 cases of porous TTA with flange: A prospective clinical study in dogs. BMC Vet. Res. 2020, 16, 279. [Google Scholar] [CrossRef]
- Stein, S.; Schmoekel, H. Short-term and eight to 12 months results of a tibial tuberosity advancement as treatment of canine cranial cruciate ligament damage. J. Small Anim. Pract. 2008, 49, 398–404. [Google Scholar] [CrossRef]
- Voss, K.; Damur, D.M.; Guerrero, T.; Hoessig, M.; Montavon, R.M. Force plate gait analysis to assess limb function after tibial tuberosity advancement in dogs with cranial cruciate ligament disease. Vet. Comp. Orthop. Traumatol. 2008, 21, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Castañón García, F. Estudio Comparativo de las Técnicas Quirúrgicas, TTA Clásica Securos®, TTA Porous® y TTA Porous® con PRP, Para el tratamiento de la Rotura del Ligamento Cruzado Anterior en el Perro. Ph.D. Thesis, University of León, León, Spain, 2015. [Google Scholar]
- Ross, M.; Worrell, T.W. Thigh and calf girth following knee injury and surgery. J. Orthop. Sports Phys. Ther. 1998, 27, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Millis, D.; Levine, D.; Mynatt, T. Changes in muscle mass following transection of the cranial cruciate ligament and immediate stifle stabilization. In Proceedings of the First International Symposium on Rehabilitation and Physical Therapy in Veterinary Medicine, Oregon State University, Corvallis, OR, USA, 7–11 August 1999; pp. 7–11. [Google Scholar]
- MacDonald, T.L.; Allen, D.A.; Monteith, G.J. Clinical assessment following tibial tuberosity advancement in 28 stifles at 6 months and 1 year after surgery. Can. Vet. J. 2013, 54, 249–254. [Google Scholar]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; Strauss, J.E.; Georgeff, K.R. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 638–646. [Google Scholar] [CrossRef]
- Martínez, C.E.; Smith, P.C.; Palma Alvarado, V.A. The influence of platelet-derived products on angiogenesis and tissue repair: A concise update. Front. Physiol. 2015, 6, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, R.E.; Scavelli, T.D.; Hoelzler, M.G.; Fulcher, R.P.; Bastian, R.P. CCLSurgical and postoperative complications associated with tibial tuberosity advancement for cranial cruciate ligament rupture in dogs: 458 cases (2007–2009). J. Am. Vet. Med. Assoc. 2012, 240, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Bisgard, S.K.; Barnhart, M.D.; Shiroma, J.T.; Kennedy, S.C.; Schertel, E.R. The Effect of Cancellous Autograft and Novel Plate Design on Radiographic Healing and Postoperative Complications in Tibial Tuberosity Advancement for Cranial Cruciate-Deficient Canine Stifles. Vet. Surg. 2011, 40, 402–407. [Google Scholar] [CrossRef]
- Brunel, L.; Etchepareborde, S.; Barthélémy, N.; Farnir, F.; Balligand, M. Mechanical testing of a new osteotomy design for tibial tuberosity advancement using the modified Maquet technique. Vet. Comp. Orthop. Traumatol. 2013, 26, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Retallack, L.M.; Daye, R.M. A modified Maquet-tibial tuberosity advancement technique for treatment of canine cranial cruciate ligament disease: Short term outcome and complications. Vet. Surg. 2018, 47, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefebvre, M.D.; Broux, O.R.; Barthélémy, N.P.; Hamon, M.; Moyse, E.V.; Bouvy, B.M.; Balligand, M.H. Risk factors for tibial damage associated with the modified Maquet technique in 174 stifles. Vet. Surg. 2018, 47, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ness, M.G. The Modified Maquet Procedure (MMP ) in Dogs: Technical Development and Initial Clinical Experience. J. Am. Anim. Hosp. Assoc. 2016, 52, 242–250. [Google Scholar] [CrossRef]
- Meyer, F.; Wardale, J.; Best, S.; Cameron, R.; Rushton, N.; Brooks, R. Effects of lactic acid and glycolic acid on human osteoblasts: A way to understand PLGA involvement in PLGA/calcium phosphate composite failure. J. Orthop. Res. 2012, 30, 864–871. [Google Scholar] [CrossRef]
- Lin, P.L.; Fang, H.W.; Tseng, T.; Lee, W.H. Effects of hydroxyapatite dosage on mechanical and biological behaviors of polylactic acid composite materials. Mater. Lett. 2007, 61, 3009–3013. [Google Scholar] [CrossRef]
- Wei, G.; Ma, P.X. Structure and properties of nano-hydroxyapatite / polymer composite scaffolds for bone tissue engineering. Biomaterials 2009, 25, 4749–4757. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.C.; Roberts, S.J.; Schrooten, J.; Luyten, F.P. Probing the osteoinductive effect of calcium phosphate by using an in vitro biomimetic model. Tissue Eng. Part A 2010, 17, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Barradas, A.M.C.; Fernandes, H.A.M.; Groen, N.; Chai, Y.C.; Schrooten, J.; Van de Peppel, J.; Van Leeuwen, J.P.T.M.; Van Blitterswijk, C.A.; De Boer, J. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials 2012, 33, 3205–3215. [Google Scholar] [CrossRef]

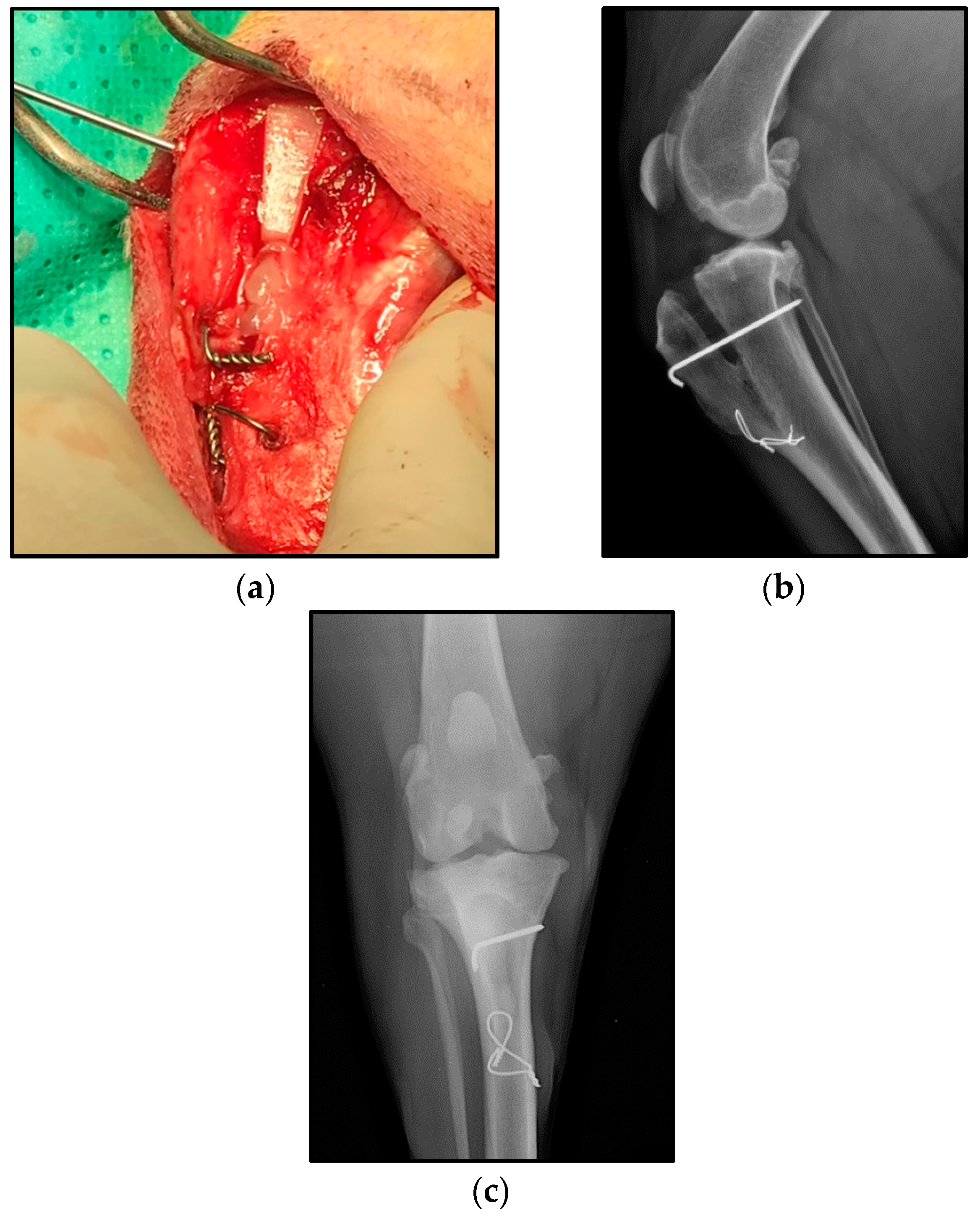
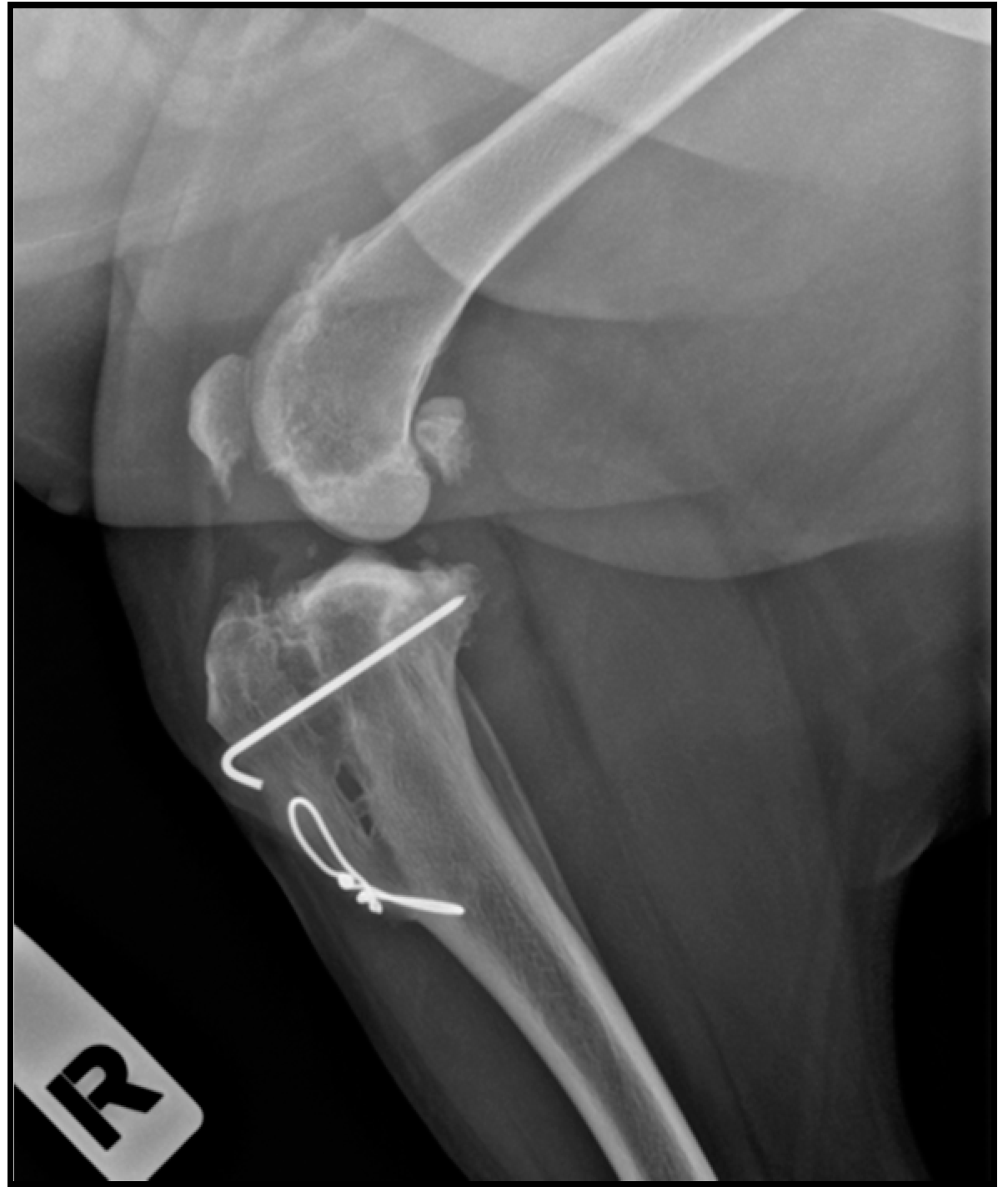
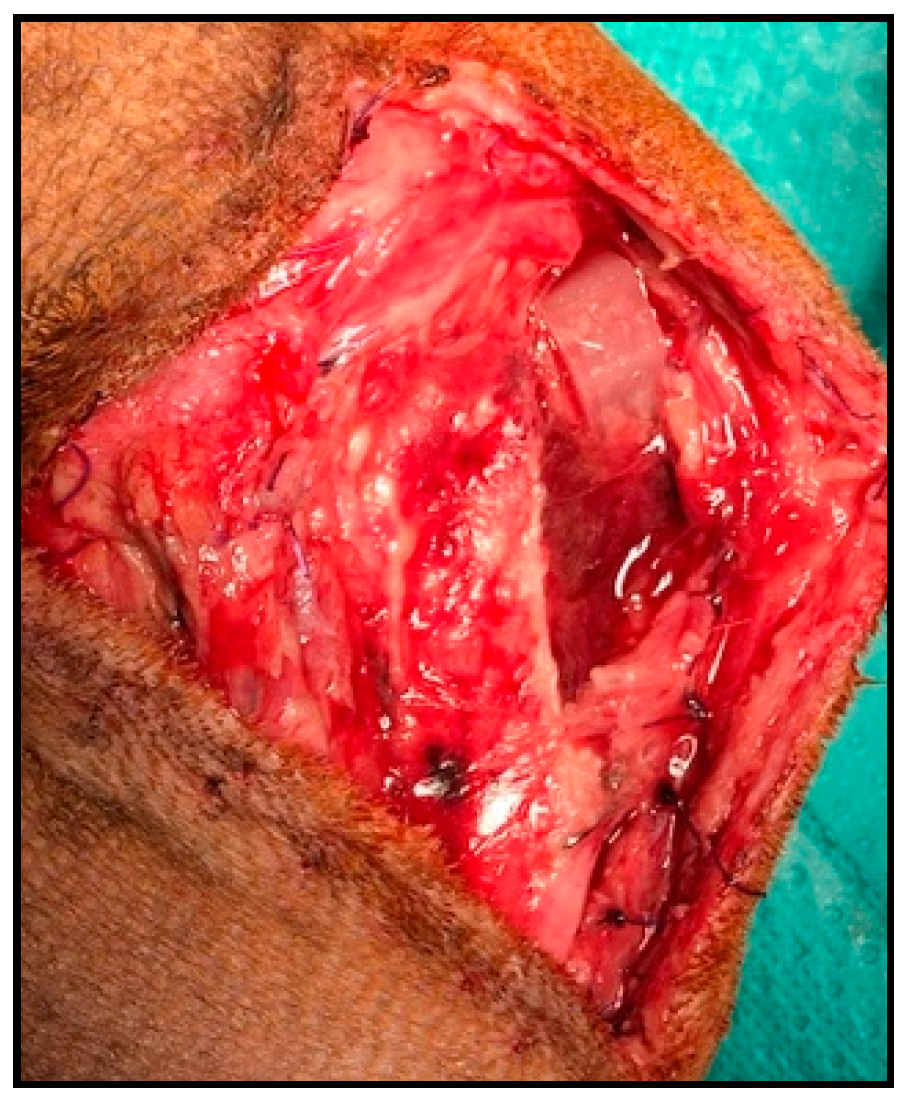
| Groups (n) | Age (months) | Weight (kg) | Cage Size (mm) | Affected Knee (n) 1 | Sex (n) 2 |
|---|---|---|---|---|---|
| PRGF group (29) | 17 (L) 58.62% | 18 (F) (6 n) | |||
| 75.10 ± 45.63 * | 26.27 ± 7.41 * | 9.20 ± 1.52 * | |||
| 12 (R) 41.37% | 11 (M) (2 n) | ||||
| Control group (24) | 12 (L) 50% | 15 (F) (2 n) | |||
| 70.16 ± 34.18 * | 30.13 ± 13.82 * | 9.41 ± 1.95 * | 12 (R) 50% | 9 (M) (2 n) | |
| Total patients (53) | 29 (L) 54.71% | 33 (F) (8 n) | |||
| 72.86 ± 40.54 | 28.02 ± 10.85 | 9.30 ± 1.71 | |||
| 24 (R) 45.28% | 20 (M) (4 n) |
| Follow-Up | PRPGF Group (n = 17) | Control Group (n = 18) | Total Patient (n = 35) |
|---|---|---|---|
| First follow-up (days) | 32.17 ± 5.34 | 35.22 ± 7.51 | 33.74 ± 6.63 |
| Second follow-up (days) | 68.64 ± 12.98 | 72.27 ± 9.71 | 70.51 ± 11.39 |
| Third follow-up (days) | 165.11 ± 14.90 | 152.61 ± 21.74 | 158.68 ± 19.52 |
| Ossification Degree | n of Patients | |||||
|---|---|---|---|---|---|---|
| PRGF Group (n = 17) | Control Group (n = 18) | |||||
| 1Fup | 2Fup | 3Fup | 1Fup | 2Fup | 3Fup | |
| 0 | 5 | 1 | 0 | 5 | 0 | 0 |
| 1 | 8 | 3 | 0 | 6 | 2 | 0 |
| 2 | 2 | 7 | 1 | 3 | 5 | 0 |
| 3 | 2 | 3 | 10 | 4 | 9 | 8 |
| 4 | 0 | 3 | 6 | 0 | 2 | 10 |
| Mean | 1.06 ± 0.96 | 2.23 ± 1.14 | 3.29 ± 0.58 | 1.33 ± 1.13 | 2.61 ± 0.84 | 3.55 ± 0.51 |
| Lameness Degree | n of Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| PRGF Group (n = 17) | Control Group (n = 18) | |||||||
| Ps | 1 Fup | 2 Fup | 3 Fup | Ps | 1 Fup | 2 Fup | 3 Fup | |
| 0 | 0 | 3 | 11 | 14 | 0 | 3 | 9 | 16 |
| 1 | 0 | 9 | 2 | 2 | 0 | 3 | 4 | 2 |
| 2 | 2 | 3 | 3 | 1 | 1 | 5 | 4 | 0 |
| 3 | 8 | 1 | 1 | 0 | 10 | 5 | 1 | 0 |
| 4 | 4 | 1 | 0 | 0 | 3 | 1 | 0 | 0 |
| 5 | 3 | 0 | 0 | 0 | 5 | 1 | 0 | 0 |
| Mean | 3.47 ± 0.94 | 1.29 ± 1.04 | 0.64 ± 0.99 | 0.23 ± 0.56 | 3.55 ± 0.92 | 2.05 ± 1.39 | 0.83 ± 0.98 | 0.11 ± 0.32 |
| PRGF | Control Group | |||
|---|---|---|---|---|
| Minor | 3 | Fracture of the distal cortical of the tibial crest without displacement (3) | 2 | Fracture of the distal cortical of the tibial crest without displacement (1) |
| Apparition of vesicles in the incision region (1) | ||||
| Major | 2 | Tension band wiring rupture with or without tibial crest displacement (1) | 3 | Tension band wiring rupture with or without tibial crest displacement (3) |
| Implant rupture (1) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valiño-Cultelli, V.; Varela-López, Ó.; González-Cantalapiedra, A. Does PRGF Work? A Prospective Clinical Study in Dogs with A Novel Polylactic Acid Scaffold Injected with PRGF Using the Modified Maquet Technique. Animals 2021, 11, 2404. https://doi.org/10.3390/ani11082404
Valiño-Cultelli V, Varela-López Ó, González-Cantalapiedra A. Does PRGF Work? A Prospective Clinical Study in Dogs with A Novel Polylactic Acid Scaffold Injected with PRGF Using the Modified Maquet Technique. Animals. 2021; 11(8):2404. https://doi.org/10.3390/ani11082404
Chicago/Turabian StyleValiño-Cultelli, Victoria, Óscar Varela-López, and Antonio González-Cantalapiedra. 2021. "Does PRGF Work? A Prospective Clinical Study in Dogs with A Novel Polylactic Acid Scaffold Injected with PRGF Using the Modified Maquet Technique" Animals 11, no. 8: 2404. https://doi.org/10.3390/ani11082404






