Dietary Copper Improves Intestinal Morphology via Modulating Intestinal Stem Cell Activity in Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Treatments
2.2. Sample Collection
2.3. Morphological Analysis
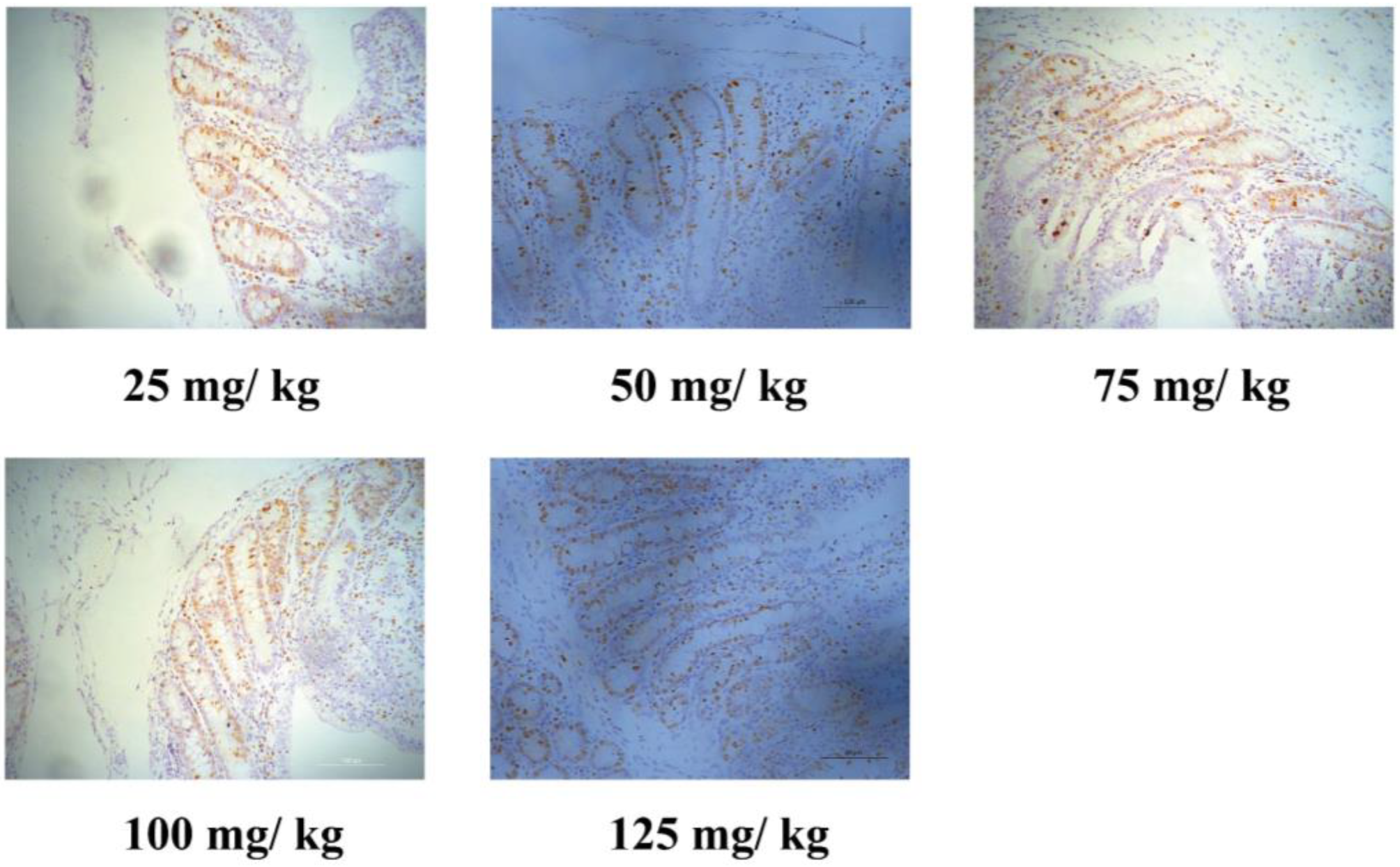
2.4. Immunohistochemistry for Ki67
2.5. Cell Shedding Analysis
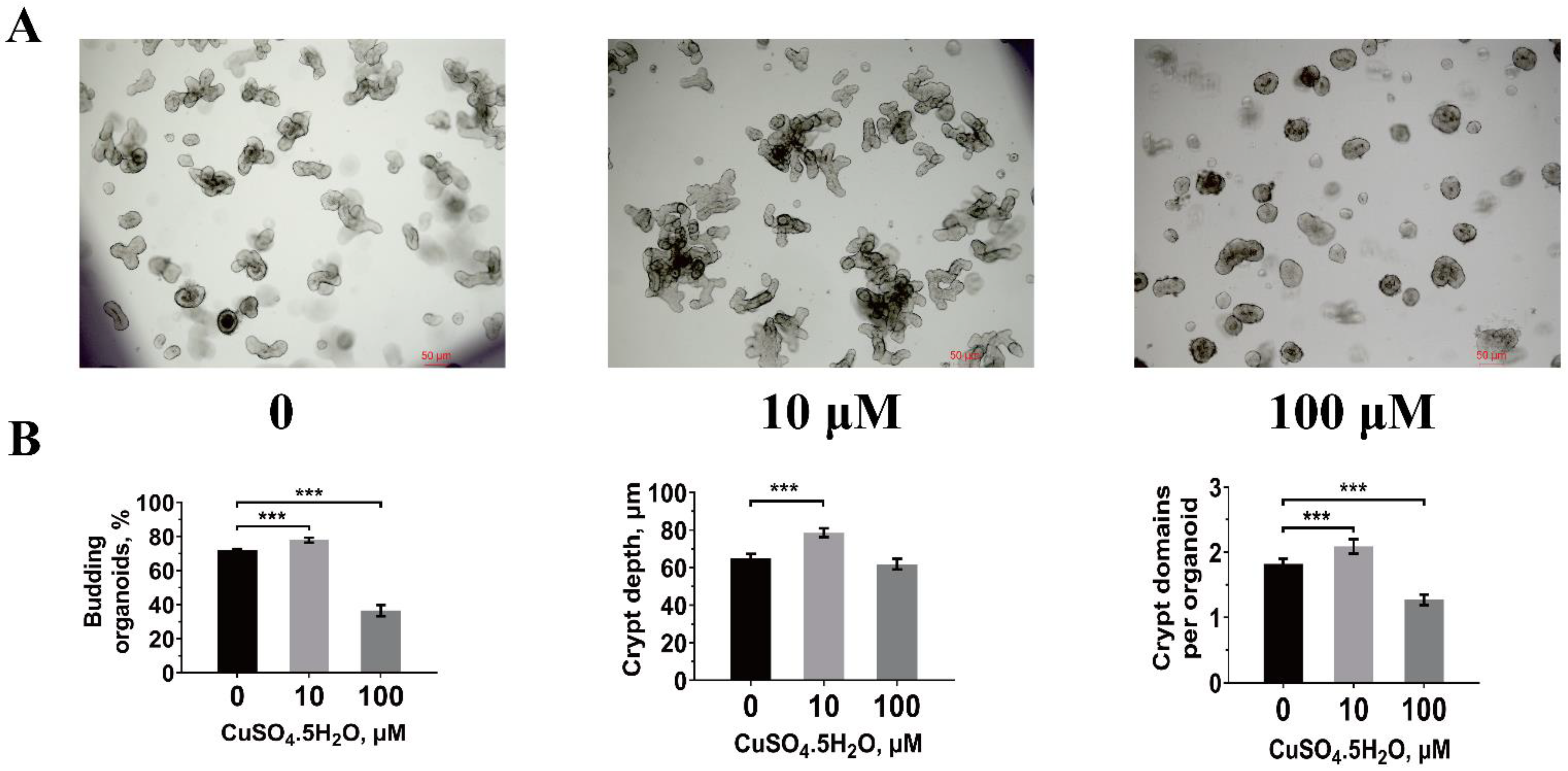
2.6. Porcine Crypt Isolation, Organoid Culture, and Measurement
2.7. Statistical Analysis
3. Results
3.1. Relative Small Intestinal Index
3.2. Intestinal Morphology
3.3. Intestinal Epithelium Cell Proliferation and Cell Shedding
3.4. Jejunal Intestinal Organoid Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, P.; Pu, B.; Yu, B.; He, J.; Yu, J.; Mao, X.B.; Luo, Y.H.; Luo, J.Q.; Huang, Z.Q.; Luo, C.G.; et al. The differences between copper sulfate and tribasic copper chloride on growth performance, redox status, deposition in tissues of pigs, and excretion in feces. Asian-Australas J. Anim. Sci. 2018, 31, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, A.C.; Ku, P.K.; Miller, E.R.; Keahey, K.K.; Ullrey, D.E. Copper requirement of baby pigs fed purified diets. J. Nutr. 1979, 109, 939–948. [Google Scholar] [CrossRef]
- Cromwell, G.L.; Monegue, H.J.; Stahly, T.S. Long-term effects of feeding a high copper diet to sows during gestation and lactation. J. Anim. Sci. 1993, 71, 2996–3002. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.W.; Tokach, M.D.; Goodband, R.D.; Nelssen, J.L.; Richert, B.T. Effects of the interrelationship between zinc oxide and copper sulfate on growth performance of early-weaned pigs. J. Anim. Sci. 1997, 75, 1861–1866. [Google Scholar] [CrossRef]
- Pérez, V.G.; Waguespack, A.M.; Bidner, T.D.; Southern, L.L.; Fakler, T.M.; Ward, T.L.; Steidinger, M.; Pettigrew, J.E. Additivity of effects from dietary copper and zinc on growth performance and fecal microbiota of pigs after weaning. J. Anim. Sci. 2011, 89, 414–425. [Google Scholar] [CrossRef]
- Luo, X.G.; Dove, C.R. Effect of Dietary Copper and Fat on Nutrient Utilization, Digestive Enzyme Activities, and Tissue Mineral Levels in Weanling Pigs. J. Anim. Sci. 1996, 74, 1888–1896. [Google Scholar] [CrossRef]
- Gonzales-Eguia, A.; Fu, C.M.; Lu, F.Y.; Lien, T.F. Effects of nanocopper on copper availability and nutrients digestibility, growth performance and serum traits of piglets. Livest. Sci. 2009, 126, 122–129. [Google Scholar] [CrossRef]
- Coble, K.F.; Burnett, D.D.; DeRouchey, J.M.; Tokach, M.D.; Gonzalez, J.M.; Wu, F.Z.; Dritz, S.S.; Goodband, R.D.; Woodworth, J.C.; Pluske, J.R. Effect of diet type and added copper on growth performance, carcass characteristics, energy digestibility, gut morphology, and mucosal mRNA expression of finishing pigs. J. Anim. Sci. 2018, 96, 3288–3301. [Google Scholar] [CrossRef] [Green Version]
- Rochell, S.J.; Usry, J.L.; Parr, T.M.; Parsons, C.M.; Dilger, R.N. Effects of dietary copper and amino acid density on growth performance, apparent metabolizable energy, and nutrient digestibility in Eimeria acervulina-challenged broilers. Poult. Sci. 2016, 96, 602–610. [Google Scholar] [CrossRef]
- Cappai, M.G.; Alesso, G.A.; Nieddu, G.; Sanna, M.; Pinna, W. Electron microscopy and composition of raw acorn starch in relation to in vivo starch digestibility. Food Funct. 2013, 4, 917–922. [Google Scholar] [CrossRef]
- Di Giancamillo, A.; Rossi, R.; Martino, P.A.; Aidos, L.; Maghin, F.; Domeneghini, C.; Corino, C. Copper sulphate forms in piglet diets: Microbiota, intestinal morphology and enteric nervous system glial cells. Anim. Sci. J. 2018, 89, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Fry, R.S.; Ashwell, M.S.; Lloyd, K.E.; O’Nan, A.T.; Flowers, W.L.; Stewart, K.R.; Spears, J.W. Amount and source of dietary copper affects small intestine morphology, duodenal lipid peroxidation, hepatic oxidative stress, and mRNA expression of hepatic copper regulatory proteins in weanling pigs. J. Anim. Sci. 2012, 90, 3112–3119. [Google Scholar] [CrossRef] [PubMed]
- Radecki, S.V.; Ku, P.K.; Bennink, M.R.; Yokoyama, M.T.; Miller, E.R. Effect of dietary copper on intestinal mucosa enzyme activity, morphology, and turnover rates in weanling pigs. J. Anim. Sci. 1992, 70, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.A. The experimental analysis of changes in proliferative and morphological status on the intestine. Scand. J. Gastroenterol. Suppl. 1982, 74, 3–10. [Google Scholar]
- Saqui-Salces, M.; Huang, Z.; Ferrandis Vila, M.; Li, J.; Mielke, J.A.; Urriola, P.E.; Shurson, G.C. Modulation of intestinal cell differentiation in growing pigs is dependent on the fiber source in the diet. J. Anim. Sci. 2017, 95, 1179–1190. [Google Scholar] [CrossRef]
- Kuo, Y.M.; Zhou, B.; Cosco, D.; Gitschier, J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc. Natl. Acad. Sci. USA 2001, 98, 6836–6841. [Google Scholar] [CrossRef] [Green Version]
- Barker, N.; van de Wetering, M.; Clevers, H. The intestinal stem cell. Genes Dev. 2008, 22, 1856–1864. [Google Scholar] [CrossRef] [Green Version]
- Verdile, N.; Mirmahmoudi, R.; Brevini, T.A.L.; Gandolfi, F. Evolution of pig intestinal stem cells from birth to weaning. Animal 2019, 13, 2830–2839. [Google Scholar] [CrossRef]
- Pierson, H.; Yang, H.J.; Lutsenko, S. Copper Transport and Disease: What Can We Learn from Organoids? Annu. Rev. Nutr. 2019, 39, 75–94. [Google Scholar] [CrossRef]
- Remus, A.; Del Castillo, J.R.E.; Pomar, C. Improving the estimation of amino acid requirements to maximize nitrogen retention in precision feeding for growing-finishing pigs. Animal 2020, 14, 2032–2041. [Google Scholar] [CrossRef]
- Zhou, X.H.; Liu, Y.H.; Zhang, L.Y.; Kong, X.F.; Li, F.N. Serine-to-glycine ratios in low-protein diets regulate intramuscular fat by affecting lipid metabolism and myofiber type transition in the skeletal muscle of growing-finishing pigs. Anim. Nutr. 2021, 7, 384–392. [Google Scholar] [CrossRef]
- Wu, Y.P.; Jiang, Z.Y.; Zheng, C.T.; Wang, L.; Zhu, C.; Yang, X.F.; Wen, X.L.; Ma, X.Y. Effects of protein sources and levels in antibiotic-free diets on diarrhea, intestinal morphology, and expression of tight junctions in weaned piglets. Anim. Nutr. 2015, 1, 170–176. [Google Scholar] [CrossRef]
- Zong, E.Y.; Yan, S.L.; Wang, M.W.; Yin, L.M.; Wang, Q.Y.; Yin, J.; Li, J.Z.; Li, Y.L.; Ding, X.Q.; Huang, P.F.; et al. The effects of dietary supplementation with hyodeoxycholic acid on the differentiation and function of enteroendocrine cells and the serum biochemical indices in weaned piglets1. J. Anim. Sci. 2019, 97, 1796–1805. [Google Scholar] [CrossRef]
- Wang, L.X.; Yan, S.L.; Li, J.Z.; Li, Y.L.; Ding, X.Q.; Yin, J.; Xiong, X.; Yin, Y.L.; Yang, H.S. Rapid Communication: The relationship of enterocyte proliferation with intestinal morphology and nutrient digestibility in weaning piglets. J. Anim. Sci. 2019, 97, 353–358. [Google Scholar] [CrossRef]
- Bullen, T.F.; Forrest, S.; Campbell, F.; Dodson, A.R.; Hershman, M.J.; Pritchard, D.M.; Turner, J.R.; Montrose, M.H.; Watson, A.J. Characterization of epithelial cell shedding from human small intestine. Lab. Investig. 2006, 86, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Duckworth, C.A.; Burkitt, M.D.; Watson, A.J.; Campbell, B.J.; Pritchard, D.M. Epithelial cell shedding and barrier function: A matter of life and death at the small intestinal villus tip. Vet. Pathol. 2015, 52, 445–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, H.A.; Lei, N.Y.; Brinkley, G.; Scott, A.; Wang, J.F.; Kar, U.K.; Jabaji, Z.B.; Lewis, M.; Martin, M.G.; Dunn, J.C.; et al. A novel culture system for adult porcine intestinal crypts. Cell Tissue Res. 2016, 365, 123–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Y.B.; Bijvelds, M.; Dang, W.; Xu, L.; van der Eijk, A.A.; Knipping, K.; Tuysuz, N.; Dekkers, J.F.; Wang, Y.; de Jonge, J.; et al. Modeling rotavirus infection and antiviral therapy using primary intestinal organoids. Antivir. Res. 2015, 123, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Huang, D.G.; Qin, Y.C.; Li, X.G.; Gao, C.Q.; Yan, H.C.; Wang, X.Q. mTORC1 signaling activation increases intestinal stem cell activity and promotes epithelial cell proliferation. J. Cell Physiol. 2019, 234, 19028–19038. [Google Scholar] [CrossRef]
- Li, R.X.; Wen, Y.; Lin, G.; Meng, C.Z.; He, P.L.; Wang, F.L. Different Sources of Copper Effect on Intestinal Epithelial Cell: Toxicity, Oxidative Stress, and Metabolism. Metabolites 2019, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Li, X.G.; Zhu, M.; Chen, M.X.; Fan, H.B.; Fu, H.L.; Zhou, J.Y.; Zhai, Z.Y.; Gao, C.Q.; Yan, H.C.; Wang, X.Q. Acute exposure to deoxynivalenol inhibits porcine enteroid activity via suppression of the Wnt/β-catenin pathway. Toxicol. Lett. 2019, 305, 19–31. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Wang, Z.; Zhang, S.W.; Lin, H.L.; Gao, C.Q.; Zhao, J.C.; Yang, C.B.; Wang, X.Q. Methionine and Its Hydroxyl Analogues Improve Stem Cell Activity To Eliminate Deoxynivalenol-Induced Intestinal Injury by Reactivating Wnt/beta-Catenin signaling. J. Agric. Food Chem. 2019, 67, 11464–11473. [Google Scholar] [CrossRef]
- Han, F.; Hu, L.; Xuan, Y.; Ding, X.M.; Luo, Y.H.; Bai, S.P.; He, S.Y.; Zhang, K.Y.; Che, L.Q. Effects of high nutrient intake on the growth performance, intestinal morphology and immune function of neonatal intra-uterine growth-retarded pigs. Br. J. Nutr. 2013, 110, 1819–1827. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yin, L.M.; Wang, L.; Huang, P.F.; Yang, H.S.; Yin, Y.L. Effects of vitamin B6 on growth, diarrhea rate, intestinal morphology, function, and inflammatory factors expression in a high-protein diet fed to weaned piglets1. J. Anim. Sci. 2019, 97, 4865–4874. [Google Scholar] [CrossRef] [PubMed]
- Chivukula, R.R.; Shi, G.; Acharya, A.; Mills, E.W.; Zeitels, L.R.; Anandam, J.L.; Abdelnaby, A.A.; Balch, G.C.; Mansour, J.C.; Yopp, A.C.; et al. An essential mesenchymal function for miR-143/145 in intestinal epithelial regeneration. Cell 2014, 157, 1104–1116. [Google Scholar] [CrossRef] [Green Version]
- Ito, S.; Nada, S.; Yamazaki, D.; Kimura, T.; Kajiwara, K.; Miki, H.; Okada, M. p18/Lamtor1-mTORC1 Signaling Controls Development of Mucin-producing Goblet Cells in the Intestine. Cell Struct. Funct. 2020, 45, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Bartfeld, S.; Clevers, H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell 2010, 7, 656–670. [Google Scholar] [CrossRef] [Green Version]
- Efird, R.C.; Armstrong, W.D.; Herman, D.L. The development of digestive capacity in young pigs: Effects of age and weaning system. J. Anim. Sci. 1983, 55, 1380–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Yang, C.; Wang, Q.Y.; Li, J.Z.; Li, Y.L.; Ding, X.Q.; Yin, J.; Yang, H.S.; Yin, Y.L. The growth performance, intestinal digestive and absorptive capabilities in piglets with different lengths of small intestines. Animal 2019, 14, 1196–1203. [Google Scholar] [CrossRef]
- Langley-Evans, S.C. Developmental programming of health and disease. Proc. Nutr. Soc. 2006, 65, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Meyer, A.M.; Caton, J.S. Role of the small intestine in developmental programming: Impact of maternal nutrition on the dam and offspring. Adv. Nutr. 2016, 7, 169–178. [Google Scholar] [CrossRef]
- Yin, Y.B.; de Jonge, H.R.; Wu, X.; Yin, Y.L. Enteroids for Nutritional Studies. Mol. Nutr. Food Res. 2019, 63, e1801143. [Google Scholar] [CrossRef]
- Fuller, M.K.; Faulk, D.M.; Sundaram, N.; Shroyer, N.F.; Henning, S.J.; Helmrath, M.A. Intestinal crypts reproducibly expand in culture. J. Surg. Res. 2012, 178, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.B.; Guo, S.G.; Wan, D.; Wu, X.; Yin, Y.L. Enteroids: Promising in Vitro Models for Studies of Intestinal Physiology and Nutrition in Farm Animals. J. Agric. Food Chem. 2019, 67, 2421–2428. [Google Scholar] [CrossRef]
| Ingredients | Content (%) |
|---|---|
| Corn grain | 37.16 |
| Extruded corn | 20.00 |
| Soybean meal (43% CP) | 8.00 |
| Soy protein concentrate powder | 7.00 |
| Whey powder | 10.00 |
| Fish meal (63% CP) | 5.00 |
| Spray-dried plasma protein | 4.50 |
| Glucose | 2.00 |
| Soybean oil | 2.00 |
| Vitamin and mineral premix a | 4.34 |
| Total | 100.00 |
| Calculated nutrient levels | |
| Crude protein, % | 19.00 |
| ME, MJ/kg | 13.25 |
| NDF, % | 6.40 |
| ADF, % | 2.30 |
| Calcium, % | 0.75 |
| Available phosphorous, % | 0.38 |
| Lysine, % | 1.38 |
| Metionine, % | 0.40 |
| Met + Cys, % | 0.80 |
| Threonine, % | 0.86 |
| Triptophane, % | 0.25 |
| Cu, mg/kg | 5.04 |
| Variables | Dietary Copper, mg/kg Dry Matter | SEM 4 | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 75 | 100 | 125 | ANOVA | Linear | Quadratic | ||
| Phase 1 (day 1 to 21) 2 | |||||||||
| Small intestine length, m | 12.4 | 12.5 | 11.5 | 11.7 | 11.8 | 0.83 | 0.064 | 0.026 | 0.244 |
| Small intestine weight, g | 516.3 | 537.7 | 494.6 | 529.8 | 478.4 | 55.84 | 0.190 | 0.179 | 0.355 |
| Relative small intestine length, m/kg | 1.6 | 1.9 | 1.7 | 1.8 | 1.8 | 0.06 | 0.464 | 0.402 | 0.624 |
| Relative small intestine weight, g/kg | 71.0 | 83.1 | 76.4 | 76.8 | 74.5 | 2.41 | 0.705 | 0.965 | 0.309 |
| Phase 2 (day 22 to 163) 3 | |||||||||
| Small intestine length, m | 19.0 | 18.6 | 19.0 | 18.3 | 18.7 | 0.24 | 0.897 | 0.624 | 0.671 |
| Small intestine weight, kg | 1.7 | 1.7 | 1.8 | 1.6 | 1.7 | 0.04 | 0.768 | 0.521 | 0.573 |
| Relative small intestine length, m/kg | 18.9 | 20.3 | 20.2 | 19.1 | 20.9 | 0.34 | 0.296 | 0.259 | 0.987 |
| Relative small intestine weight, kg/kg | 16.8 | 18.6 | 18.9 | 16.9 | 18.3 | 0.35 | 0.198 | 0.551 | 0.306 |
| Variables | Dietary Copper, mg/kg DM | SEM 4 | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 75 | 100 | 125 | ANOVA | Linear | Quadratic | ||
| Phase 1 (day 1 to 21) 2 | |||||||||
| Villus height, μm | 305.9 | 359.5 | 303.5 | 333.4 | 346.0 | 9.30 | 0.160 | 0.413 | 0.959 |
| Crypt depth, μm | 319.6 | 324.5 | 327.4 | 321.6 | 296.0 | 10.39 | 0.753 | 0.521 | 0.449 |
| Villus height: crypt depth, μm:μm | 1.0 | 1.2 | 1.0 | 1.1 | 1.1 | 0.05 | 0.646 | 0.587 | 0.864 |
| Villus width, μm | 175.9 a | 146.0 bc | 153.7 bc | 161.3 ac | 147.7 bc | 3.42 | 0.037 | 0.075 | 0.226 |
| Intestinal villus surface area, mm2 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 0.01 | 0.598 | 0.706 | 0.471 |
| Phase 2 (day 22 to 163) 3 | |||||||||
| Villus height, μm | 409.7 b | 379.1 b | 393.8 b | 368.6 b | 512.1 a | 13.83 | <0.001 | 0.016 | 0.002 |
| Crypt depth, μm | 443.1 | 402.5 | 414.9 | 407.6 | 467.3 | 9.93 | 0.158 | 0.424 | 0.030 |
| Villus height:crypt depth, μm:μm | 0.9 | 0.9 | 1.0 | 0.9 | 1.1 | 0.03 | 0.245 | 0.175 | 0.368 |
| Variables | Dietary Copper, mg/kg DM | SEM 2 | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 75 | 100 | 125 | ANOVA | Linear | Quadratic | ||
| Ki67-positive cells per crypt, n | 22.3 b | 27.4 a | 25.5 ac | 24.6 bc | 27.7 ad | 0.50 | <0.001 | 0.007 | 0.381 |
| Cell shedding rate, % | 25.4 | 29.1 | 28.8 | 28.4 | 28.3 | 0.48 | 0.134 | 0.135 | 0.060 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, L.; Yang, Q.; Zhang, Y.; Wan, D.; Yin, Y.; Wang, Q.; Huang, J.; Li, J.; Yang, H.; Yin, Y. Dietary Copper Improves Intestinal Morphology via Modulating Intestinal Stem Cell Activity in Pigs. Animals 2021, 11, 2513. https://doi.org/10.3390/ani11092513
Yin L, Yang Q, Zhang Y, Wan D, Yin Y, Wang Q, Huang J, Li J, Yang H, Yin Y. Dietary Copper Improves Intestinal Morphology via Modulating Intestinal Stem Cell Activity in Pigs. Animals. 2021; 11(9):2513. https://doi.org/10.3390/ani11092513
Chicago/Turabian StyleYin, Lanmei, Qing Yang, Yiming Zhang, Dan Wan, Yuebang Yin, Qiye Wang, Jing Huang, Jianzhong Li, Huansheng Yang, and Yulong Yin. 2021. "Dietary Copper Improves Intestinal Morphology via Modulating Intestinal Stem Cell Activity in Pigs" Animals 11, no. 9: 2513. https://doi.org/10.3390/ani11092513






