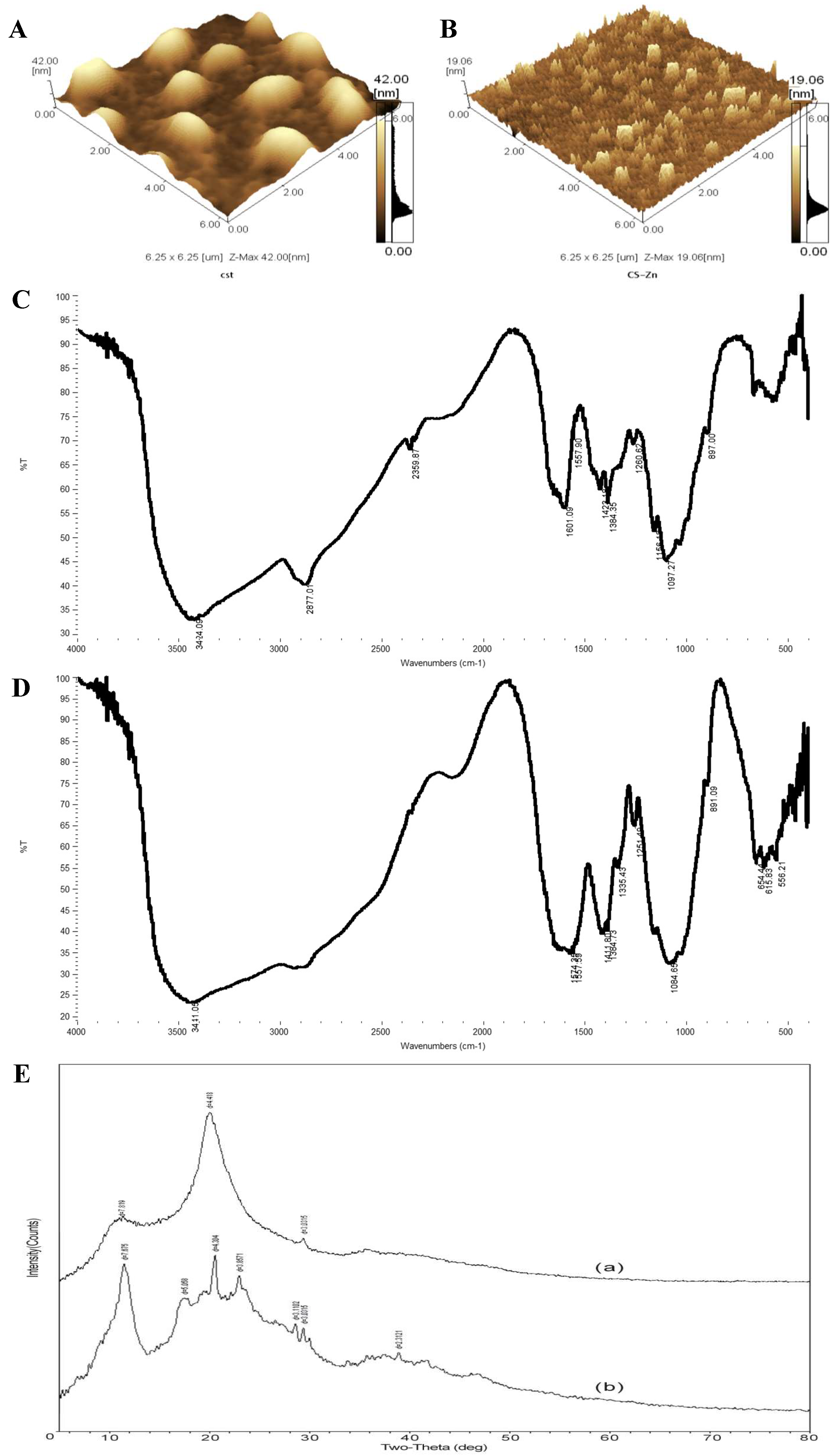
3.5. Bioavailability Analysis
Growth performance of weaned piglets affected by dietary Zn source and level was shown in
Table 2. Zn source, added Zn level, and their interaction between Zn source and added Zn level significantly affected ADG, ADFI, and F/G (
p < 0.014). Compared with the piglet’s diets supplemented with ZnSO
4, the piglets fed diets supplemented with CS–Zn had higher ADG and ADFI and a lower F/G (
p < 0.05). The piglets fed diets supplemented with 150 mg kg
−1 of Zn had higher ADG and ADFI than those fed diets supplemented with either 50 or 100 mg kg
−1 regardless of Zn source (
p < 0.01). Diarrhea incidence of weaned piglets fed dietary CS–Zn or ZnSO
4 is presented in
Figure 2A. The interaction between Zn source and added Zn level affected diarrhea incidence significantly. By adding 100 mg kg
−1 Zn as CS–Zn, 150 mg kg
−1 Zn as CS–Zn, and 150 mg kg
−1 Zn as ZnSO
4, the diarrhea incidence of weaned piglets was obviously decreased, compared with control treatment (
p < 0.01). Moreover, the piglets receiving 100 mg kg
−1 Zn as CS–Zn and 150 mg kg
−1 Zn as CS–Zn had a lower diarrhea incidence than that of the piglets fed the diets containing the same level of ZnSO
4 (
p < 0.001). In CS–Zn treatment, the diarrhea incidence was significantly decreased with the increase in Zn level.
In the present study, dietary Zn improved growth performance and decreased diarrhea incidence apparently; moreover, diets supplemented with CS–Zn were better than those supplemented with ZnSO
4 in general. Buff et al. (2005) [
21] reported that dietary supplementation of zinc–polysaccharide improved growth performance in weaned piglets. However, Case and Carlson (2002) [
6] found that there was no significant difference in growth performance between the piglets fed 500 mg kg
−1 of Zn as zinc–amino acid chelate and those of control group. Another study observed that organic zinc either as polysaccharide or proteinate complex had no effect on the growth performance [
4]. The differences among the results may relate to the health level of trial animal, gene differential expression, breeding environment, feeding schedule, and especially, to differences in chelating agent. In recent years, domestic and international studies have reported that organic zinc increases the growth performance only in the growth stage, mainly to increase the feed intake [
22]. In actual production, the addition of Zn to the diet of weaned piglets has the effect of promoting weight gain. One of the reasons is likely to be generated by participating in the taste element to affect the structure and function of oral mucosal epithelial cells. Moreover, Zn takes part in the synthesis of a variety of metabolic enzymes in the body, improves the digestion function, and enhances appetite. At the weaned stage, piglets suffer from one of the most stressful events, as they are fed with solid diets instead of liquid milk, which results in increased susceptibility to diarrhea. It was reported that zinc ion could inhibit the respiratory chain of pathogenic
Escherichia coli, leading to diarrhea [
22]. The results that CS–Zn reduced the incidence of diarrhea might depend on not only the function of zinc ion but also the antimicrobial activity of chelated chitosan. Another possible reason may be the improvement of the intestinal microflora and the immune function of weaned piglets fed diets containing CS–Zn [
13]. The reduction in diarrhea may make a contribution to the improved growth performance.
The effect of dietary Zn source and level on the content of Zn in the liver and pancreas of weaned piglets was shown in
Table 3. Compared with the piglets fed the control diet, the piglets fed diets supplemented with Zn had higher Zn contents in the liver and pancreas (
p < 0.05). The interaction between Zn source and added Zn level affected pancreas Zn content (
p < 0.001), but had no effect on Zn content in the liver (
p = 0.732). Zn source and added Zn level significantly affected Zn contents in the liver (
p < 0.007). The piglets fed diets supplemented with CS–Zn had higher Zn contents in the liver than those fed diets supplemented with ZnSO
4 (
p < 0.05). Compared with ZnSO
4, all levels of CS–Zn remarkably increased the zinc content in pancreas (
p < 0.05). There were significant differences in the zinc content in pancreas among 50 mg kg
−1 CS–Zn, 100 mg kg
−1 CS–Zn, and 150 mg kg
−1 CS–Zn groups (
p < 0.05), which was appropriate for ZnSO
4 groups. The Zn contents in the liver and pancreas increased linearly with added Zn increasing (
p < 0.001). The effects of dietary Zn source and level on the content of Cu in the liver and pancreas was shown in
Table S3. The Zn source and added Zn level had no significant influence on the content of Cu in the liver, but had a significant effect on that in pancreas. The concentrations of pancreatic Cu in 50 mg kg
−1, 100 mg kg
−1, and 150 mg kg
−1 CS–Zn groups and the 150 mg kg
−1 ZnSO
4 group were significantly increased, which was similar with the study of Hill et al. (2014) [
23] who observed that adding organic zinc can increase the concentration of Cu in kidney. CS–Zn can not only affect the homeostasis of Zn metabolism in the pancreas, but also affect the metabolism of Cu. While, ZnSO
4 had a significant effect on Cu metabolism in the pancreas only when the dose of ZnSO
4 reached 150 mg kg
−1. Compared with the same dose of ZnSO
4, 50 mg kg
−1 and 100 mg kg
−1 CS–Zn significantly increased the copper content in the pancreas, indicating that CS–Zn reduced the competitive inhibitory effect of zinc and copper in the process of zinc absorption and transport.
Figure 2B showed the intestinal zinc transporters protein expression of weaned piglets fed dietary CS–Zn or ZnSO
4. Compared with control group, dietary zinc had a great effect on the protein expressions of ZnT1 and ZIP5 in duodenal mucosa (
p < 0.05), while the protein expressions of ZnT1 in duodenal mucosa from CS–Zn treatment was enhanced than ZnSO
4 treatment (
p < 0.05) (
Table 4). The Zn source and added Zn level had significant influence on the protein expression of ZnT1 in duodenal mucosa (
p < 0.001). The interaction between Zn source and added Zn level affected ZIP5 protein expression (
p < 0.001). The protein expressions of ZIP5 in duodenal mucosa of piglets fed the diets supplemented with 100 mg kg
−1 CS–Zn and 150 mg kg
−1 CS–Zn treatments were higher than piglets from the same level of ZnSO
4 treatments (
p < 0.05), whereas there was no difference for that between the 50 mg kg
−1 CS–Zn and 50 mg kg
−1 ZnSO
4 groups (
p > 0.05). Compared to the other two levels of Zn, the dietary contained 150 mg kg
−1 Zn, as both CS–Zn and ZnSO
4 increased the expression of ZnT5 significantly. The protein expressions of ZnT1 and ZIP5 in duodenal mucosa increased linearly with the added Zn level increasing (
p < 0.001).
Under normal physiological conditions, zinc is a charged bivalent cation, which cannot be transmitted through the cytoplasmic membrane or the endothelium by passive diffusion. Therefore, the transfer of zinc in the body requires a series of proteins to participate in the metabolism. The intestine is the site of excretion and absorption of zinc, which plays a crucial role in maintaining zinc homeostasis. The absorption of zinc in the intestine is shown as unsaturated and saturated kinetics, while the latter process requires a mediated carrier [
24]. It is well known that there are two families of zinc transporter protein, the ZIP (SLC39) family and the ZnT (SLC30) family, which play opposite roles in homeostasis. Therefore, the expression of ZnT1 and ZIP5 protein in duodenal mucosa can reflect the ability of the body to absorb and transport Zn as CS–Zn or ZnSO
4 and, then, deduce the bioavailability of CS–Zn. In the present study, the results showed that CS–Zn or ZnSO
4 both increased the expression of zinc transporter protein, and the expression of zinc transporter protein increased linearly with supplemental zinc. ZnT1 is distributed on the basolateral membrane of enterocytes; the function of ZnT1 is to transport excess intestinal zinc into extracellular matrix or organelles for the sake of reducing the zinc level of cytoplasm [
25]. Increased zinc concentrations significantly elevated intestinal ZnT1 mRNA and protein expression [
26]. The current study found that the ZnT1 protein expressions in CS–Zn treatment were more elevated than that in ZnSO
4 treatment and reached the highest expression in duodenal mucosa when adding 150 mg Zn/kg. Liuzzi et al. (2004) [
27] found that the mRNA expression abundance of ZnT1 was decreased in rats with zinc deficiency (<1 mg kg
−1), while high zinc (180 mg kg
−1) significantly increased the mRNA expression. ZIP5 is located on the basement membrane of the intestinal epithelial cells, which transports zinc through the basement membrane into the intestinal epithelial cells to maintain the zinc homeostasis. In the absence of zinc, the protein expression of ZIP5 was inhibited, and once the zinc concentration increased rapidly, it was involved in the protein translation process of ZIP5. The current study showed that the Zn source, added Zn level, and their interaction between Zn source and added Zn level had a significant influence on the protein expression of ZIP5 in duodenal mucosa. Weaver et al. (2007) [
28] found that the mRNA expression of ZIP5 did not respond to zinc concentration, and zinc ion regulated the translation process of ZIP5 mRNA. Furthermore, the expression of zinc transporter protein in the CS–Zn group was higher than that in the ZnSO
4 group, indicating that CS–Zn increased the protein expression of ZnT1 and ZIP5, so as to transport zinc to blood, facilitating the zinc transshipment to various tissues and organs.
Relative bioavailability of CS–Zn based on multiple linear regression of zinc content in the liver and pancreas was shown in
Table 5. Slope-ratio methodology was used to estimate relative bioavailability of CS–Zn, with the relative bioavailability of ZnSO
4 standard set at 100%. Using the zinc content in the liver and pancreas as a response parameter, the bioavailability of CS–Zn was 110.9% (
p < 0.01) and 149.0% (
p < 0.01), respectively. The zinc content in feces of weaned piglets fed dietary CS–Zn or ZnSO
4 is shown in
Figure 2C. Compared with the same level of Zn as ZnSO
4, the zinc contents in the feces of weaned piglets fed dietary CS–Zn were reduced by above 30% (
p < 0.05).
Bioavailability is defined as the proportion of the ingested nutrients being absorbed and available for use or storage. Currently, researchers use the method of determining the accumulation of mineral elements in specific sensitive tissues to assess the bioavailability of mineral element additives. It is well known that the liver and pancreas are sensitive to zinc concentration in a diet [
29]; therefore, zinc content in the liver and pancreas are used to estimate zinc bioavailability from different zinc sources. Cao et al. (2000) [
30] estimated the bioavailability of polysaccharides–zinc chelate when compared with ZnSO
4 via multiple linear regression slope-ratio analysis; the results were 110% (liver) and 113% (pancreas), respectively. The bioavailability of the current study was higher for CS–Zn compared with ZnSO
4. Moreover, fecal zinc content of piglets fed CS–Zn was decreased significantly, which might be associated with the higher bioavailability of CS–Zn in body. Including all concentrations of CS–Zn groups and ZnSO
4 groups increased zinc concentration in the liver significantly; moreover, there was a linear relationship between them. When the zinc supplementation exceeds the requirement of pigs, the liver zinc concentration is reduced inversely [
23], indicating that zinc supplementation was not in excess in the current study. For weaned piglets, the normal digestive function of the pancreas is essential to maintain intestinal health and integrity. Pieper et al. (2015) [
31] reported that the activity of intestinal digestive enzymes increased under the high concentration of zinc. Using the pancreas zinc concentration as an index, the present study showed that organic zinc sources had a higher bioavailability than inorganic sources. The results indicated that the pancreas was sensitive to the zinc concentration in a diet. The mechanism of absorption for CS–Zn in the small intestine has not yet been identified. The reason why the bioavailability of CS–Zn in weaned piglets is higher than that of ZnSO
4 might be related to the absorption and metabolism mechanism of CS–Zn in the small intestine. In brief, CS–Zn harbors its own characters, accelerating the expression of ZnT1 and ZIP5 in the small intestine to increase the concentration of zinc in the liver and pancreas.