Validation of On-Farm Bacteriological Systems for Endometritis Diagnosis in Postpartum Dairy Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
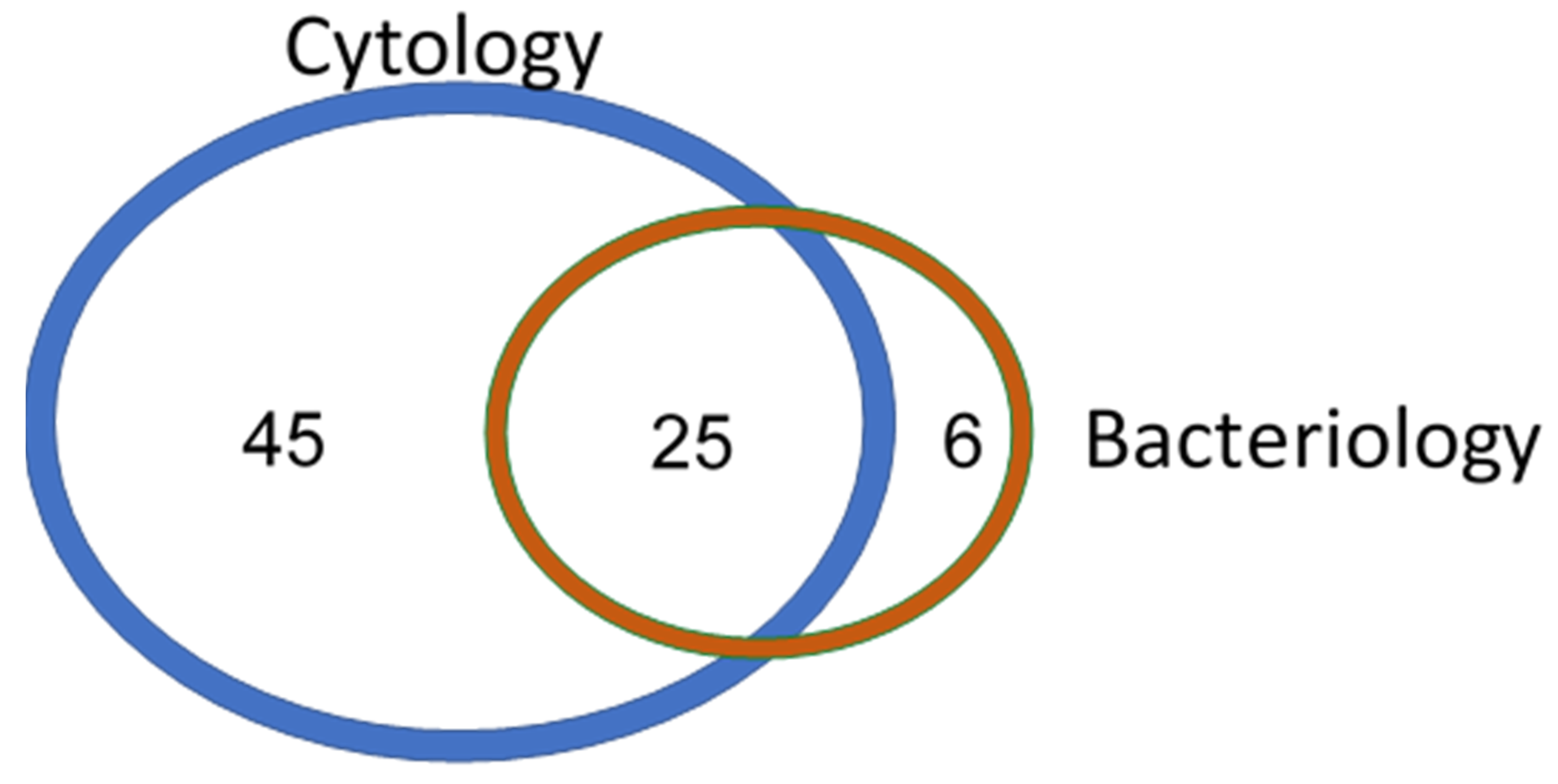
3. Results
3.1. Petrifilm System
3.2. Tri-Plate System
3.3. VBLAB Results and PMNL Count
4. Discussion
4.1. On-Farm Bacteriological Systems
4.2. VBLAB Results and PMNL Count Comparison
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheldon, I.M.; Lewis, G.S.; LeBlanc, S.; Gilbert, R. Defining postpartum uterine disease in cattle. Theriogenology 2006, 65, 1516–1530. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Williams, E.; Miller, A.N.; Nash, D.M.; Herath, S. Uterine diseases in cattle after parturition. Vet. J. 2008, 176, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Barlund, C.; Carruthers, T.; Waldner, C.; Palmer, C. A comparison of diagnostic techniques for postpartum endometritis in dairy cattle. Theriogenology 2008, 69, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Dubuc, J.; Denis-Robichaud, J. A dairy herd-level study of postpartum diseases and their association with reproductive performance and culling. J. Dairy Sci. 2017, 100, 3068–3078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, L.C.; Cronin, J.G.; Sheldon, I.M. Mechanisms linking bacterial infections of the bovine endometrium to disease and infertility. Reprod. Biol. 2016, 16, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Dubuc, J.; Duffield, T.; Leslie, K.; Walton, J.; LeBlanc, S. Definitions and diagnosis of postpartum endometritis in dairy cows. J. Dairy Sci. 2010, 93, 5225–5233. [Google Scholar] [CrossRef]
- Wagener, K.; Gabler, C.; Drillich, M. A review of the ongoing discussion about definition, diagnosis and pathomechanism of subclinical endometritis in dairy cows. Theriogenology 2017, 94, 21–30. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Duffield, T.F.; Foster, R.A.; Gartley, C.J.; Leslie, K.E.; Walton, J.S.; Johnson, W.H. The effect of a single administration of cephapirin or cloprostenol on the reproductive performance of dairy cows with subclinical endometritis. Theriogenology 2005, 63, 818–830. [Google Scholar] [CrossRef]
- McDougall, S. Effect of intrauterine antibiotic treatment on reproductive performance of dairy cows following periparturient disease. N. Z. Vet. J. 2001, 49, 150–158. [Google Scholar] [CrossRef]
- Leblanc, S.; Duffield, T.; Leslie, K.; Bateman, K.; Keefe, G.; Walton, J.; Johnson, W. The Effect of Treatment of Clinical Endometritis on Reproductive Performance in Dairy Cows. J. Dairy Sci. 2002, 85, 2237–2249. [Google Scholar] [CrossRef]
- Tison, N.; Bouchard, E.; DesCôteaux, L.; Lefebvre, R. Effectiveness of intrauterine treatment with cephapirin in dairy cows with purulent vaginal discharge. Theriogenology 2017, 89, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Kabera, F.; Dufour, S.; Keefe, G.; Cameron, M.; Roy, J.P. Evaluation of quarter-based selective dry cow therapy using Petrifilm on-farm milk culture: A randomized controlled trial. J. Dairy Sci. 2020, 103, 7276–7287. [Google Scholar] [CrossRef] [PubMed]
- Vries, E.M.M.-D.; Knorr, N.; Paduch, J.-H.; Zinke, C.; Hoedemaker, M.; Krömker, V. A field study evaluation of Petrifilm™ plates as a 24-h rapid diagnostic test for clinical mastitis on a dairy farm. Prev. Vet. Med. 2014, 113, 620–624. [Google Scholar] [CrossRef] [PubMed]
- McCarron, J.; Keefe, G.; McKenna, S.; Dohoo, I.; Poole, D. Laboratory evaluation of 3M Petrifilms and University of Minnesota Bi-plates as potential on-farm tests for clinical mastitis. J. Dairy Sci. 2009, 92, 2297–2305. [Google Scholar] [CrossRef] [PubMed]
- Dubuc, J. Short communication: Diagnostic performance of on-farm bacteriological culture systems for identification of uterine Escherichia coli in postpartum dairy cows. J. Dairy Sci. 2017, 100, 3079–3082. [Google Scholar] [CrossRef] [Green Version]
- Flahault, A.; Cadilhac, M.; Thomas, G. Sample size calculation should be performed for design accuracy in diagnostic test studies. J. Clin. Epidemiol. 2005, 58, 859–862. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Duffield, T.F.; Foster, R.A.; Gartley, C.J.; Leslie, K.E.; Walton, J.S.; Johnson, W.H. Endometrial cytology and ultrasonography for the detection of subclinical endometritis in postpartum dairy cows. Theriogenology 2004, 62, 9–23. [Google Scholar] [CrossRef]
- Madoz, L.V.; Prunner, I.; Jaureguiberry, M.; Gelfert, C.C.; de la Sota, R.L.; Giuliodori, M.J.; Drillich, M. Application of a bacteriological on-farm test to reduce antimicrobial usage in dairy cows with purulent vaginal discharge. J. Dairy Sci. 2017, 100, 3875–3882. [Google Scholar] [CrossRef]
- Williams, E.J.; Fischer, D.P.; Pfeiffer, D.U.; England, G.C.; Noakes, D.E.; Dobson, H.; Sheldon, I.M. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology 2005, 63, 102–117. [Google Scholar] [CrossRef]
- Gilbert, R.O.; Santos, N.R. Dynamics of postpartum endometrial cytology and bacteriology and their relationship to fertility in dairy cows. Theriogenology 2016, 85, 1367–1374. [Google Scholar] [CrossRef] [Green Version]
- Földi, J.; Kulcsár, M.; Pécsi, A.; Huyghe, B.; de Sa, C.; Lohuis, J.A.C.M.; Cox, P.; Huszenicza, G. Bacterial complications of postpartum uterine involution in cattle. Anim. Reprod. Sci. 2006, 96, 265–281. [Google Scholar] [CrossRef] [PubMed]
| Threshold (Colony Count) | Sensitivity (%) [95% CI] | Specificity (%) [95% CI] | Sensitivity + Specificity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) | Apparent Prevalence (%) |
|---|---|---|---|---|---|---|
| ˃20 | 83.3 [65.3;94.4] | 28.9 [22.0;36.6] | 112.3 | 18.1 | 90.2 | 73.0 |
| ˃40 | 63.3 [43.9;80.1] | 48.4 [40.4;56.5] | 111.8 | 18.8 | 87.5 | 53.4 |
| ˃60 | 60.0 [40.6;77.3] | 61.0 [53.0;68.6] | 121.0 | 22.5 | 89.0 | 42.3 |
| ˃80 | 56.7 [37.4;74.5] | 68.6 [60.7;75.7] | 125.2 | 25.4 | 89.3 | 35.4 |
| ˃100 * | 56.7 [37.4;74.5] | 72.3 [64.7;79.1] | 129.0 | 27.9 | 89.8 | 32.2 |
| ˃120 | 50.0 [31.3;68.7] | 76.7 [69.4;83.1] | 126.7 | 28.8 | 89.1 | 27.5 |
| ˃140 | 46.7 [28.3;65.7] | 80.5 [73.5;86.4] | 127.2 | 31.1 | 88.9 | 23.8 |
| ˃160 | 43.3 [25.5;62.6] | 83.0 [76.3;88.5] | 126.4 | 32.5 | 88.6 | 21.2 |
| ˃180 | 43.3 [25.5;62.6] | 84.9 [78.4;90.1] | 128.2 | 35.1 | 88.8 | 19.6 |
| ˃200 | 40.0 [22.7;59.4] | 86.8 [80.5;91.6] | 126.8 | 36.4 | 88.5 | 17.5 |
| Threshold (Colony Count) | Sensitivity (%) [95% CI] | Specificity (%) [95% CI] | Sensitivity + Specificity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) | Apparent Prevalence (%) |
|---|---|---|---|---|---|---|
| ˃10 | 76.7 [57.7;90.1] | 51.6 [43.5;59.6] | 128.2 | 23.0 | 92.1 | 52.9 |
| ˃20 | 73.3 [54.1;87.7] | 73.0 [65.4;79.7] | 146.3 | 33.8 | 93.5 | 34.4 |
| ˃30 | 73.3 [54.1;87.7] | 82.4 [75.6;88.0] | 155.7 | 44.0 | 94.2 | 26.5 |
| ˃40 | 73.3 [54.1;87.7] | 86.2 [79.8;91.1] | 159.5 | 50.0 | 94.5 | 23.3 |
| ˃50 | 73.3 [54.1;87.7] | 89.9 [84.2;94.1] | 163.3 | 57.9 | 94.7 | 20.1 |
| ˃60 | 73.3 [54.1;87.7] | 90.6 [84.9;94.6] | 163.9 | 59.5 | 94.7 | 19.6 |
| ˃70 | 73.3 [54.1;87.7] | 91.8 [86.4;95.6] | 165.2 | 62.9 | 94.8 | 18.5 |
| ˃80 | 73.3 [54.1;87.7] | 92.5 [87.2;96.0] | 165.8 | 64.7 | 94.8 | 18.0 |
| ˃90 * | 73.3 [54.1;87.7] | 94.3 [87.2;96.0] | 167.7 | 71.0 | 94.9 | 16.4 |
| ˃100 | 70.0 [50.6;85.3] | 95.6 [91.1;98.2] | 165.6 | 75.0 | 94.4 | 14.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbeau-Grégoire, N.; Boyer, A.; Rousseau, M.; Gauthier, M.-L.; Dubuc, J. Validation of On-Farm Bacteriological Systems for Endometritis Diagnosis in Postpartum Dairy Cows. Animals 2021, 11, 2695. https://doi.org/10.3390/ani11092695
Barbeau-Grégoire N, Boyer A, Rousseau M, Gauthier M-L, Dubuc J. Validation of On-Farm Bacteriological Systems for Endometritis Diagnosis in Postpartum Dairy Cows. Animals. 2021; 11(9):2695. https://doi.org/10.3390/ani11092695
Chicago/Turabian StyleBarbeau-Grégoire, Nicolas, Alexandre Boyer, Marjolaine Rousseau, Marie-Lou Gauthier, and Jocelyn Dubuc. 2021. "Validation of On-Farm Bacteriological Systems for Endometritis Diagnosis in Postpartum Dairy Cows" Animals 11, no. 9: 2695. https://doi.org/10.3390/ani11092695






