RNA-Seq Analysis of the Key Long Noncoding RNAs and mRNAs Related to the Regulation of Acute Heat Stress in Rainbow Trout
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ethics Statement
2.3. Transcriptome Sequencing and Analysis
3. Results
3.1. Behavior Evaluation
3.2. Oxidative Stress Index and Pro-Inflammatory Cytokine Index
3.3. DEGs
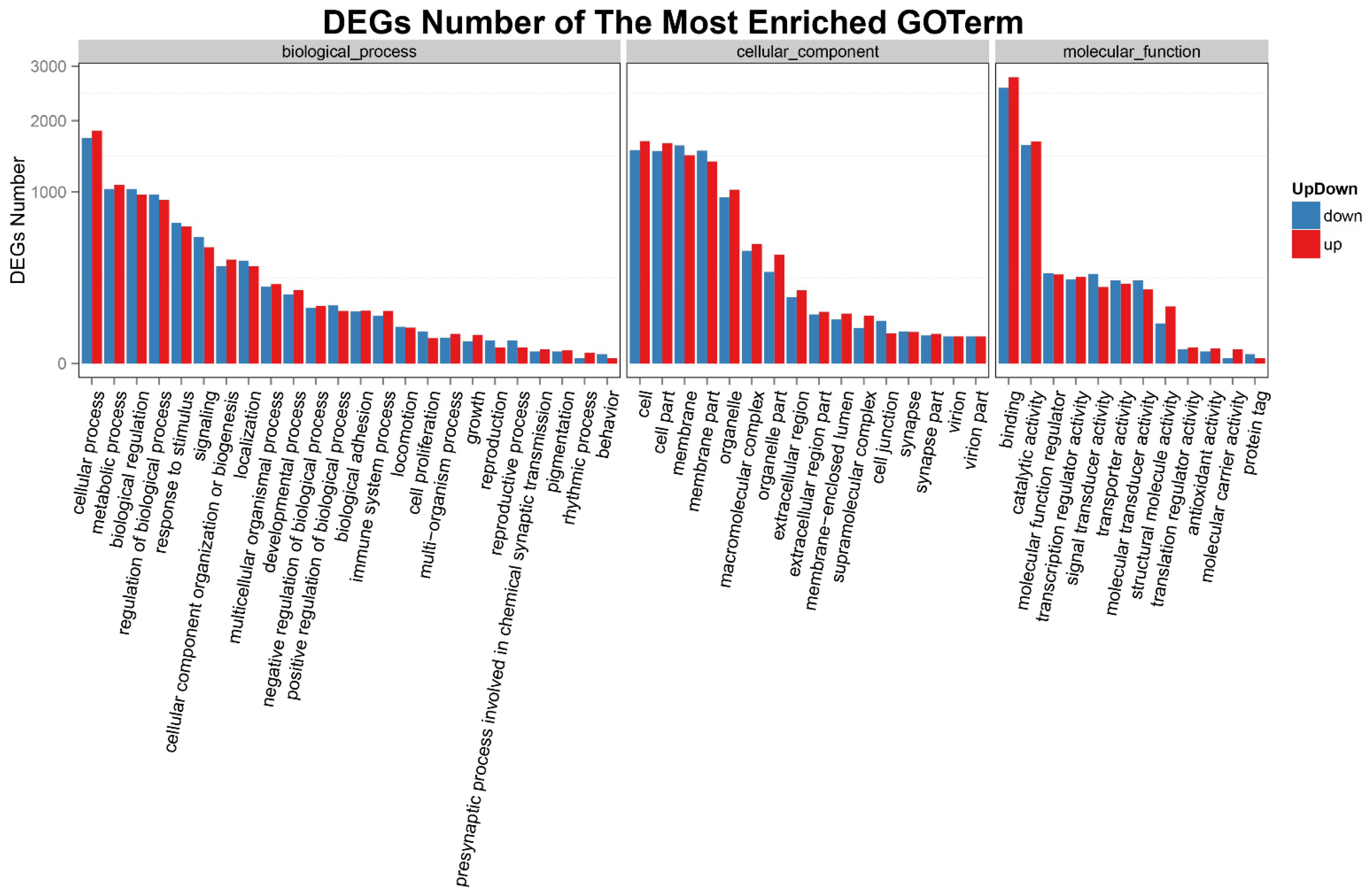
3.4. Analysis of DEGs
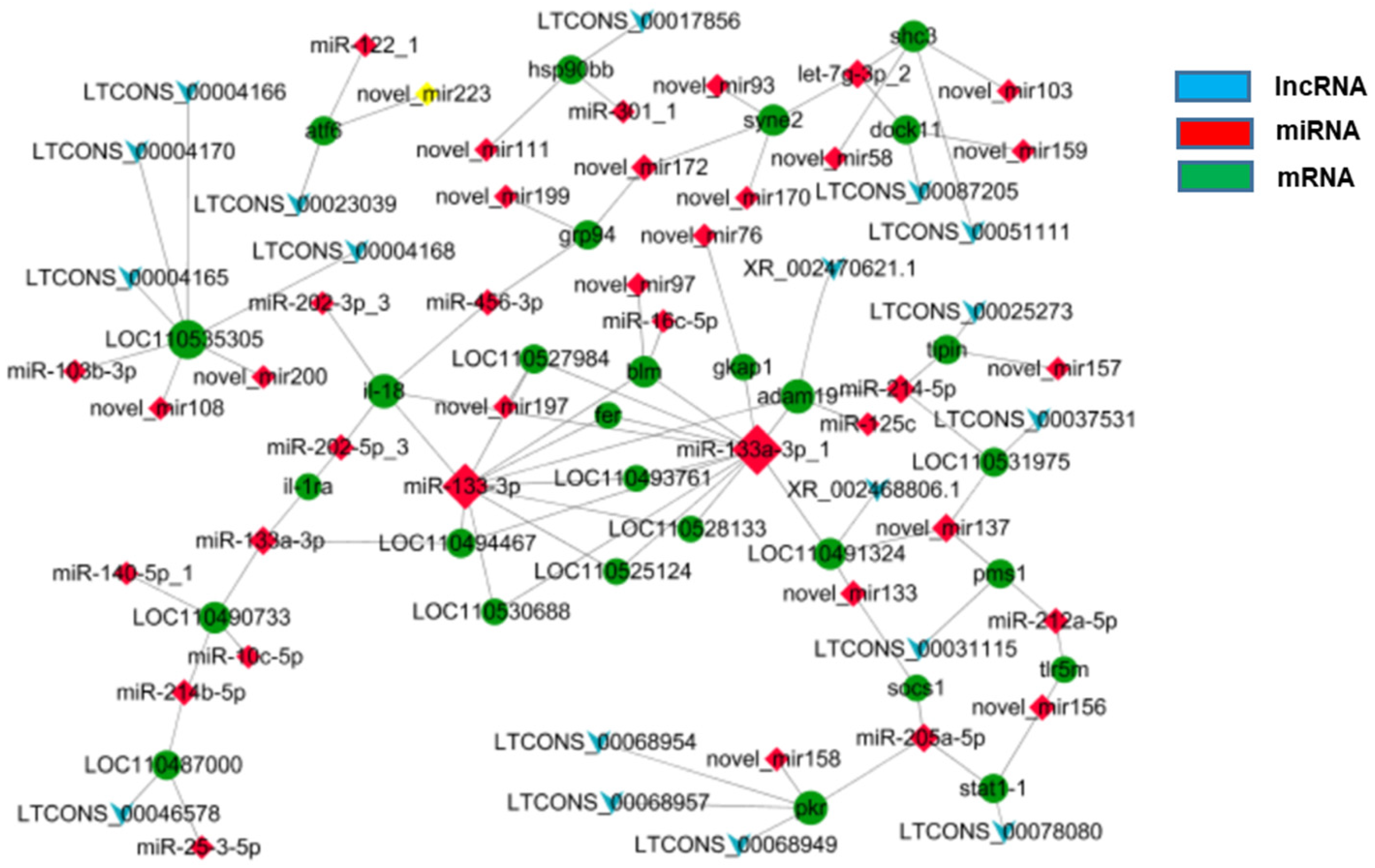
3.5. Regulatory Network of Analysis
4. Discussion
4.1. Immune Regulation
4.2. Apoptosis
4.3. Metabolic
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, C.Q.; Zhou, P.; Ren, Y.L.; Cao, L.H.; Wang, J.L. Physiological response and miRNA-mRNA interaction analysis in the head kidney of rainbow trout exposed to acute heat stress. J. Therm. Biol. 2019, 83, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Geven, E.J.W.; Klaren, P.H.M. The teleost head kidney: Integrating thyroid and immune signalling. Dev. Comp. Immunol. 2017, 66, 73–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uribe, C.; Folch, H.; Enriquez, R.; Moran, G.J. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011, 56, 486–503. [Google Scholar] [CrossRef] [Green Version]
- Kemenade, B.M.L.; Stolte, H.H.; Metz, J.R. Neuroendocrine-immune interactions in teleost fish. Fish Neuroendocrinol. 2009, 28, 313–364. [Google Scholar]
- Velculescu, V.E.; Vogelstein, B.; Kinzler, K.W. Characterization of the Yeast Transcriptome. Cell 1997, 88, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Jiang, S.; Wu, W.; Yu, F.; Chang, W.; Li, P.; Wang, K. Non-coding RNAs Function as Immune Regulators in Teleost Fish. Front. Immunol. 2018, 9, 2801. [Google Scholar] [CrossRef]
- Alexander, R.P.; Fang, G.; Rozowsky, J.; Snyder, M.; Gerstein, M.B. Annotating non-coding regions of the genome. Nat. Rev. Genet. 2010, 11, 559–571. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Zhang, J.; Zhao, X.; Xu, P.; Liu, X.; Li, M.; Lv, C.; Song, X. Crosstalk of mRNA, miRNA, lncRNA, and circRNA and Their Regulatory Pattern in Pulmonary Fibrosis. Mol. Ther. Nucleic Acids 2019, 18, 204–218. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.L.; Guo, R.S.; Hao, G.J.; Xiao, J.; Bao, Y.; Zhou, J.; Chen, Q.; Wei, X. The expression profiling and ontology analysis of noncoding RNAs in peritoneal fibrosis induced by peritoneal dialysis fluid. Gene 2015, 564, 210–219. [Google Scholar] [CrossRef]
- Zhou, C.Q.; Ka, W.; Yuan, W.K.; Wang, J.L. The effect of acute heat stress on the innate immune function of rainbow trout based on the transcriptome. J. Therm. Biol. 2021, 96, 102834. [Google Scholar] [CrossRef]
- Wang, L.K.; Feng, Z.X.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Wendelaar, B.S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef] [PubMed]
- Petitjean, Q.; Jean, S.; Gandar, A.; Côte, J.; Laffaille, P.; Jacquin, L. Stress responses in fish: From molecular to evolutionary processes. Sci. Total Environ. 2019, 684, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.D.; Hsu, L.W.; Goto, S.; Yeh, C.W.; Nakano, T.; Lai, C.Y.; Chang, Y.C.; Hou, C.H.; Wang, C.C.; Cheng, Y.F.; et al. Adaptor protein Shc acts as an immune-regulator for the LPS-stimulated maturation of bone marrow-derived dendritic cells. BMC Immunol. 2011, 12, 32. [Google Scholar] [CrossRef] [Green Version]
- Bagci, H.; Sriskandarajah, N.; Robert, A.; Boulais, J.; Elkholi, I.E.; Tran, V.; Lin, Z.Y.; Thibault, M.P.; Dubé, N.; Faubert, D.; et al. Mapping the proximity interaction network of the Rho-family GTPases reveals signalling pathways and regulatory mechanisms. Nat. Cell Biol. 2020, 22, 120–134. [Google Scholar] [CrossRef]
- Tolar, P. Cytoskeletal control of B cell responses to antigens. Nat. Rev. Immunol. 2017, 17, 621–634. [Google Scholar] [CrossRef]
- Harwood, N.E.; Batista, F. Early events in B cell activation. Annu. Rev. Immunol. 2010, 28, 185–210. [Google Scholar] [CrossRef]
- Burbage, M.; Keppler, S.J.; Gasparrini, F.; Martínez-Martín, N.; Gaya, M.; Feest, C.; Domart, M.C.; Brakebusch, C.; Collinson, L.; Bruckbauer, A.; et al. Cdc42 is a key regulator of B cell differentiation and is required for antiviral humoral immunity. J. Exp. Med. 2015, 212, 53–72. [Google Scholar] [CrossRef]
- Burbage, M.; Keppler, S.J.; Montaner, B.; Mattila, P.K.; Batista, F.D. The Small Rho GTPase TC10 Modulates B Cell Immune Responses. J. Immunol. 2017, 199, 1682–1695. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, A.; Maruyama, M. Contribution of DOCK11 to the Expansion of Antigen-Specific Populations among Germinal Center B Cells. Immunohorizons 2020, 4, 520–529. [Google Scholar] [CrossRef]
- Ma, F.; Liu, Z.; Kang, Y.; Quan, J. Genome-Wide Identification of hsp90 Gene in Rainbow Trout (Oncorhynchus mykiss) and Their Regulated Expression in Response to Heat Stress. DNA Cell Biol. 2020, 39, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J. Proteins as molecular chaperones. Nature 1987, 328, 378–379. [Google Scholar] [CrossRef] [PubMed]
- Demers, N.E. Immediate Effects of Acute Stress on Innate Immunity in Rainbow Trout (Oncorhynchus mykiss). Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 1996. [Google Scholar]
- Daugaard, M.; Rohde, M.; Jäättelä, M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007, 581, 3702–3710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.J.; Huang, J.Q.; Liu, Z.; Zhou, Y.; Xia, B.; Wang, Y.; Kang, Y.; Wang, J. Transcriptome analysis provides insights into hepatic responses to moderate heat stress in the rainbow trout (Oncorhynchus mykiss). Gene 2017, 619, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H. Research progress on the structure and function of STAT1. Int. Transplant. Hemopurif. 2008, 6, 39–41. [Google Scholar]
- Ouchi, T.; Lee, S.W.; Ouchi, M.; Aaronson, S.A.; Horvath, C.M. Collaboration of signal transducer and activator of transcription 1 (STAT1) and BRCA1 in differential regulation of IFN-γ target genes. Proc. Natl. Acad. Sci. USA 2000, 97, 5208–5213. [Google Scholar] [CrossRef] [Green Version]
- Gil, J.; Esteban, M. Induction of apoptosis by the dsRNA-dependent protein kinase (PKR): Mechanism of action. Apoptosis 2000, 5, 107–114. [Google Scholar] [CrossRef]
- Li, S.D.; Peters, G.A.; Ding, K.Y.; Zhang, X.; Qin, J.; Sen, G.C. Molecular basis for PKR activation by PACT or dsRNA. Proc. Natl. Acad. Sci. USA 2006, 103, 10005–10010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, M.A.; Meurs, E.F.; Esteban, M. The dsRNA protein kinase PKR: Virus and cell control. Biochimie 2007, 89, 799–811. [Google Scholar] [CrossRef]
- Hugon, J.; Mouton-Liger, F.; Dumurgier, J.; Paquet, C. PKR involvement in Alzheimer’s disease. Alzheimers Res. Ther. 2017, 9, 83. [Google Scholar] [CrossRef]
- Wanatabe, T.; Imamura, T.; Hiasa, Y. Roles of protein kinase R in cancer: Potential as a therapeutic target. Cancer Sci. 2018, 109, 919–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gal-Ben-Ari, S.; Barrera, I.; Ehrlich, M.; Rosenblum, K. PKR: A Kinase to Remember. Front. Mol. Neurosci. 2019, 11, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillary, R.F.; FitzGerald, U. A lifetime of stress: ATF6 in development and homeostasis. J. Biomed. Sci. 2018, 25, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFarlane, R.J.; Mian, S.; Dalgaard, J.Z. The many facets of the Tim-Tipin protein families’ roles in chromosome biology. Cell Cycle 2010, 9, 700–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leman, A.R.; Noguchi, E. Local and global functions of Timeless and Tipin in replication fork protection. Cell Cycle 2012, 11, 3945–3955. [Google Scholar] [CrossRef] [Green Version]
- Chou, D.M.; Elledge, S.J. Tipin and Timeless form a mutually protective complex required for genotoxic stress resistance and checkpoint function. Proc. Natl. Acad. Sci. USA 2006, 103, 18143–18147. [Google Scholar] [CrossRef] [Green Version]
- Baldeyron, C.; Brisson, A.; Tesson, B.; Némati, F.; Koundrioukoff, S.; Saliba, E.; De Koning, L.; Martel, E.; Ye, M.; Rigaill, G.; et al. TIPIN depletion leads to apoptosis in breast cancer cells. Mol. Oncol. 2015, 9, 1580–1598. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P.J. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Ma, A.; Yang, S.; Huang, Z. Integrated metabolome and transcriptome analyses revealing the effects of thermal stress on lipid metabolism in juvenile turbot Scophthalmus maximus. J. Therm. Biol. 2021, 99, 102937. [Google Scholar] [CrossRef]
- Huovila, A.P.J.; Turner, A.J.; Pelto-Huikko, M.; Kärkkäinen, I.; Ortiz, R.M. Shedding light on ADAM metalloproteinases. Trends Biochem. Sci. 2005, 30, 413–422. [Google Scholar] [CrossRef]
- Weerasekera, L.; Rudnicka, C.; Sang, Q.X.; Curran, J.E.; Johnson, M.P.; Moses, E.K.; Göring, H.H.; Blangero, J.; Hricova, J.; Schlaich, M.; et al. ADAM19: A Novel Target for Metabolic Syndrome in Humans and Mice. Mediat. Inflamm. 2017, 2017, 7281986. [Google Scholar] [CrossRef] [PubMed]
- Casciola-Rosen, L.A.; Pluta, A.F.; Plotz, P.H.; Cox, A.E.; Morris, S.; Wigley, F.M.; Petri, M.; Gelber, A.C.; Rosen, A. The DNA mismatch repair enzyme PMS1 is a myositis-specific autoantigen. Arthritis Rheum. 2001, 44, 389–396. [Google Scholar] [CrossRef]
- Li, X.B.; Wu, Y.L.; Suo, P.S.; Liu, G.; Li, L.; Zhang, X.; Chen, S.; Xu, M.; Song, L. Identification of a novel germline frameshift mutation p.D300fs of PMS1 in a patient with hepatocellular carcinoma: A case report and literature review. Medicine 2020, 99, e19076. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.-Q.; Ka, W.; Zhang, H.-J.; Li, Y.-L.; Gao, P.; Long, R.-J.; Yang, S.-W.; Wang, J.-L. RNA-Seq Analysis of the Key Long Noncoding RNAs and mRNAs Related to the Regulation of Acute Heat Stress in Rainbow Trout. Animals 2022, 12, 325. https://doi.org/10.3390/ani12030325
Zhou C-Q, Ka W, Zhang H-J, Li Y-L, Gao P, Long R-J, Yang S-W, Wang J-L. RNA-Seq Analysis of the Key Long Noncoding RNAs and mRNAs Related to the Regulation of Acute Heat Stress in Rainbow Trout. Animals. 2022; 12(3):325. https://doi.org/10.3390/ani12030325
Chicago/Turabian StyleZhou, Chang-Qing, Wei Ka, Hui-Jun Zhang, Ya-Lan Li, Pan Gao, Rui-Jun Long, Shun-Wen Yang, and Jian-Lin Wang. 2022. "RNA-Seq Analysis of the Key Long Noncoding RNAs and mRNAs Related to the Regulation of Acute Heat Stress in Rainbow Trout" Animals 12, no. 3: 325. https://doi.org/10.3390/ani12030325
APA StyleZhou, C.-Q., Ka, W., Zhang, H.-J., Li, Y.-L., Gao, P., Long, R.-J., Yang, S.-W., & Wang, J.-L. (2022). RNA-Seq Analysis of the Key Long Noncoding RNAs and mRNAs Related to the Regulation of Acute Heat Stress in Rainbow Trout. Animals, 12(3), 325. https://doi.org/10.3390/ani12030325




