Anaemia in Lambs Caused by Mycoplasma ovis: Global and Australian Perspectives
Abstract
:Simple Summary
Abstract
1. Introduction
2. Epidemiology: Transmission, Distribution, Prevalence and Zoonotic Potential
2.1. Transmission of M. ovis
2.2. Distribution of M. ovis
2.3. Zoonotic Potential of M. ovis
3. Pathogenesis: Clinical Signs, Pathology and Diagnosis of M. ovis Infection
3.1. Pathogenesis of M. ovis Infection
3.2. Clinical Signs of M. ovis Infection
3.3. Pathology of M. ovis Infection
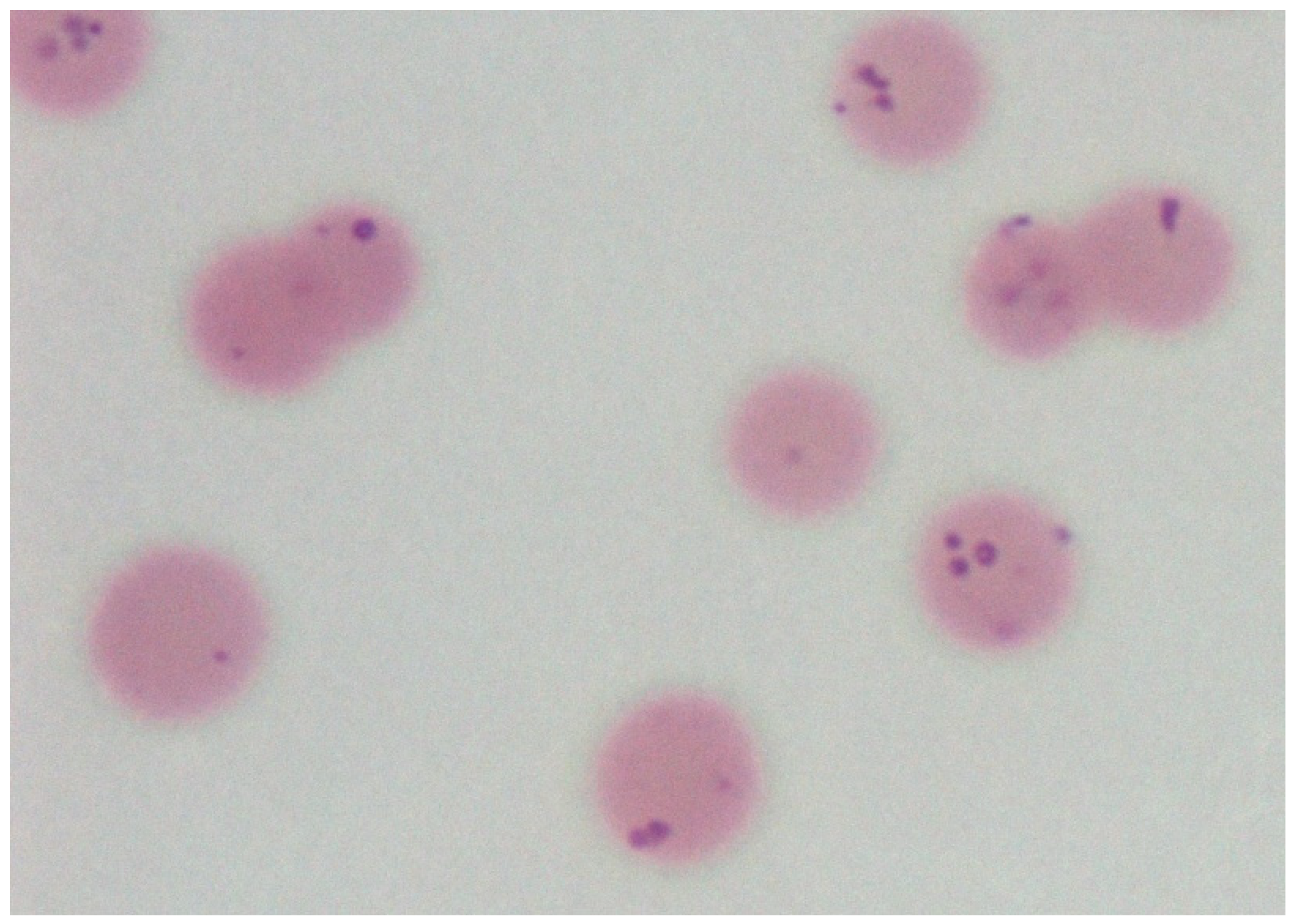
3.4. Diagnosis of M. ovis Infection
3.5. Concurrent Infections and the Differential Diagnosis of M. ovis
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neimark, H.; Hoff, B.; Ganter, M. Mycoplasma ovis comb. nov. (formerly Eperythrozoon ovis), an epierythrocytic agent of haemolytic anaemia in sheep and goats. Int. J. Syst. Evol. Microbiol. 2004, 54, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Jesse, F.F.A.; Chung, E.L.T.; Che-Amat, A.; Lila, M.A.M.; Hashi, H.A.; Norsidin, M.J. Review of clinical aspects, epidemiology and diagnosis of haemotropic Mycoplasma ovis in small ruminants: Current status and future perspectives in tropics focusing on Malaysia. Trop. Anim. Health Prod. 2020, 52, 2829–2844. [Google Scholar] [CrossRef] [PubMed]
- Urie, N.J.; Highland, M.A.; Knowles, D.P.; Branan, M.A.; Herndon, D.R.; Marshall, K.L. Mycoplasma ovis infection in domestic sheep (Ovis aries) in the United States: Prevalence, distribution, associated risk factors, and associated outcomes. Prev. Veter. Med. 2019, 171, 104750. [Google Scholar] [CrossRef]
- Windsor, P.; Nampanya, S.; Tagger, A.; Keonam, K.; Gerasimova, M.; Putthana, V.; Bush, R.; Khounsy, S. Is orf infection a risk to expanding goat production in developing countries? A case study from Lao PDR. Small Rumin. Res. 2017, 154, 123–128. [Google Scholar] [CrossRef]
- Sykes, J.E.; Lindsay, L.A.L.; Maggi, R.G.; Breitschwerdt, E.B. Human coinfection with Bartonella henselae and two hemotropic mycoplasma variants resembling Mycoplasma ovis. J. Clin. Microbiol. 2010, 48, 3782–3785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Windsor, P.A.; Lomax, S. Addressing welfare concerns regarding control of cutaneous myiosis in Australia. Small Rum. Res. 2013, 110, 165–169. [Google Scholar] [CrossRef]
- Neimark, H.; Johansson, K.E.; Rikihisa, Y.; Tully, J.G. Proposal to transfer some members of the genera Haemobartonella and Eperythrozoon to the genus Mycoplasma with descriptions of ‘Candidatus Mycoplasma haemofelis’, ‘Candidatus Mycoplasma haemomuris’, ‘Candidatus Mycoplasma haemosuis’ and ‘Candidatus Mycoplasma wenyonii’. Int. J. Syst. Evol. Microbiol. 2001, 51, 891–899. [Google Scholar] [CrossRef]
- Daddow, K.N. Culex annulirostris as a vector of Eperythrozoon ovis infection in sheep. Vet. Parasitol. 1980, 7, 313–317. [Google Scholar] [CrossRef]
- Aktas, M.; Ozubek, S.A. Molecular survey of small ruminant hemotropic mycoplasmosis in Turkey, including first laboratory confirmed clinical cases caused by Mycoplasma ovis. Vet. Microbiol. 2017, 208, 217–222. [Google Scholar] [CrossRef]
- Mason, R.W.; Statham, P. The determination of the level of Eperythrozoon ovis parasitaemia in chronically infected sheep and its significance to the spread of infection. Aust. Vet. J. 1991, 68, 115–116. [Google Scholar] [CrossRef]
- Hornok, S.; Micsutka, A.; Meli, M.; Lutz, H.; Hofmann-Lehmann, R. Molecular investigation of transplacental and vector-borne transmission of bovine haemoplasmas. Vet. Microbiol. 2011, 152, 411–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, R.W.; Sloan, C.A.; Harbutt, P.R. Observations on mortality in lambs in Victoria associated with Eperythrozoon ovis. Aust. Vet. J. 1971, 47, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.W.; Corbould, A.; Statham, P. A serological survey of Eperythrozoon ovis in goats and sheep in Tasmania. Aust. Vet. J. 1989, 66, 122–123. [Google Scholar] [CrossRef] [PubMed]
- Kabay, M.J.; Richards, R.B.; Ellis, T.E. A cross-sectional study to show Eperythrozoon ovis infection is prevalent in Western Australian sheep farms. Aust. Vet. J. 1991, 68, 170–173. [Google Scholar] [CrossRef]
- Rjeibi, M.R.; Darghouth, M.A.; Omri, H.; Souidi, K.; Rekik, M.; Gharbi, M. First molecular isolation of Mycoplasma ovis from small ruminants in North Africa. Onderst. J. Vet. Res. 2015, 82, a912. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Hernández, J.M.; Ballados-González, G.G.; Fernández-Bandala, D.; Martínez-Soto, S.; Velázquez-Osorio, V.; Martínez-Rodríguez, P.B.; Cruz-Romero, A.; Grostieta, E.; Lozano-Sardaneta, Y.; Salas, P.C.; et al. Molecular detection of Mycoplasma ovis in an outbreak of hemolytic anemia in sheep from Veracruz, Mexico. Trop. Anim. Health Prod. 2018, 51, 243–248. [Google Scholar] [CrossRef]
- Yang, D.; Xiuzheng, T.; Ying, Q.; Sheng, Y. Prevalence of Eperythrozoon spp. infection and congenital eperythrozoonosis in humans in inner Mongolia, China. Epidem. Infect. 2000, 125, 421–426. [Google Scholar] [CrossRef]
- Maggi, R.G.; Compton, S.M.; Trull, C.L.; Mascarelli, P.E.; Mozayeni, B.R.; Breitschwerdt, E.B. Infection with Hemotropic Mycoplasma Species in Patients with or without Extensive Arthropod or Animal Contact. J. Clin. Microbiol. 2013, 51, 3237–3241. [Google Scholar] [CrossRef] [Green Version]
- Neitz, W.; Alexander, R.; Du Toit, P. Eperythrozoon ovis (sp. nov.) infection in sheep. Onderst. J. Vet. Res. 1934, 11, 263–271. [Google Scholar]
- Littlejohns, I.R. Eperythrozoonosis in sheep. Aust. Vet. J. 1960, 366, 260–265. [Google Scholar] [CrossRef]
- Gulland, F.M.; Doxey, D.L.; Scott, G.R. Changing morphology of Eperythrozoon ovis. Res. Vet. Sci. 1987, 43, 88–91. [Google Scholar] [CrossRef]
- Norris, M.J.; Rahaley, R.S.; Whittaker, R.G. Effect of Eperythrozoon ovis on the lysis of sheep erythrocytes in the complement fixation test. Vet. Immunol. Immunopathol. 1987, 16, 283–288. [Google Scholar] [CrossRef]
- Philbey, A.W.; Barron, R.C.J.; Gounden, A. Chronic eperythrozoonosis in an adult ewe. Vet. Rec. 2006, 158, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Theiss, P.; Karpas, A.; Wise, K.S. Antigenic topology of the P29 surface lipoprotein of Mycoplasma fermentans: Differential display of epitopes results in high-frequency phase variation. Infect. Immun. 1996, 64, 1800–1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katharina Hoelzle, K.; Ade, J.; Hoelzle, L.E. Persistence in Livestock Mycoplasmas—A Key Role in Infection and Pathogenesis. Curr. Clin. Microbiol. Rep. 2020, 7, 81–89. [Google Scholar] [CrossRef]
- Robson, S.; Kemp, B. Eperythrozoonosis in Sheep. NSW Department of Primary Industry Prime Fact 466, 2007. Available online: https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0013/111154/eperythrozoonosis-in-sheep.pdf (accessed on 25 May 2022).
- Welker Biondo, A.; Pires dos Santos, A.; Marcia Sá Guimarães, A.; Felipe da Costa Vieira, R.; Vidotto, O.; de Barros Macieira, D.; Regina Pereira Almosny, N.; Beltrão Molento, M.; Timenetsky, J.; Autran de Morais, H.; et al. A review of the occurrence of hemoplasmas (hemotrophic mycoplasmas) in Brazil. Rev. Bras. Parasitol. Vet. 2009, 18, 1–7. [Google Scholar] [CrossRef]
- Hampel, J.A.; Spath, S.N.; Bergin, I.L.; Lim, A.; Bolin, S.R.; Dyson, M.C. Prevalence and diagnosis of hemotrophic mycoplasma infection in research sheep and its effects on hematology variables and erythrocyte membrane fragility. Comp. Med. 2014, 64, 478–485. [Google Scholar]
- Mascarelli, P.E.; Keel, M.K.; Yabsley, M.; Last, L.A.; Breitschwerdt, E.B.; Maggi, R.G. Hemotropic mycoplasmas in little brown bats (Myotis lucifugus). Parasite Vectors 2014, 7, 117. [Google Scholar] [CrossRef] [Green Version]
- Varanat, M.; Maggi, R.G.; Linder, K.E.; Breitschwerdt, E.B. Molecular Prevalence of Bartonella, Babesia, and Hemotropic Mycoplasma sp. in Dogs with Splenic Disease. J. Vet. Intern. Med. 2011, 25, 1284–1291. [Google Scholar] [CrossRef]
- Jesse, F.; Jazid, N.; Mohammed, K.; Tijjani, A.; Chung, E.; Abba, Y.; Sadiq, M.; Saharee, A. Hemotropic Mycoplasma ovis infection in goats with concurrent gastrointestinal parasitism in Malaysia. J. Adv. Vet. Anim. Res. 2015, 2, 464–468. [Google Scholar] [CrossRef]
- Souza, U.A.; Oberrather, K.; Fagundes-Moreira, R.; de Almeida, B.A.; Valle, S.D.F.; Girotto-Soares, A.; Soares, J.F. First molecular detection of Mycoplasma ovis (Hemotropic mycoplasmas) from Sheep in Brazil. Rev. Bras. Parasitol. Vet. 2019, 28, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Archer, J.F.; Littlejohns, I.R. Eperythrozoon ovis and copper poisoning as causes of jaundice in lamb carcases. Aust. Vet. J. 1984, 61, 312–313. [Google Scholar] [CrossRef] [PubMed]
- Lacasta, D.; Ferrer, L.M.; Sanz, S.; Labanda, R.; González, J.M.; Benito, A.; Ruiz, H.; Rodríguez-Largo, A.; Ramos, J.J. Anaplasmosis Outbreak in Lambs: First Report Causing Carcass Condemnation. Animals 2020, 10, 1851. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, J.A.L. Eperythrozoan ovis infection in Western Australia. Aust. Vet. J. 1969, 45, 436. [Google Scholar] [CrossRef]
- Searle, L. A Case of Mycoplasmosis. Flock and Herd Case Studies. 2015. Available online: https://www.flockandherd.net.au/sheep/reader/mycoplasma-ovis-I.html (accessed on 29 April 2022).
- Meat and Livestock Australia. The Number of Merino Breeding Ewes is Falling. MLA. 2021. Available online: https://www.mla.com.au/news-and-events/industry-news/the-number-of-merino-breeding-ewes-is-falling/# (accessed on 28 April 2022).
- Meat and Livestock Australia. Sheep Projections. MLA. 2022. Available online: https://www.mla.com.au/prices-markets/Trends-analysis/sheep-projections/# (accessed on 28 April 2022).
- Lomax, S.; Sheil, M.; Windsor, P.A. Impact of topical anaesthesia on pain alleviation and wound healing in lambs after mulesing. Aust. Vet. J. 2008, 86, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Windsor, P.A.; Lomax, S.; White, P. Progress in pain management to improve small ruminant farm welfare. Small Rum. Res. 2016, 142, 55–57. [Google Scholar] [CrossRef]
- Roberts, C.D.; Windsor, P.A. Innovative pain management solutions in animals may provide improved wound pain reduction during debridement in humans: An opinion informed by veterinary literature. Int. Wound J. 2019, 16, 968–973. [Google Scholar] [CrossRef]
- Windsor, P.A. Progress with Livestock Welfare in Extensive Production Systems: Lessons From Australia. Front. Vet. Sc. 2021, 8, 674482. [Google Scholar] [CrossRef]
- Lacasta, D.; Reina, R.; de Arcaute, M.R.; Ferrer, L.M.; Benito, A.A.; Tejedor, M.T.; Echeverria, I.; Ruiz, H.; Cardenas, S.M.; Windsor, P.A. Effect of a Topical Formulation on Infective Viral Load in Lambs Naturally Infected with Orf Virus. Vet. Med. Res. Rep. 2021, 12, 149–158. [Google Scholar] [CrossRef]
- Ferrer, L.M.; Lacasta, D.; Ortín, A.; Ramos, J.J.; Tejedor, M.T.; Borobia, M.; Pérez, A.O.; Castells, E.; De Arcaute, M.R.; Ruiz, H.; et al. Impact of a topical anaesthesia wound management formulation on pain, inflammation and reduction of secondary infections after tail docking in lambs. Animals 2020, 10, 1255. [Google Scholar] [CrossRef]
- Temple, D.; Manteca, X. Animal Welfare in Extensive Production Systems Is Still an Area of Concern. Front. Sustain. Food Syst. 2020, 4, 545902. [Google Scholar] [CrossRef]
- Windsor, P.; Whittington, R. Ovine Paratuberculosis Control in Australia Revisited. Animals 2020, 10, 1623. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Windsor, P.A. Anaemia in Lambs Caused by Mycoplasma ovis: Global and Australian Perspectives. Animals 2022, 12, 1372. https://doi.org/10.3390/ani12111372
Windsor PA. Anaemia in Lambs Caused by Mycoplasma ovis: Global and Australian Perspectives. Animals. 2022; 12(11):1372. https://doi.org/10.3390/ani12111372
Chicago/Turabian StyleWindsor, Peter A. 2022. "Anaemia in Lambs Caused by Mycoplasma ovis: Global and Australian Perspectives" Animals 12, no. 11: 1372. https://doi.org/10.3390/ani12111372
APA StyleWindsor, P. A. (2022). Anaemia in Lambs Caused by Mycoplasma ovis: Global and Australian Perspectives. Animals, 12(11), 1372. https://doi.org/10.3390/ani12111372





