1. Introduction
Coccidiosis, induced by the apicomplexan protozoan parasites of the genus
Eimeria, accounts for more than USD 3 billion in economic loss in the poultry industry annually [
1]. The nine identified
Eimeria spp. in chickens include
E. acervulina,
E. brunetti,
E. maxima,
E. necatrix,
E. praecox,
E. mitis,
E. tenella,
E. mivati, and
E. hagani to date [
2,
3].
Eimeria spp. infect and multiply within the mucosal epithelial layers in the different parts of the gastrointestinal tract of chickens [
4]. After several sets of asexual and sexual replications, oocysts are excreted with feces, can be sporulated in the appropriate environment (temperature, humidity, and access to oxygen), and infect chickens repeatedly when they are ingested by chickens [
5]. Infection of
E. maxima, which propagates in the jejunum, can cause severe reduction in nutrient digestion and absorption along with inflammation caused by immune system in broilers [
6,
7]. Currently, prophylactic coccidiostats and anti-coccidial drugs are provided to control coccidiosis in broilers [
8]. However, due to the concern of spread of resistant bacteria and
Eimeria strains, numerous studies have been conducted to find alternatives for anticoccidial drugs including amino acids [
9], prebiotics [
10], plant extracts [
11], organic acids [
12], and nitro compounds [
13] in poultry production.
Tannins, polyphenol compounds that can precipitate proteins, are categorized into hydrolysable and condensed tannins. Diverse tannin sources such as chestnut (
Castanea sativa; hydrolysable tannin) and quebracho (
Schinopsis lorentzii; condensed tannin) are known to control
Eimeria infections [
14,
15,
16]. Hydrolysable and condensed tannins have different bioavailability because condensed tannins cannot be hydrolyzed into small molecules in chickens [
17]. Although high doses (>5 g/kg) of tannins have cytotoxicity and are considered as anti-nutritional factors in chickens, tannins at appropriate dosages are also known to show beneficial effects by exhibiting strong antimicrobial, antioxidant, and anti-inflammatory effects in chickens [
17]. Tannins can limit the growth of microorganisms by directly inhibiting activities of microbial enzymes and by indirectly forming complex with metal ions [
18,
19]. Moreover, immunomodulatory and antioxidant properties of tannins have potentials to reduce the parasitic infection and attenuate negative impacts of parasitic infections, respectively, in chickens [
20,
21]. Tannic acid (TA) is considered as the typical and standard of hydrolysable tannins. Tonda et al. [
22] reported that supplementation of 500 mg/kg gallnut TA extract reduced total oocysts in excreta and decreased intestinal lesion scores in broilers infected with
E. acervulina,
E. maxima, and
E. tenella. In contrast, Mansoori and Modirsanei [
23] showed that supplemental TA (10 g/kg) increased the total number of oocysts in excreta in broilers infected with
Eimeria spp., which indicates that high dosages of tannins can impair the immune system against coccidiosis and create better gut environment for
Eimeria propagation. Whether supplementation of TA at appropriate dosages can show antiparasitic effects against
E. maxima is still unknown. Hence, the purpose of the study was to evaluate effects of different dosages of supplemental TA on growth performance, oocyst shedding, gut permeability, intestinal morphology, number of goblet cells, immune system, and antioxidant capacity in broilers infected with
E. maxima.
4. Discussion
The purpose of the current study was to investigate effects of TA on growth performance, intestinal morphology, goblet cell number, gut barrier integrity, antioxidant capacity, oocyst shedding, and immune system in broilers infected with
E. maxima. E. maxima infection significantly decreased BW, ADG, and ADFI in the acute phase, which is consistent with our previous study [
24]. During the recovery phase, the CC group had statistically similar BW to the SCC group by enhancing feed intake and nutrient digestibility in the current study, which implies that there was compensatory growth in the CC group. Supplementation of TA reduced BW and ADG during the pre-challenge period via decreasing the feed intake. The TA–saliva protein complexes are known to induce astringent and bitter taste, which may decrease feed intake of broilers [
34]. Moreover, compromised gut ecosystem due to the high concentrations of TA potentially reduces feed intake of broilers [
35]. During the acute phase, supplementation of TA linearly decreased BW of broilers without affecting feed intake, intestinal morphology, and nutrient digestibility of broilers, which suggests that reduced growth performance by supplementation of TA at young ages may continuously affect growth performance of broilers at later growing phases. However, the TA0.5 group had the numerically highest BW among the
E. maxima-infected groups, which suggests that supplementation of 500 mg/kg TA may have potential to improve growth performance of broilers infected with
E. maxima. In contrast, supplementation of TA linearly reduced BW, ADG, and FCR of broilers infected with
E. maxima in the recovery phase. In the current study, supplementation of TA did not reduce ADG, ADFI, and FCR in the acute phase potentially because TA exhibited defensive effects against
E. maxima infection in broilers.
Supplementation of TA decreased oocyst shedding of
E. maxima in broilers at 5 to 9 dpi. Consistent with a previous study [
36], around 7 dpi was the peak point for oocyst shedding of
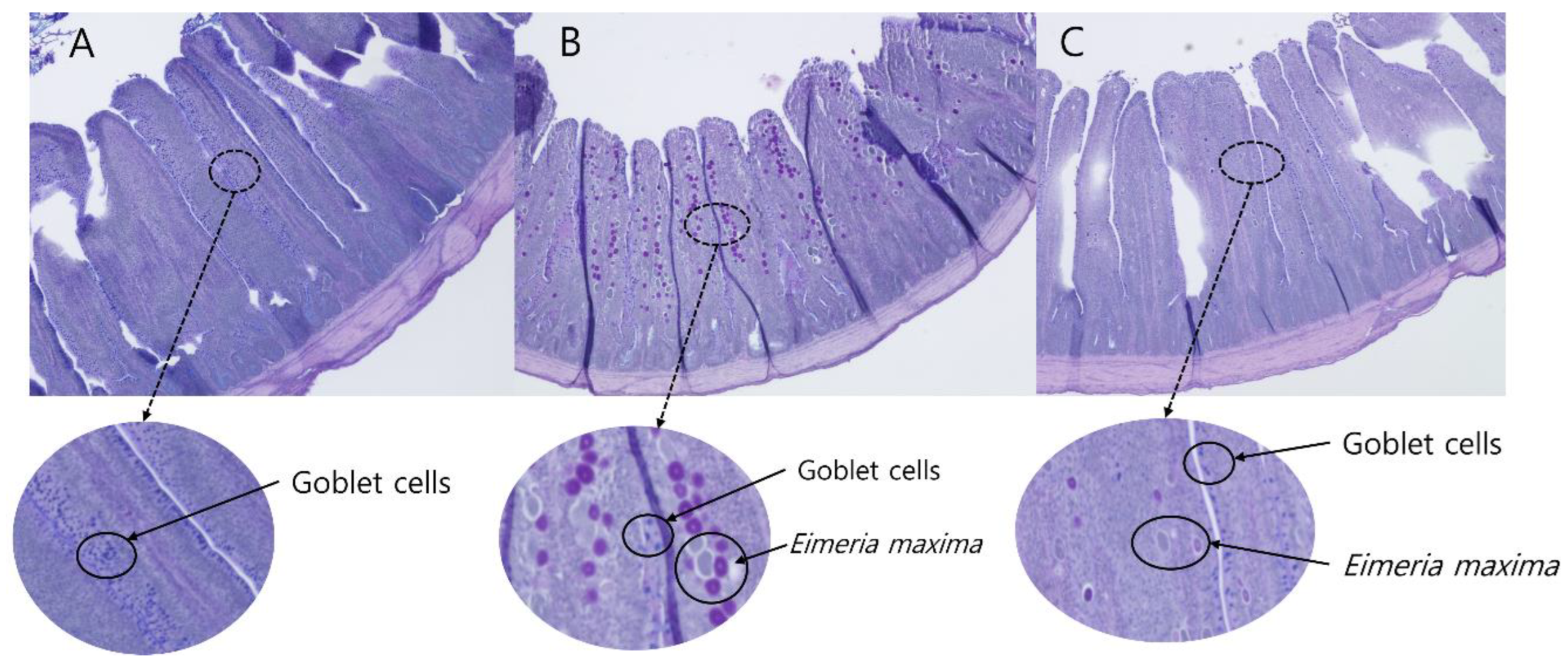
E. maxima. Moreover, pictures of jejunum morphology showed visually less
E. maxima in the TA0.5 group compared to the CC group (
Figure 1). In agreement with this, Tonda et al. [
22] and Kaleem et al. [
37] reported that tannin extract from plants decreased oocyst shedding in broilers infected with
Eimeria spp. Because there were no differences in the jejunal lesion score among the groups infected with
E. maxima, thereby, TA could potentially not inhibit the invasion of
E. maxima into the enterocytes but instead inhibit the sexual reproduction of
E. maxima to produce oocysts. Sexual reproduction of
Eimeria spp., following at least two cycles of asexual reproductions, is required to produce oocysts [
38]. Potentially, TA may directly inhibit enzymes activities and interact with proteins related to sexual reproduction or deprive nutrients (e.g., iron and proteins) from parasites for their reproduction by forming complex with proteins and metals. Reducing oocyst shedding is an important trait as an anti-coccidial agent because this possibly reduces continuous exposure of
E. maxima in a chicken flock [
36].
We hypothesized that
E. maxima infection may increase ileal and fecal moisture content because
E. maxima is known to induce diarrhea in broilers [
39]. However, the inoculation dosage used in the current study induced only mild infection and did not lead to watery digesta and feces. Because
E. maxima infection did not induce diarrhea in the present study, decreased ileal and fecal moisture content might not have been due to anti-diarrhea effects of TA. Reduced moisture content in ileal digesta (9 dpi) and feces (9 to 13 dpi) could be closely associated with drastically reduced AID of ash in the current study. Tannins form a complex with minerals (zinc, iron, copper, etc.), which potentially results in decreased mineral utilization and increased endogenous loss of minerals in chickens [
40]. Secretion and absorption of electrolytes are closely related to water secretion and absorption in the intestine, and ileal and fecal moisture contents were reduced with the similar trends with the AID of ash in the current study. Reducing digesta and fecal moisture content via reducing AID of ash are not considered beneficial effects because loss of ash can result in reduced growth performance and bone development in broilers.
Gut permeability, measured by the FITC-D4 gavage method, was increased by
E. maxima infection, consistent with our previous study [
11]. Increased gut permeability, which is mainly modulated by tight junction proteins (ZO2, CLDN4, JAM2, etc.) and mucus (main protein in the intestine, MUC2) [
41], indicates that pathogenic bacteria and toxins are more likely to enter the bloodstream of broilers. [
42]. Consistent with the FITC-D4 permeability assay result,
E. maxima infection reduced mRNA expression of ZO2, CLDN4, and MUC2 in the present study. Teng et al. [
24] reported that during the
E. maxima asexual and sexual replication, tight junction proteins between enterocytes can be damaged. Supplementation of TA quadratically decreased gut permeability in broilers infected with
E. maxima, and the TA2.75 group had significantly lower gut permeability compared to the CC group in the present study. In agreement with this, supplementation of TA tended to quadratically increase relative mRNA expression of ZO2 in the current study. Along with increased mRNA expression of tight junction proteins, supplementation of TA quadratically increased relative mRNA expression of IL1β and NFκB. Increased mRNA expression of IL1β and NFκB levels have been considered as negative factors for intestinal barrier integrity [
43,
44]. However, potentially pro-inflammatory cytokines are still important in controlling several pathogens by activating the immune system and maintaining gut barrier integrity as defensive mechanisms in broilers infected with
Eimeria spp. [
45]. However, broilers fed 5000 mg/kg TA (TA5) increased gut permeability compared to the broilers fed 2750 mg/kg TA (TA2.75) in the present study. This may have been because high concentrations of TA can show cytotoxicity and impair tight junction proteins. These results indicate that supplementation of TA at appropriate dosages improves gut barrier integrity via modulating mRNA expression of tight junction proteins and stimulating the immune system of broilers infected with
E. maxima.
In the recovery phase, AID of DM, OM, and ash were significantly increased in the CC group compared to the SCC group, which supports the fact that there was a compensatory growth in the recovery phase after mild infection of
E. maxima in the study. The AID of DM and CP were linearly reduced and quadratically increased due to supplementation of TA in the present study. It is well known that TA decreases nutrient digestibility by compromising gut health and forming a complex with proteins in monogastric animals [
46]. However, the TA2.75 group had the higher AID of DM and OM compared to the CC group, indicating that TA at appropriate dosages has the potential to increase nutrient digestibility in broilers, depending on the conditions.
The GSH is the major endogenous antioxidant in the cells of broilers [
47]. A decrease in total GSH, reduced GSH, and GSH/GSSG and an increase in GSSG in the jejunum and liver by supplementation of TA in the current study indicates that TA can impair the endogenous antioxidant system and induce oxidative stress in broilers infected with
E. maxima. However, the TA0.5 group had numerically similar or even better values in GSH, GSSG, reduced GSH, and reduced GSH/GSSG compared to the CC group. Many studies showed that tannins have antioxidant capacity in animals [
48,
49]. However, our current study showed that cytotoxic and proteolytic effects [
50] of TA at high concentrations can induce oxidative stress and impair the endogenous antioxidant system in broilers infected with
E. maxima.
In the current study,
E. maxima infection reduced jejunal morphology at 6 and 13 dpi in broilers, indicating restricted nutrient absorption and digestion, which is in agreement with our previous study [
24]. Supplementation of TA quadratically increased CD of broilers infected with
E. maxima at 6 dpi; however, this did not lead to changes in the VH and VH/CD ratio in the current study. At 13 dpi, supplementation of TA linearly increased jejunal VH in the current study, which potentially explains the increased nutrient digestibility. Wang et al. [
51] reported that supplementation of TA increased jejunal development in mice challenged with diquat (an oxidative stress model). The differences were due to different animals, challenge models (parasitic infection vs. oxidative stress model), and ways to provide TA (feeding vs. oral gavage). Broilers infected with
E. maxima had increased goblet cell density in the present study. Goblet cells, which produce mucus, play important roles in cytoprotective functions against colonization of pathogens in the epithelium of the small intestine [
26]. However, over-produced mucus can increase bacterial pathogenesis because some pathogens can use mucus as their food and habitat [
52,
53]. Collier et al. [
54] reported that
E. maxima caused a host mucogenic response, which can make the intestine vulnerable to be infected with pathogens (e.g.,
Clostridium perfringens) that utilize mucus for their growth and proliferation in the intestine of broilers. However, supplementation of TA did not modulate the concentrations of goblet cells in the villus and crypts in the present study.