Effects of Bacillus licheniformis on Growth Performance, Diarrhea Incidence, Antioxidant Capacity, Immune Function, and Fecal Microflora in Weaned Piglets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Feedings
2.2. Sample Collection
2.3. Growth Performance and Diarrhea Rate
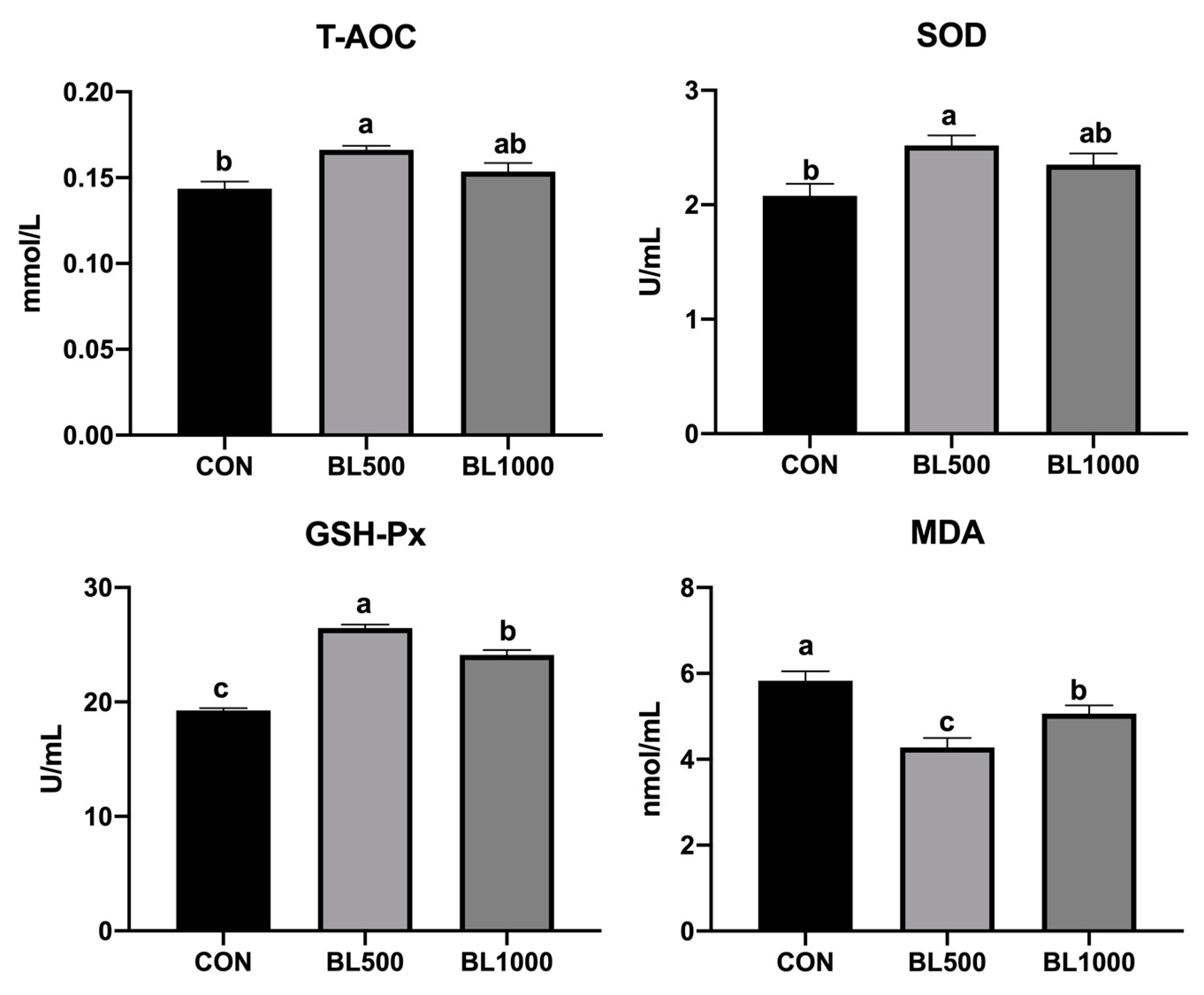
2.4. Serum Antioxidant Indexes
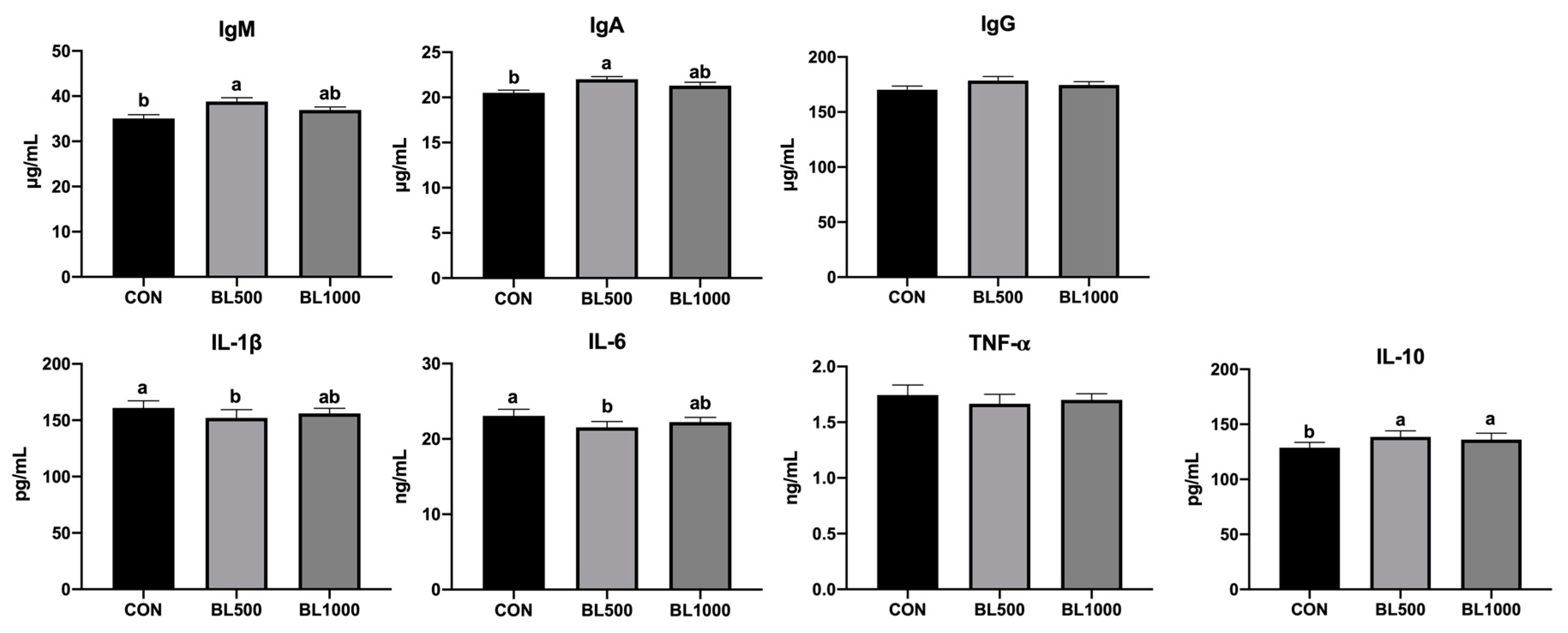
2.5. Immune Indexes
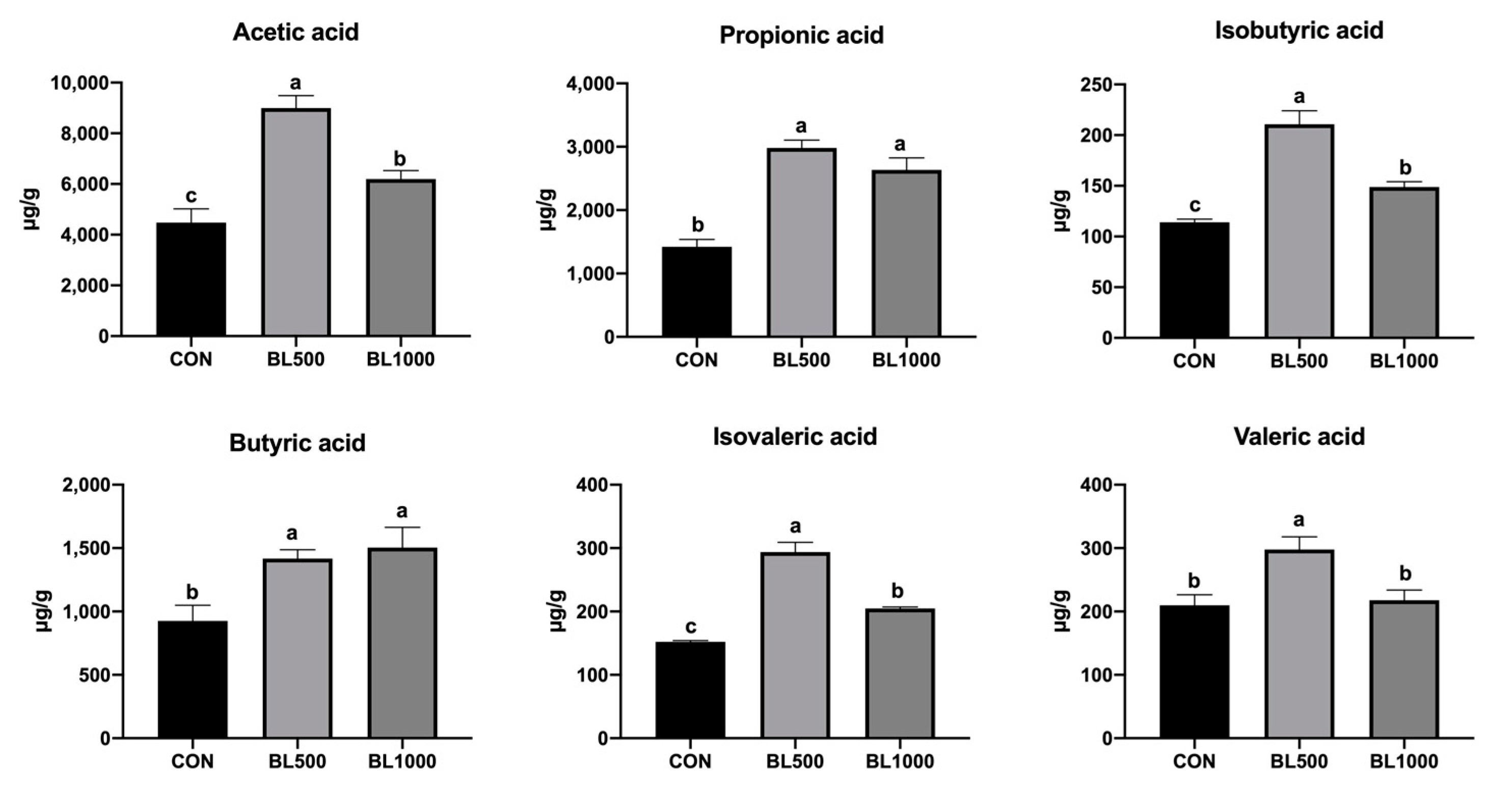
2.6. Fecal Volatile Fatty Acids (VFAs)
2.7. Fecal Flora Structure
2.8. Statistical Analysis
3. Results
3.1. Growth Performance and Diarrhea Rate
3.2. Serum Antioxidant Indexes
3.3. Serum Immune Cytokine
3.4. Concentrations of VFAs
3.5. Microflora Structure in the Colonic Contents
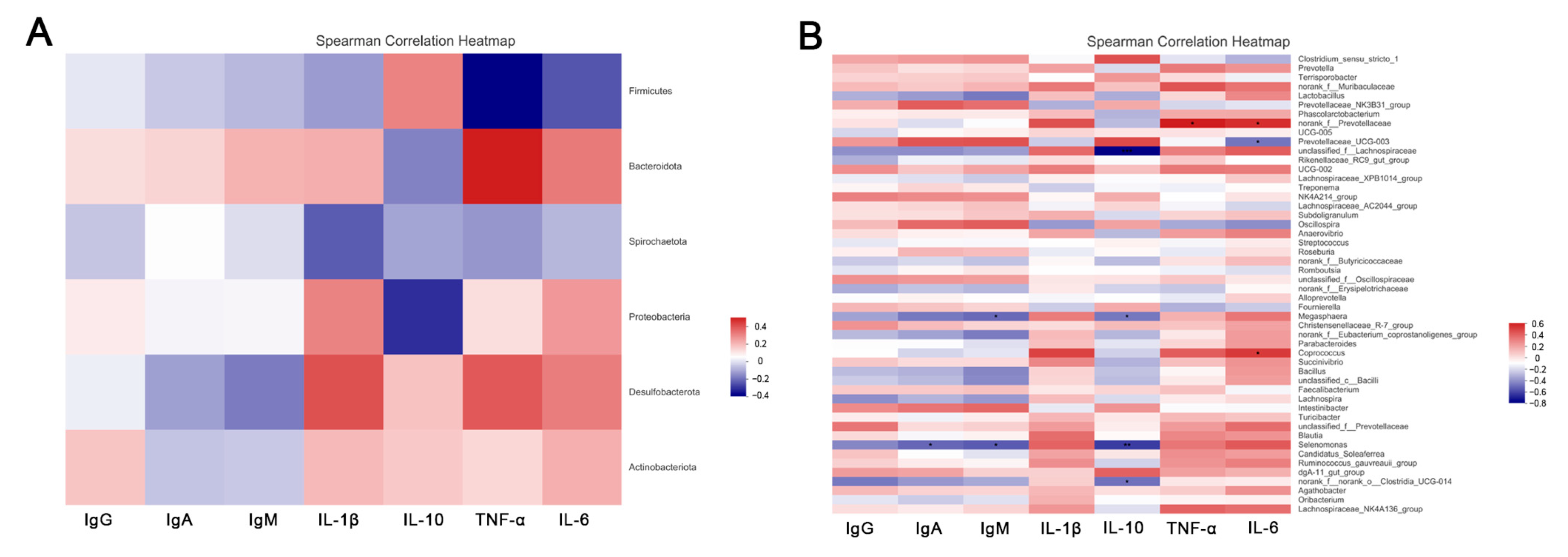
3.6. Spearman’s Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dunsford, B.R.; Knabe, D.A.; Haensly, W.E. Effect of dietary soybean meal on the microscopic anatomy of the small intestine in the early-weaned pig. J. Anim. Sci. 1989, 67, 1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, X.; Yin, Y.; Wu, G.; Liu, H.; Yin, F.; Li, T.; Huang, R.; Ruan, Z.; Xiong, H.; Deng, Z. Dietary supplementation with Acanthopanax senticosus extract modulates cellular and humoral immunity in weaned piglets. Asian-Australas. J. Anim. Sci. 2007, 20, 1453–1461. [Google Scholar] [CrossRef]
- Levy, S. Reduced antibiotic use in livestock: How Denmark tackled resistance. Environ. Health Perspect. 2014, 122, A160–A165. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Khan, A.U. Global economic impact of antibiotic resistance: A review. J. Glob. Antimicrob. Resist. 2019, 19, 313–316. [Google Scholar] [CrossRef]
- Simon, O. An interdisciplinary study on the mode of action of probiotics in pigs. J. Anim. Feed. Sci. 2010, 19, 230–243. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Shi, H.; Xie, W.; Zhao, L.; Zhang, J.; Ji, C.; Ma, Q. Dietary supplementation with acidifiers improves the growth performance, meat quality and intestinal health of broiler chickens. Anim. Nutr. 2021, 7, 762–769. [Google Scholar] [CrossRef]
- Liu, H.; Tong, J.; Zhou, D. Utilization of Chinese herbal feed additives in animal production. Agric. Sci. China 2011, 10, 1262–1272. [Google Scholar] [CrossRef]
- Liu, X.; Xia, B.; He, T.; Li, D.; Su, J.; Guo, L.; Wang, F.; Zhu, Y. Oral Administration of a Select Mixture of Lactobacillus and Bacillus alleviates inflammation and maintains mucosal barrier integrity in the ileum of pigs challenged with salmonella infantis. Microorganisms 2019, 7, 135. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zeng, D.; Wang, H.; Qing, X.; Sun, N.; Xin, J.; Luo., M.; Khalique., A.; Pan., K.; Shu, G. Dietary probiotic Bacillus licheniformis H2 enhanced growth performance, morphology of small intestine and liver, and antioxidant capacity of broiler chickens against Clostridium perfringens-induced subclinical necrotic enteritis. Probiotic Antimicrob. Proteins 2020, 12, 883–895. [Google Scholar] [CrossRef]
- Kayalvizhi, N.; Gunasekaran, P. Production and characterization of a low-molecular-weight bacteriocin from Bacillus licheniformis MKU3. Lett. Appl. Microbiol. 2008, 47, 600–607. [Google Scholar] [CrossRef]
- Lei, K.; Li, Y.; Yu, D.; Rajput, I.; Li, W. Influence of dietary inclusion of Bacillus licheniformis on laying performance, egg quality, antioxidant enzyme activities, and intestinal barrier function of laying hens. Poult. Sci. 2013, 92, 2389–2395. [Google Scholar] [CrossRef]
- Wang, S.; Hou, Q.; Guo, Q.; Zhang, J.; Sun, Y.; Wei, H.; Shen, L. Isolation and characterization of a deoxynivalenol-degrading bacterium Bacillus licheniformis YB9 with the capability of modulating intestinal microbial flora of mice. Toxins 2020, 12, 184. [Google Scholar] [CrossRef] [Green Version]
- Hsu, T.C.; Yi, P.J.; Lee, T.Y.; Liu, J.R. Probiotic characteristics and zearalenone-removal ability of a Bacillus licheniformis strain. PLoS ONE 2018, 13, e0194866. [Google Scholar] [CrossRef]
- Lan, R.; Kim, I.H. Effects of Bacillus licheniformis and Bacillus subtilis complex on growth performance and faecal noxious gas emissions in growing-finishing pigs. J. Sci. Food. Agric. 2019, 99, 1554–1560. [Google Scholar] [CrossRef]
- Lin, K.H.; Yu, Y.H. Evaluation of Bacillus licheniformis-fermented feed additive as an antibiotic substitute: Effect on the growth performance, diarrhea incidence, and cecal microbiota in weaning piglets. Sensors 2020, 10, 1649. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Yu, Y.; Shen, Y.; Li, Q.; Lan, J.; Wu, Y.; Zhang, R.; Cao, G.; Yang, C. Effects of Bacillus subtilis and Bacillus licheniformis on growth performance, immunity, short chain fatty acid production, antioxidant capacity, and cecal microflora in broilers. Poult. Sci. 2021, 100, 101358. [Google Scholar] [CrossRef]
- Yang, C.M.; Zhang, L.L.; Cao, G.T.; Feng, J.; Yue, M.; Xu, Y.; Dai, B.; Han, Q.; Guo, X. Effects of dietary supplementation with essential oils and organic acids on the growth performance, immune system, fecal volatile fatty acids, and microflora community in weaned piglets. J. Anim. Sci. 2019, 97, 133–143. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras, D.F.; Fleury, M.A.; Van, W.T.; Forano, E.; Blanquet, D.S. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Zhou, D.; Zhu, Y.H.; Zhang, W.; Wang, M.L.; Fan, W.Y.; Song, D.; Yang, G.Y.; Jensen, B.B.; Wang, J.F. Oral administration of a select mixture of Bacillus probiotics generates Tr1 cells in weaned F4ab/acR- pigs challenged with an F4+ ETEC/VTEC/EPEC strain. Vet. Res. 2015, 46, 95. [Google Scholar] [CrossRef] [Green Version]
- Sahu, M.K.; Swarnakumar, N.S.; Sivakumar, K.; Thangaradjou, T.; Kannan, L. Probiotics in aquaculture: Importance and future perspectives. Indian J. Microbiol. 2008, 48, 299–308. [Google Scholar] [CrossRef]
- Ren, C.; Zhou, Q.; Guan, W.; Lin, X.; Wang, Y.; Song, H.; Zhang, Y. Immune response of piglets receiving mixture of formic and propionic acid alone or with either capric acid or Bacillus licheniformis after escherichia coli challenge. BioMed Res. Int. 2019, 2019, e6416187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Lin, Y.; Zeng, D.; Zhou, M.; Zeng, Y.; Wang, H.; Zhou, Y.; Zhu, H.; Pan, K.; Jing, B.; et al. Bacillus licheniformis normalize the ileum microbiota of chickens infected with necrotic enteritis. Sci. Rep. 2018, 8, 1744. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Wang, T.H.; Lu, Z.Q.; Song, D.G.; Zhao, J.; Wang, Y.Z. Effects of Clostridium Butyricum or in combination with Bacillus Licheniformis on the growth performance, blood indexes, and intestinal barrier function of weanling piglets. Livest. Sci. 2019, 220, 137–142. [Google Scholar] [CrossRef]
- Iqbal, M.; Pumfor, N.R.; Tang, Z.X.; Lassiter, K.; Ojano, D.C.; Wing, T.; Cooper, M.; Bottje, W. Compromised liver mitochondrial function and complex activity in low feed efficient broilers are associated with higher oxidative stress and differential protein expression1. Poult. Sci. 2005, 84, 933–941. [Google Scholar] [CrossRef]
- Fu, R.; Chen, D.; Tian, G.; Zheng, P.; Mao, X.; Yu, J.; He, J.; Huang, Z.; Luo, Y.; Yu, B. Effect of dietary supplementation of Bacillus coagulans or yeast hydrolysates on growth performance, antioxidant activity, cytokines and intestinal microflora of growing-finishing pigs. Anim. Nutr. 2019, 5, 366–372. [Google Scholar] [CrossRef]
- Xu, J.; Xu, C.; Chen, X.; Cai, X.; Yang, S.; Sheng, Y.; Wang, T. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition 2014, 30, 584–589. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Duan, X.; Feng, X.; Wang, T.; Wang, P.; Ding, M.; Wang, W.; Zhou, X.; Yao, W. The interaction effects of coke oven emissions exposure and metabolic enzyme Gene variants on total antioxidant capacity of workers. Environ. Toxicol. Pharmacol. 2019, 70, 103197. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Z.; Heng, J.; Song, H.; Tian, M.; Chen, F.; Guan, W. Combined yeast culture and organic selenium supplementation during late gestation and lactation improve preweaning piglet performance by enhancing the antioxidant capacity and milk content in nutrient-restricted sows. Anim. Nutr. 2020, 6, 160–167. [Google Scholar] [CrossRef]
- Jia, P.; Cui, K.; Ma, T.; Wan, F.; Wang, W.; Yang, D.; Wang, Y.; Guo, B.; Zhao, L.; Diao, Q. Influence of dietary supplementation with Bacillus licheniformis and Saccharomyces cerevisiae as alternatives to monensin on growth performance, antioxidant, immunity, ruminal fermentation and microbial diversity of fattening lambs. Sci. Rep. 2018, 8, 16712. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, Y.; Du, M.; Wen, C.; Zhou, Y. Evaluation of dietary synbiotic supplementation on growth performance, muscle antioxidant ability and mineral accumulations, and meat quality in late-finishing pigs. Kafkas. Univ. Vet. Fak. Derg. 2018, 24, 673–679. [Google Scholar] [CrossRef]
- Cerami, A. Inflammatory cytokines. Clin. Immunol. Immunopathol. 1992, 62, S3–S10. [Google Scholar] [CrossRef]
- Kc, K. Nutritional aspects of leukocytic cytokines. J. Nutr. 1988, 118, 1436–1446. [Google Scholar] [CrossRef]
- Kwak, M.J.; Tan, P.L.; Oh, J.K.; Chae, K.S.; Kim, J.; Kim, S.H.; Eun, J.S.; Chee, S.W.; Kang, D.K.; Kim, S.H. The effects of multispecies probiotic formulations on growth performance, hepatic metabolism, intestinal integrity and fecal microbiota in growing-finishing pigs. Anim. Feed. Sci. Technol. 2021, 274, 114833. [Google Scholar] [CrossRef]
- Huang, S.; Mao, J.; Zhou, L.; Xiong, X.; Deng, Y. The imbalance of gut microbiota and its correlation with plasma inflammatory cytokines in pemphigus vulgaris patients. Scand. J. Immunol. 2019, 90, e12799. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhu, N.; Zheng, X.; Liu, Y.; Lu, H.; Yin, X.; Hao, H.; Tan, Y.; Wang, D.; Hu, H. Intratumor microbiome analysis identifies positive association between Megasphaera and survival of Chinese patients with pancreatic ductal adenocarcinomas. Front. Immunol. 2022, 13, 785422. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, L.; Fang, J.; Jiang, H.; Liu, G. Effects of IQW and IRW on inflammation and gut microbiota in etec-induced diarrhea. Mediators. Inflamm. 2021, 2021, e2752265. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Xu, P.; Ma, C.; Tang, J.; Zhang, X. Gut microbiota, host health, and polysaccharides. Biotechnol. Adv. 2013, 31, 318–337. [Google Scholar] [CrossRef]
- Long, S.; Liu, S.; Wang, J.; Mahfuz, S.; Piao, X. Natural capsicum extract replacing chlortetracycline enhances performance via improving digestive enzyme activities, antioxidant capacity, anti-inflammatory function, and gut health in weaned pigs. Anim. Nutr. 2021, 7, 305–314. [Google Scholar] [CrossRef]
- García, K.E.; Souza, T.; Landín, G.M.; Barreyro, A.; Santos, M.; Soto, J. Microbial fermentation patterns, diarrhea incidence, and performance in weaned piglets fed a low protein diet supplemented with probiotics. Food. Nutr. Sci. 2014, 5, 1776. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Tao, F.; Hu, Y.; Li, Z.; Zhang, Y.; Deng, B.; Zhan, X. Positive effects of a Clostridium butyricum-based compound probiotic on growth performance, immune responses, intestinal morphology, hypothalamic neurotransmitters, and colonic microbiota in weaned piglets. Food. Funct. 2019, 10, 2926–2934. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wen, Z.; Liu, W.; Meng, L.; Huang, H. Oscillospira—A candidate for the next-generation probiotics. Gut Microbes 2021, 13, 1987783. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; García, M.; Alférez, M.; Pérez, V.; Sanchez, V.; Linde, Á.; Ortiz, M.; Soriano, M.; García, J.A.; López, I. Gut microbiome–short-chain fatty acids interplay in the context of iron deficiency anaemia. Eur. J. Nutr. 2022, 61, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pan, L.; Shang, Q.; Ma, X.; Long, S.; Xu, Y.; Piao, X. Effects of isomalto-oligosaccharides as potential prebiotics on performance, immune function and gut microbiota in weaned pigs. Anim. Feed. Sci. Technol. 2017, 230, 126–135. [Google Scholar] [CrossRef]
- Raabis, S.; Li, W.; Cersosimo, L. Effects and immune responses of probiotic treatment in ruminants. Vet. Immunol. Immunopathol. 2019, 208, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.; Gong, J.; Lange, C. The gastrointestinal microbiota and its role in monogastric nutrition and health with an emphasis on pigs: Current understanding, possible modulations, and new technologies for ecological studies. Can. J. Anim. Sci. 2005, 85, 421–435. [Google Scholar] [CrossRef]
- Zeng, Z.; Xu, X.; Zhang, Q.; Li, P.; Zhao, P.; Li, Q.; Liu, J.; Piao, X. Effects of essential oil supplementation of a low-energy diet on performance, intestinal morphology and microflora, immune properties and antioxidant activities in weaned pigs. Anim. Sci. J. 2015, 86, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, H.; Yin, J.; He, X.; Tang, Z.; Li, T.; Yao, K.; Yin, Y. D- and L-Aspartate regulates growth performance, inflammation and intestinal microbial community in young pigs. Food. Funct. 2019, 10, 1028–1037. [Google Scholar] [CrossRef]
- Forssten, S.; Sindelar, C.; Ouwehand. A. Probiotics from an industrial perspective. Anaerobe 2011, 17, 410–413. [Google Scholar] [CrossRef]
- Wlodarska, M.; Willing, B.; Bravo, D.; Finlay, B. Phytonutrient diet supplementation promotes beneficial Clostridia species and intestinal mucus secretion resulting in protection against enteric infection. Sci. Rep. 2015, 5, 9253. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Tan, Z.; Shi, L.; Xun, W. Resveratrol attenuates diquat-induced oxidative stress by regulating gut microbiota and metabolome characteristics in piglets. Front. Microbiol. 2021, 12, 695155. [Google Scholar] [CrossRef]
- Zhou, J.; Luo, J.; Yang, S.; Xiao, Q.; Wang, X.; Zhou, Z.; Xiao, Y.; Shi, D. Different responses of microbiota across intestinal tract to enterococcus faecium hdrsef1 and their correlation with inflammation in weaned piglets. Microorganisms 2021, 9, 1767. [Google Scholar] [CrossRef]


| Ingredients | Content, % | Nutrient Level | Content |
|---|---|---|---|
| Corn | 55.00 | DE, MJ/kg | 14.17 |
| Wheat midding | 3.50 | CP, % | 20.35 |
| Phospholipid | 2.00 | Lys, % | 1.34 |
| Whey powder | 5.00 | Met+Cys, % | 0.77 |
| Extruded soybean | 7.30 | Thr, % | 0.80 |
| Soybean meal | 18.50 | Ca, % | 0.95 |
| Fish meal | 5.00 | TP, % | 0.65 |
| Dicalcium phosphate | 1.00 | AP, % | 0.48 |
| Limestone | 1.10 | ||
| NaCl | 0.10 | ||
| L-Lysine HCl | 0.35 | ||
| DL- methionine | 0.15 | ||
| Vitamin-mineral premix 1 | 1.00 | ||
| Total | 100 |
| Item 1 | Diet | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | BL500 | BL1000 | |||
| Initial BW, kg | 8.938 | 9.428 | 8.7050 | 0.327 | 0.714 |
| Final BW, kg | 16.482 | 19.045 | 17.521 | 0.613 | 0.251 |
| ADG, kg | 0.269 b | 0.343 a | 0.315 ab | 0.013 | 0.023 |
| ADFI, kg | 0.475 | 0.569 | 0.524 | 0.018 | 0.079 |
| F:G | 1.765 | 1.656 | 1.664 | 0.028 | 0.216 |
| Diarrhea rate, % | 9.52 a | 7.54 b | 5.56 b | 0.006 | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Cui, Z.; Qin, S.; Zhang, R.; Wu, Y.; Liu, J.; Yang, C. Effects of Bacillus licheniformis on Growth Performance, Diarrhea Incidence, Antioxidant Capacity, Immune Function, and Fecal Microflora in Weaned Piglets. Animals 2022, 12, 1609. https://doi.org/10.3390/ani12131609
Yu X, Cui Z, Qin S, Zhang R, Wu Y, Liu J, Yang C. Effects of Bacillus licheniformis on Growth Performance, Diarrhea Incidence, Antioxidant Capacity, Immune Function, and Fecal Microflora in Weaned Piglets. Animals. 2022; 12(13):1609. https://doi.org/10.3390/ani12131609
Chicago/Turabian StyleYu, Xiaorong, Zhenchuan Cui, Songke Qin, Ruiqiang Zhang, Yanping Wu, Jinsong Liu, and Caimei Yang. 2022. "Effects of Bacillus licheniformis on Growth Performance, Diarrhea Incidence, Antioxidant Capacity, Immune Function, and Fecal Microflora in Weaned Piglets" Animals 12, no. 13: 1609. https://doi.org/10.3390/ani12131609
APA StyleYu, X., Cui, Z., Qin, S., Zhang, R., Wu, Y., Liu, J., & Yang, C. (2022). Effects of Bacillus licheniformis on Growth Performance, Diarrhea Incidence, Antioxidant Capacity, Immune Function, and Fecal Microflora in Weaned Piglets. Animals, 12(13), 1609. https://doi.org/10.3390/ani12131609





