Simple Summary
Oxidative stress may reduce the growth performance and intestinal health status of weanling piglets. Due to the fact that the body can synthesize glycine, it is generally treated as an amino acid which is nonessential for nourishment. However, previous research has demonstrated that synthesized glycine was unable to support piglets’ newborn growth and development. Moreover, according to several findings, glycine is crucial for relieving oxidative stress and intestinal damage. The purpose of this investigation was to determine whether glycine could lessen the intestinal damage caused by diquat in weanling piglets and the relationship between ferroptosis and diquat-induced intestinal epithelial cell death. The results showed that dietary glycine reduced intestinal oxidative stress induced by diquat in weanling piglets. Furthermore, with increasing anti-oxidative capacity, dietary glycine was able to restrain intestinal epithelial cell ferroptosis triggered by diquat.
Abstract
The purpose of this research was to examine the impact of glycine on intestinal injury caused by oxidative stress in piglets. A 2 × 2 factorial experiment with diets (basic diet vs. 1% glycine diet) and oxidative stress (saline vs. diquat) was conducted on 32 weanling piglets. On day 21, all piglets received an injection of either saline or diquat. After 7 days, all pigs were slaughtered and intestinal samples were collected. Dietary glycine supplementation improved intestinal mucosal morphology, increased the activities of disaccharidases and enhanced intestinal mucosal antioxidant capacity, while regulating the expression of ferroptosis mediators in the piglets under oxidative stress. These findings suggested that dietary glycine supplementation improved the morphology and function of the intestinal mucosa, which was involved in regulating antioxidant capacity and ferroptosis.
1. Introduction
Changes in dietary components, the use of medications and vaccines, and mycotoxin contamination of feeds, among other things, may result in the excessive formation of reactive oxide species (ROS), which causes oxidative stress in pigs in the current intensive swine production [1]. In particular, intestinal damage can result from severe oxidative stress [2]. The primary sources of ROS generation are found in abundance in intestinal epithelial cells’ mitochondria [3]. In addition to inducing apoptosis and preventing cell proliferation, ROS also interferes with intestinal function and retards intestinal development [4,5]. Therefore, dietary regulation is crucial to reducing intestinal damage brought on by oxidative stress.
The most recent type of cell death to be discovered is ferroptosis, which has recently been linked to oxidative stress [6,7]. The major features of ferroptosis are the buildup of iron ions in cells, the decreased ability of glutathione peroxidase 4 (GPX4) to repair lipid peroxidation injury, and the oxidation of polyunsaturated fatty acids including phospholipids [8]. Ferroptosis cells exhibit morphological traits such as ruptured mitochondrial outer membranes, reduced or absent mitochondrial cristae, and damaged cell membrane integrity [6]. In terms of biochemistry, ferroptosis may cause glutathione to be depleted and GPX4 activity to decline [4].
Glycine is typically viewed as a nutritionally unnecessary amino acid because the body can produce it [9]. However, a wealth of data has demonstrated that synthesized de novo glycine was unable to support piglets’ neonatal growth and development [10,11,12]. More and more studies have recently claimed that glycine is vital for relieving oxidative stress and liver damage [13,14,15]. Glycine is an important part of the antioxidant glutathione peroxidase (GSH-PX), which is a crucial regulator of ferroptosis [16]. Glycine may, therefore, have the ability to alleviate ferroptosis.
In order to induce intestinal oxidative stress and damage in the weanling piglets, the pigs received an intraperitoneal injection of diquat. Intraperitoneal injection of diquat is a common and mature model to establish oxidative stress [17]. Some studies showed that an injection of diquat induced a reduction in productive performance, organ injury and increased production of ROS and so on [18,19]. The goal was to determine if glycine might enhance intestinal health by modulating the anti-oxidative capability and ferroptosis signaling pathway in piglets’ intestinal mucosa.
2. Materials and Methods
2.1. Experimental Animals and Design
An animal trial was conducted according to the Animal Scientific Procedures Act 1986 (Home Office Code of Practice. HMSO: London January 1997) and EU regulation (Directive 2010/63/EU). The whole procedure was approved by the Animal Care and Use Committee of Wuhan Polytechnic University (Wuhan, China). A total of 32 weanling piglets purchased from a commercial herd (Wuhan Charoen Pokphand Co., Ltd., Wuhan, China) with the following breeds-Duroc, Landrace, and Large White-with ages of 28 days and initial body weights (BW) of 7.18 ± 0.70 kg were employed in this study. Piglets were housed separately in 1.80 × 1.10 m2 stainless steel metabolic cages with unrestricted access to food and water in a climate-controlled environment. According to the specifications of the National Research Council, the experimental basal diet was designed [20].
A 2 × 2 factorial trial was used in the design of this investigation. Following a 21-day feeding of either a basal diet or one containing 1% glycine, all pigs received intraperitoneal injections of diquat (dibromide monohydrate, Chem Service, West Chester, PA, USA), either at a dosage of 10 mg/kg BW in saline or the same amount of saline. Diet type (basal or glycine diet) and oxidative stress were the determining variables for treatment (diquat or saline).
2.2. Sample Collection
All piglets were painlessly put to death by sodium pentobarbital injections at 80 mg/kg body weight a week after receiving diquat or saline injections. In accordance with our earlier work, the mid-jejunum and mid-ileum segments measuring 3 cm and 10 cm, respectively, were cut [21]. The fresh, 4% paraformaldehyde/PBS solution was used to gently flush the 3 cm intestine segments before storing them for histological observation [22]. The luminal chyme was gently washed out of the 10 cm intestinal samples after they were opened longitudinally. The mucosa samples were taken by scraping them off sterile glass slides, quickly freezing them in liquid nitrogen, and then storing them at −80 °C for analysis of the disaccharidases’ activities, the antioxidases’ activities, the protein, DNA, and RNA contents, and the levels of mRNA and protein expression.
2.3. Intestinal Morphology
The intestinal segments were fixed for 24 h, then dehydrated, embedded, and stained with hematoxylin and eosin. According to our prior research [23], a microscope (Olympus CX31, Japan) was used to measure the crypt depth and villus height at a magnification of 40×. At least 10 well-oriented and intact villi were chosen. Villus height was measured from the villus tip to the crypt mouth, and crypt depth was measured from the crypt mouth to the base.
2.4. Disaccharidase Activities of the Intestinal Mucosa
Using glucose kits, disaccharidase activities in the intestinal mucosa were measured with our prior investigation [22]. (No. A082-1 for lactase, No. A082-2 for sucrase, and No. A082-3 for maltase; Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.5. Protein, DNA, and RNA Contents of the Intestinal mucosa
After homogenizing frozen mucosal samples, the supernatant was collected by centrifuging the mixture at 2500 rpm for 10 min. In accordance with earlier investigations, the supernatant’s protein, DNA, and RNA contents were examined [24].
2.6. Antioxidative Capacity of the Intestinal Mucosa
Total antioxidative capacity (T-AOC), activities of glutathione peroxidases (GSH-PX), contents of reductive glutathione (GSH), and malondialdehyde (MDA) of intestinal mucosa were determined using commercial kits from Nanjing Jiancheng Bioengineering Co. according to the previous study [1].
2.7. Gene Expression Analysis
The methods used in the previous study were followed for the isolation of total RNA, quantification, reverse transcription, and real-time PCR [23]. Table 1 displays the primer pairings for target gene amplification. Using the 2−△△CT approach, the expression of the target genes in comparison to the housekeeping gene (glyceraldehyde-3-phosphate dehydrogenase; GAPDH) was examined. The piglets fed a basic diet and given saline injections were used to standardize the relative mRNA abundance of each target gene.

Table 1.
Primer sequences used for real-time PCR.
2.8. Protein Abundance Analysis
The methods for protein abundance analysis in intestinal mucosa were according to previous research [20]. Specific primary antibodies included rabbit anti-transferrin receptor protein 1 (TFR1, 1:1000; 86 kDa, #70R-50471; Fitzgerald, Rd. Sudbury, Acton, MA, USA), goat anti-solute carrier family 7 member 11 (SLC7A11, 1:1000; 55 kDa, #ab60171; Abcam, Cambridge, MA, USA), rabbit anti-glutathione peroxidase 4 (GPX4, 1:1000; 20 kDa, #10005258; Cayman Chemical Company, Rd. Ellsworth, Ann Arbor, MI, USA) and mouse anti-β-actin antibody (1:1000, 43 kDa, #A2228; Sigma-Aldrich, St. Louis, MO, USA). As a ratio of target protein/β-actin protein, the relative protein abundance of the target proteins (TFR1, SLC7A11, and GPX4) was expressed.
2.9. Statistical Analyses
Using the general linear model techniques (GLM) of SAS (SAS Inst. Inc., Cary, NC, USA), the data were analyzed as a 2 × 2 factorial experiment by ANOVA. The statistical model took into account the impacts of oxidative stress (saline or diquat), diet type (basal diet or glycine diet), and their interactions. Data were presented as means and SEMs. A post hoc analysis was carried out using Duncan’s multiple comparison tests when the interaction between diet and stress was significant. Differences were considered to be significant if p < 0.05.
3. Results
3.1. Intestinal Morphology
According to Table 2, a significant interaction was observed, which was related to the interaction between diet and stress on the crypt depth of jejunum and villus height of ileum (p < 0.05). Dietary glycine significantly enhanced the crypt depth of jejunum in the piglets that suffered oxidative stress induced by diquat (p < 0.05). In addition, oxidative stress significantly decreased the ratio of the villus height to the crypt depth of ileum and jejunum, as well as the height of jejunal villus (p < 0.05). Compared with the piglets fed the control diet, the piglets fed dietary glycine had significantly enhanced villus height of ileum (p < 0.05).

Table 2.
The intestinal morphology of the piglets fed glycine diets under oxidative stress.
3.2. Disaccharidases Activities of the Intestinal Mucosa
As shown in Table 3, a significant interaction existed between stress and diet for the activities of jejunal sucrase and maltase, and ileal maltase (p < 0.05). Compared with the piglets reared on the basal diet, the piglets reared with the glycine supplementation diet had increased the activities of jejunal mucosal disaccharidases of sucrase and maltase and enhanced the activity of ileal mucosal disaccharidases of maltase (p < 0.05).

Table 3.
The activities of intestinal mucosal disaccharidases of the piglets fed glycine diets under oxidative stress (U/mg protein).
3.3. Protein, DNA, and RNA Contents of the Intestinal Mucosa
As exhibited in Table 4, a significant interaction was presented between diet and stress on the contents of intestinal mucosal protein of the jejunum and ileum, ileal mucosal RNA/DNA, and protein/DNA (p < 0.05). Oxidative stress significantly decreased jejunal RNA/DNA and protein/DNA (p < 0.05). Overall, compared with the piglets treated with the control diet, the piglets treated with the glycine supplementation diet had increased the contents of protein of jejunum and ileum, and enhanced ileal RNA/DNA and protein/DNA (p < 0.05).

Table 4.
The contents of intestinal mucosal protein (mg/g tissue), RNA/DNA, and protein/NDA (mg/μg) of the piglets fed glycine diets under oxidative stress.
3.4. Antioxidative Capacity of the Intestinal Mucosa
Table 5 illustrated a significant interaction arisen in stress and diet for the activities of jejunal GSH-PX and GSH, MDA concentration of jejunum (p < 0.05), and the activity of ileal GSH (p < 0.05). Furthermore, oxidative stress inspired by injected diquat dramatically dropped the activity of T-AOC of jejunum, T-AOC, and GSH of ileum (p < 0.05). Compared with the piglets fed the basal diet, the piglets fed glycine supplementation significantly enhanced the activities of jejunal GSH-PX and GSH, increased the activity of GSH of ileum, and reduced the concentration of MDA of jejunum (p < 0.05).

Table 5.
The intestinal mucosal antioxidative capacity of the piglets fed glycine diets under oxidative stress.
3.5. Intestinal Mucosal Gene Expressions of the Key Genes Related to Ferroptosis
As displayed in Table 6, there was a significant interaction that was linked to stress and diet for the gene expressions of TFR1, SLC7A11, and GPX4 of ferroptosis-related signals of jejunum in the piglets (p < 0.05). Compared with the piglets fed the control diet, the piglets fed glycine supplementation significantly improved the gene expression of jejunal SLC7A11 and GPX4 and reduced the gene expression of TFR1 of jejunum (p < 0.05). Similarly, a significant interaction existed between stress and diet on the ileal gene expression of TFR1, SLC7A11, and GPX4 of ferroptosis-related signals (p < 0.05). Moreover, oxidative stress triggered by injected diquat significantly increased the gene expression of HSPB1 of ileum (p < 0.05). Interestingly, the glycine supplementation function in the ileum as in the jejunum about the gene expressions of ferroptosis-related signals in the piglets. Compared with the piglets reared on the basal diet, the piglets reared with glycine supplementation significantly prompted the gene expression of SLC7A11 and GPX4 of ileum and decreased ileal TFR1 gene expression (p < 0.05).

Table 6.
The intestinal mucosal gene expressions of ferroptosis-related signals of the piglets fed glycine diets under oxidative stress.
3.6. Intestinal Mucosal Protein Abundance of the Key Proteins Related to Ferroptosis
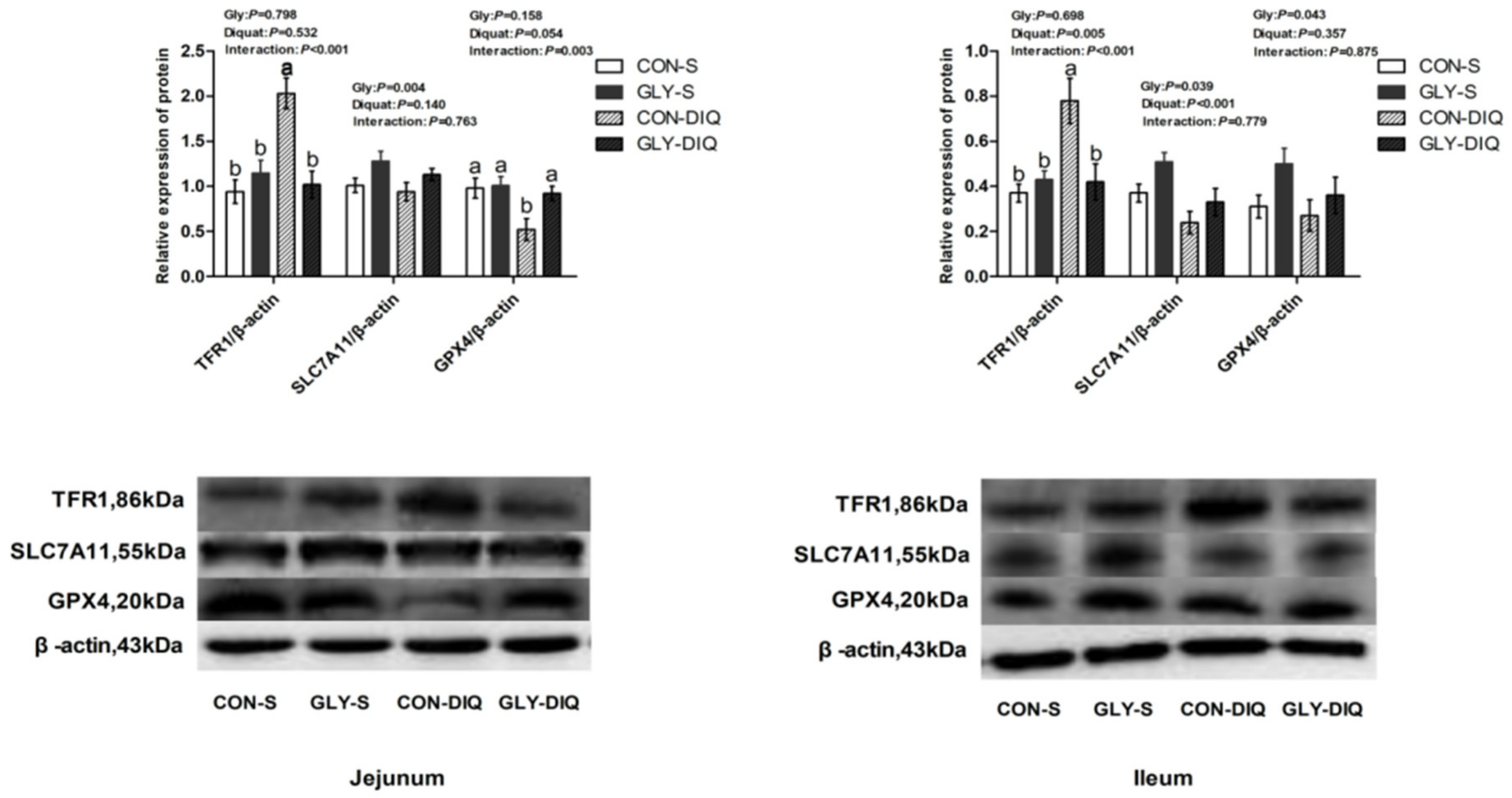
A significant interaction existed between stress and diet on the protein abundance of TFR1 and GPX4 of jejunum of the key proteins related to ferroptosis in Figure 1. (p < 0.05). Compared with the piglets treated with the basal diet, the piglets treated with glycine supplementation significantly increased the protein abundance of jejunal GPX4 and decreased protein abundance of jejunal TFR1 (p < 0.05). Meanwhile, there was a significant interaction between stress and diet on protein abundance of ileal TFR1 (p < 0.05). Compared with the piglets fed the control diet, the piglets fed glycine supplementation significantly decreased the protein abundance of TFR1 of ileum (p < 0.05).
Figure 1.
The abundance of ferroptosis-related proteins of jejunum and ileum in the piglets fed glycine supplementation diets under oxidative stress inspired by injected diquat. The stripes served as representational exemplars of Western blot images. Values are mean and pooled SEM, n = 8 (1 piglet per pen). CON-S, piglets fed the basal diet and injected with saline; GLY-S, piglets fed the glycine supplementation diet and injected with saline; CON-DIQ, piglets fed the basal diet and challenged with diquat; GLY-DIQ, piglets fed the glycine supplementation diet and challenged with diquat. a, b. No identical letter appears in the same row, which demonstrates that the difference is significant, p < 0.05.
4. Discussion
This experiment aimed to investigate whether intestinal cells would undergo ferroptosis after the establishment of diquat-induced oxidative stress model in piglets, and to explore the protective effect and mechanism of glycine supplementation diet on intestinal injury in weaned piglets.
The morphological changes in intestinal tissue may be a direct reflection of how well the intestinal barrier is functioning. Intestinal VH, CD, and VH/CD are crucial indicators of intestinal mucosal morphology [25,26]. Disaccharides need to be further broken down into monosaccharides by intestinal mucosal cells (such as sucrase, lactase, and maltase) in order to be absorbed, because intestinal epithelial cells cannot do this directly. As a result, the activity of intestinal mucosal disaccharides is one of the key markers indicating intestinal digestive capacity [27,28]. Protein, RNA, and DNA are indicators of intestinal growth and development and damage repair. Protein/DNA and RNA/DNA can indicate the ability of protein synthesis [29,30]. In this study, oxidative stress induced by diquat stimulation significantly changed the morphological structure of intestinal villi, and decreased the activity of disaccharidase, protein content, RNA/DNA, and protein/DNA in piglets. Consistent with our research, Xiao et al. showed that oxidative stress can lead to impaired intestinal barrier function in piglets [31]. Xu et al. showed that oxidative stress can cause intestinal injury in piglets and significantly reduce the activity of intestinal digestive enzymes [32]. The above results were consistent with the results of this study. Interestingly, dietary glycine ameliorated intestinal morphological and structural damage, and increased disaccharidase activity, protein content, RNA/DNA, and protein/DNA, which demonstrated that glycine may be able to positively regulate intestinal structural and functional damage caused by oxidative stress in this study. Similar to our results, a variety of studies have shown that dietary glycine can improve the intestinal barrier function, and promote the villus growth of jejunum and ileum, which is beneficial to the development of small intestinal mucosa of piglets [30,33].
Intestinal antioxidant capacity is closely related to intestinal health; however, diquat can induce oxidative stress and decrease intestinal antioxidant capacity [34]. T-AOC displays the overall amount of total antioxidants in the body or organs, and MDA reflects the degree of peroxidation in the body, which is an essential marker of oxidative stress [35]. GSH is a cofactor of GSH-PX, which is a natural free radical scavenger in animals. GSH-PX plays a significant function in antioxidant damage and can accelerate the reduction of lipid peroxides [36]. In this study, it was found that under normal physiological conditions of piglets, dietary glycine significantly enhanced the activities of T-AOC in jejunum and GSH-PX in ileum. This may be because glycine is a vital precursor for the synthesis of GSH, and with the increase in dietary glycine, intestinal antioxidant capacity was enhanced. When piglets were exposed to oxidative stress induced by diquat, the activities of T-AOC and GSH-PX and GSH content in jejunum were significantly decreased, MDA content was significantly increased, and the activities of T-AOC and GSH-PX in ileum were significantly decreased. However, GSH-PX activity and GSH content in jejunum of piglets in the glycine supplementation group were significantly increased, MDA content was significantly decreased, and GSH content in ileum was significantly enhanced. These results suggested that glycine can enhance the antioxidant capacity and mitigate the damage of oxidative stress by increasing the activity of antioxidant enzymes in jejunum of piglets. Some studies have shown that supplementation of glycine in the diet can increase intestinal GSH content, enhance the intestinal antioxidant level and effectively relieve oxidative stress in mice [36,37]. Moreover, Hua et al. found that dietary glycine could increase the activities of T-AOC, GSH-PX, and GSH in the liver, reduce the content of MDA, and alleviate the oxidative stress induced by diquat injection in piglets [38]. This further illustrated that glycine may improve intestinal antioxidant capacity by increasing the GSH content [30,33].
Intestinal injury is inevitably accompanied by cell death; however, it is possible that intestinal injury brought on by oxidative stress has a distinct cell death mechanism rather than the usual classical cell death mode [39]. Ferroptosis is a non-apoptotic, iron-dependent cell death form that is intimately associated with the oxidative stress process. [6]. In this study, the mRNA and protein expression levels of intestinal ferroptosis-related signaling pathway were detected to explore its influencing mechanism. System Xc−/GPX4 signaling pathway is closely related to ferroptosis [40]. TFR1 acts as a carrier to transfer iron ions (Fe3+) into the cell’s inner membrane when cells undergo ferroptosis. HSPB1 is a molecular chaperone of several small heat shock proteins, which can reduce the concentration of Fe3+ by inhibiting the expression of TFR1, thereby alleviating the intensity of ferroptosis [41]. System Xc− is composed of SLC7A11 and SLC3A2 subunits through disulfide bonds, which can transfer glutamate out of the cell through the cell membrane and cystine into the cell membrane at the same time. Finally, cystine is converted into cysteine and GSH is synthesized. GSH can reduce ROS under the action of the antioxidant enzyme GPX4 and alleviate oxidative damage to cells [42]. In this study, under diquat stimulation, the mRNA expressions of TFR1 and GPX4 in jejunum and TFR1 and HSPB1 in ileum were significantly increased, while the mRNA expression of SLC7A11 in ileum was significantly decreased, indicating that diquat stimulation caused a large amount of Fe3+ to enter cells, leading to oxidative stress in the intestine. At this time, the body alleviated oxidative stress injury by enhancing the activities of GPX4 and HSPB1. Meanwhile, dietary glycine significantly increased the mRNA expressions of SLC7A11 and GPX4 in jejunum and ileum, and significantly decreased the mRNA expression of TFR1 in jejunum and ileum after diquat stimulation. These results indicated that glycine can enhance the intestinal antioxidant system to inhibit the occurrence of ferroptosis, which is consistent with the results of intestinal antioxidant capacity. According to the results of mRNA expression and protein expression, glycine significantly reduced the expression of TFR1 protein in jejunum and ileum after diquat stimulation, and significantly increased the expression of GPX4 protein in jejunum. Consistent with the results of many studies, it was found that the expression of the GPX4 gene could inhibit iron death and alleviate cell damage [43,44,45]. Xu et al. have shown that dietary glycine can effectively inhibit the expression of TFR1, a key gene of ferroptosis, and promote the expression of GPX4, thereby alleviating liver injury induced by diquat [38]. These results suggested that dietary glycine can enhance the synthesis of GPX4, inhibit the occurrence of ferroptosis, and then protect the gut from the damage caused by ferroptosis.
5. Conclusions
In summary, the diet supplied with 1% glycine relieved intestinal damage by inhibiting the occurrence of ferroptosis and enhancing intestinal antioxidant capacity in the piglets under oxidative stress.
Author Contributions
Conceptualization, X.X., J.Z. and Y.L.; methodology, X.X., H.H., H.Z., J.Z. and K.X.; software, H.H., J.Z. and Y.W.; validation, X.X. and Y.L.; formal analysis, H.Z. and K.X.; investigation, Y.W. and H.H; resources, X.X. and K.X.; data curation, H.H. and Y.W.; writing—original draft preparation, Y.W. and X.X.; writing—review and editing, K.X., J.Z. and Y.W.; visualization, H.Z. and J.Z.; supervision, Y.L.; project administration, X.X., J.Z. and Y.L.; funding acquisition, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research and APC were funded by the National Natural Science Foundation of China (No. 32272918), the Dawn Project in Special Project of Knowledge Innovation of Wuhan (No. 2022020801020396), the Scientific Research Project of Wuhan Polytechnic University (NO. 2022J05).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Experimental procedures and approved by the Animal Care and Use Committee of Wuhan Polytechnic University (Wuhan, China, No.WPU202000110).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- He, P.W.; Hua, H.W.; Tian, W.; Zhu, H.L.; Liu, Y.L.; Xu, X. Holly (Ilex latifolia Thunb.) polyphenols extracts alleviate hepatic damage by regulating ferroptosis following diquat challenge in a piglet model. Front. Nutr. 2020, 7, 604328. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Intestinal redox biology and oxidative stress. Semin. Cell Dev. Biol. 2012, 23, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Figueira, T.R.; Barros, M.H.; Camargo, A.A.; Castilho, R.F.; Ferreira, J.C.B.; Kowaltowski, A.J.; Sluse, F.E.; Souza-Pinto, N.C.; Vercesi, A.E. Mitochondria as a source of reactive oxygen and nitrogen species: From molecular mechanisms to human health. Antioxid. Redox Signal. 2013, 18, 2029–2074. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, 4966–4975. [Google Scholar] [CrossRef]
- Yin, J.; Wu, M.M.; Li, Y.Y.; Ren, W.K.; Xiao, H.; Chen, S.; Li, C.Y.; Tan, B.; Ni, H.J.; Xiong, X. Toxicity assessment of hydrogen peroxide on Toll-like receptor system, apoptosis, and mitochondrial respiration in piglets and IPEC-J2 cells. Oncotarget 2017, 8, 3124–3131. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Dixon, S.J.; Stockwell, B.R. The Hallmarks of Ferroptosis. Annu. Rev. Cancer Biol. 2019, 3, 35–54. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids. 2013, 45, 463–477. [Google Scholar] [CrossRef]
- Wang, W.; Dai, Z.; Wu, Z.; Lin, G.; Jia, S.; Hu, S.; Dahanayaka, S.; Wu, G. Glycine is a nutritionally essential amino acid for maximal growth of milk-fed young pigs. Amino Acids 2014, 46, 2037–2045. [Google Scholar] [CrossRef]
- Wu, G. Dietary requirements of synthesizable amino acids by animals: A paradigm shift in protein nutrition. J. Anim. Sci. Biotechno. 2014, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Flynn, N.E.; Knabe, D.A.; Mallick, B.K.; Wu, G. Postnatal changes of plasma amino acids in suckling pigs. J. Anim. Sci. 2000, 78, 2369–2375. [Google Scholar] [CrossRef]
- Barakat, H.; Hamza, A.H. Glycine alleviates liver injury induced by deficiency in methionine and or choline in rats. Aust. J. Basic Appl. Sci. 2011, 5, 1061–1070. [Google Scholar]
- Senthilkumar, R.; Viswanathan, P.; Nalini, N. Effect of glycine on oxidative stress in rats with alcohol induced liver injury. Pharmazie. 2004, 59, 55–60. [Google Scholar]
- Marsh, D.C.; Vreugdenhil, P.K.; Mack, V.E.; Belzer, F.O.; Southard, J.H. Glycine protects hepatocytes from injury caused by anoxia, cold ischemia and mitochondrial inhibitors, but not injury caused by calcium ionophores or oxidative stress. Hepatology 2010, 17, 91–98. [Google Scholar] [CrossRef]
- Angeli, J.P.F.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Doan, N.; Liu, Y.; Xiong, X.; Kim, K.; Wu, Z.; Bravo, D.M.; Blanchard, A.; Ji, P. Organic selenium supplement partially alleviated diquat-induced oxidative insults and hepatic metabolic stress in nursery pigs. Br. J. Nutr. 2020, 124, 23–33. [Google Scholar] [CrossRef]
- Wang, A.N.; Cai, C.J.; Zeng, X.F.; Zhang, F.R.; Zhang, G.L.; Thacker, P.A.; Wang, J.J.; Qiao, S.Y. Dietary supplementation with Lactobacillus fermentum I5007 improves the anti-oxidative activity of weanling piglets challenged with diquat. J. Appl. Micorbiol. 2013, 114, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.K.; Wang, H.; Yang, H.; Tan, B.; Zhou, S.; Guan, G. Effects of dietary carboxymethyl pachyman on oxidative stress and inflammation in weaned piglets challenged with diquat. Anim. Feed Sci. Technol. 2021, 276, 114922. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Pi, D.; Liu, Y.; Shi, H.; Li, S.; Odle, J.; Lin, X.; Zhu, H.; Chen, F.; Hou, Y.; Leng, W. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J. Nutr. Biochem. 2014, 25, 456–462. [Google Scholar] [CrossRef]
- Liu, Y.L.; Huang, J.J.; Hou, Y.Q.; Zhu, H.L.; Zhao, S.J.; Ding, B.Y.; Yin, Y.L.; Yi, G.F.; Shi, J.X.; Fan, W. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br. J. Nutr. 2008, 100, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, S.K.; Wang, H.B.; Tu, Z.X.; Wang, S.H.; Wang, X.Y.; Zhu, H.L.; Wang, C.W.; Zhu, J.D.; Liu, Y.L. Medium-chain TAG improve intestinal integrity by suppressing toll-like receptor 4, nucleotide-binding oligomerisation domain proteins and necroptosis signaling in weanling piglets challenged with lipopolysaccharide. Br. J. Nutr. 2018, 119, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.L.; Liu, Y.L.; Chen, S.K.; Wang, X.Y.; Pi, D.A.; Leng, W.B.; Chen, F.; Zhang, J.; Kang, P. Fish oil enhances intestinal barrier function and inhibits corticotropin-releasing hormone/corticotropin-releasing hormone receptor 1 signalling pathway in weaned pigs after lipopolysaccharide challenge. Br. J. Nutr. 2016, 115, 1947–1957. [Google Scholar] [CrossRef]
- Hong, Q.H.; Li, X.; Lin, Q.; Shen, Z.J.; Feng, J.; Hu, C.H. Resveratrol improves intestinal morphology and anti-oxidation ability in deoxynivalenol-challenged piglets. Animals 2022, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Li, C.Y.; Yin, Y.L.; Zhang, S.; Li, X.Z.; Sun, Q.P.; Wan, D. Effects of zinc oxide/zeolite on intestinal morphology, intestinal microflora, and diarrhea rates in weaned piglets. Biol. Trace Elem. Res. 2021, 199, 1405–1413. [Google Scholar] [CrossRef]
- Tsukahara, T.; Inoue, R.; Nakatani, M.; Fukuta, K.; Kishino, E.; Ito, T.; Ushida, K. Influence of weaning age on the villous height and disaccharidase activities in the porcine small intestine. Anim. Sci. J. 2016, 87, 67–75. [Google Scholar] [CrossRef]
- Hartke, J.L.; Monaco, M.H.; Wheeler, M.B.; Donovan, S.M. Effect of a short-term fast on intestinal disaccharidase activity and villus morphology of piglets suckling insulin-like growth factor-I transgenic sows. J. Anim. Sci. 2005, 83, 2404–2413. [Google Scholar] [CrossRef]
- Scharl, M.; Paul, G.; Barrett, K.E.; McCole, D.F. AMP-activated protein kinase mediates the interferon-gamma-induced decrease in intestinal epithelial barrier function. J. Biol. Chem. 2010, 284, 27952–27963. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.Y.; Wu, H.T.; Zhu, H.L.; Liu, C.C.; Hou, Y.Q.; Dai, B.; Liu, X.T.; Liu, Y.L. Glycine relieves intestinal injury by maintaining MTOR signaling and suppressing AMPK, TLR4, and NOD signaling in weaned piglets after lipopolysaccharide challenge. Int. J. Mol. Sci. 2018, 19, 1980. [Google Scholar] [CrossRef]
- Xiao, Y.X.; Huang, R.; Wang, N.; Deng, Y.K.; Tan, B.; Yin, Y.L.; Qi, M.; Wang, J. Ellagic acid alleviates oxidative stress by mediating Nrf2 signaling pathways and protects against paraquat-induced intestinal injury in piglets. Antioxidants 2022, 11, 252. [Google Scholar] [CrossRef]
- Xu, X.; Wei, Y.; Hua, H.W.; Jing, X.Q.; Zhu, H.L.; Xiao, K.; Zhao, J.C.; Liu, Y.L. Polyphenols sourced from Ilex Latifolia Thunb. relieve intestinal injury via modulating ferroptosis in weanling piglets under oxidative stress. Antioxidants 2022, 11, 966. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, X.X.; Ji, Y.; Li, J.; Dai, Z.; Wu, Z.L. Glycine represses endoplasmic reticulum stress-related apoptosis and improves intestinal barrier by activating mammalian target of rapamycin complex 1 signaling. Anim. Nutr. 2022, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Yu, B.; Mao, X.B.; Zheng, P.; He, J.; Chen, D.W. Responses of growth performance and tryptophan metabolism to oxidative stress induced by diquat in weaned pigs. Animal 2012, 6, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, L.; Zheng, F.P.; Jia, C.F.; Ruan, Y.; Li, H. Correlation between the amplitude of glucose excursion and the oxidative/antioxidative system in subjects with different types of glucose regulation. Biomed. Environ. Sci. 2011, 24, 68–73. [Google Scholar] [PubMed]
- El-Hafidi, M.; Franco, M.; Ramírez, A.R.; Sosa, J.S.; Flores, J.A.P.; Acosta, O.L.; Salgado, M.C.; Cardoso-Saldaña, G. Glycine increases insulin sensitivity and glutathione biosynthesis and protects against oxidative stress in a model of sucrose-induced insulin resistance. Oxidative Med. Cell. Longev. 2018, 2018, 2101562. [Google Scholar] [CrossRef]
- Ruiz-Ramírez, A.; Ortiz-Balderas, E.; Cardozo-Saldaña, G.; Diaz-Diaz, E.; El-Hafidi, M. Glycine restores glutathione and protects against oxidative stress in vascular tissue from sucrose-fed rats. Clin. Sci. Lond. Engl. 2014, 126, 19–29. [Google Scholar] [CrossRef]
- Hua, H.W.; Xu, X.; Tian, W.; Li, P.; Zhu, H.L.; Wang, W.J.; Liu, Y.L.; Xiao, K. Glycine alleviated diquat-induced hepatic injury via inhibiting ferroptosis in weaned piglets. Anim. Biosci. 2022, 35, 938–947. [Google Scholar] [CrossRef]
- Xu, S.; He, Y.; Lin, L.H.; Chen, P.; Chen, M.H.; Zhang, S.H. The emerging role of ferroptosis in intestinal disease. Cell Death Dis. 2021, 12, 289. [Google Scholar] [CrossRef]
- Forcina, G.C.; Dixon, S.J. GPX4 at the crossroads of lipid homeostasis and ferroptosis. Proteomics 2019, 19, 1800311. [Google Scholar] [CrossRef]
- Gao, M.H.; Monian, P.; Jiang, X.J. Metabolism and iron signaling in ferroptotic cell death. Oncotarget 2015, 6, 35145–35146. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Yang, Y.Q.; Sheng, H.H.; Tang, Q.; Han, L.; Wang, S.M.; Wu, W.Y. GPX4 plays a crucial role in Fuzheng Kang’ai decoction-induced non-small cell lung cancer cell ferroptosis. Front. Pharmacol. 2022, 13, 851680. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Carlisle, A.E.; Peppers, A.; Park, S.J.; Doshi, M.B.; Spears, M.E.; Kim, D. XCT-driven expression of GPX4 determines sensitivity of breast cancer cells to ferroptosis inducers. Antioxidants 2021, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Bai, L.L.; Fang, X.; Liu, J.; Kang, R.; Zhou, D.; Tang, D.; Dai, E. SMG9 drives ferroptosis by directly inhibiting GPX4 degradation. Biochem. Biophys. Res. Commun. 2021, 567, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.C.; Wu, Y.; Chen, Y.T.; Xiong, X.L.; Li, P.; Peng, X.M.; Li, C.M.; Weng, W.J.; Zhu, Y.F.; Zhou, D.H.; et al. Arsenic trioxide increases apoptosis of SK-N-BE (2) cells partially by inducing GPX4-mediated ferroptosis. Mol. Biol. Rep. 2022, 49, 6573–6580. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).