Elucidating the Role of Innate and Adaptive Immune Responses in the Pathogenesis of Canine Chronic Inflammatory Enteropathy—A Search for Potential Biomarkers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Innate Immune Response
2.1. Intestinal Microbiota
2.2. Mucosal Epithelial Barrier
2.3. Innate Immune Cells and Their Derived Molecules
2.3.1. Integrins
2.3.2. Cytokines
2.3.3. Metalloproteinases
2.3.4. Neutrophils
2.3.5. Macrophages
2.3.6. Eosinophils
2.3.7. Mast Cells
2.3.8. Natural Killer Lymphocytes and Natural Killer Cells
2.3.9. Natural Antibodies
2.3.10. S100/Calgranulins and RAGE Receptors
2.3.11. Pattern Recognition Receptors (PRRs): Toll-Like Receptor and NOD-Like Receptors
3. Adaptive Immunity
3.1. T Helper CD4+ Lymphocytes
3.2. Intestinal Intraepithelial Lymphocytes
3.3. B-Lymphocytes
4. Cross-Talk in the Immune Responses and Their Possible Role in the Pathogenesis of Chronic Inflammatory Enteropathy (CIE) in Dogs
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dandrieux, J.R. Inflammatory Bowel Disease Versus Chronic Enteropathy in Dogs: Are They One and the Same? J. Small Anim. Pract. 2016, 57, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Dandrieux, J.R.S.; Mansfield, C.S. Chronic Enteropathy in Canines: Prevalence, Impact and Management Strategies. Vet. Med. 2019, 10, 203–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heilmann, R.M.; Steiner, J.M. Clinical Utility of Currently Available Biomarkers in Inflammatory Enteropathies of Dogs. J. Vet. Intern. Med. 2018, 32, 1495–1508. [Google Scholar] [CrossRef] [PubMed]
- Grevenitis, P.; Thomas, A.; Lodhia, N. Medical Therapy for Inflammatory Bowel Disease. Surg. Clin. N. Am. 2015, 95, 1159–1182. [Google Scholar] [CrossRef] [PubMed]
- Cerquetella, M.; Rossi, G.; Suchodolski, J.S.; Schmitz, S.S.; Allenspach, K.; Rodríguez-Franco, F.; Furlanello, T.; Gavazza, A.; Marchegiani, A.; Unterer, S.; et al. Proposal for Rational Antibacterial Use in the Diagnosis and Treatment of Dogs with Chronic Diarrhoea. J. Small Anim. Pract. 2020, 61, 211–215. [Google Scholar] [CrossRef]
- Bastan, I.; Robinson, N.A.; Ge, X.N.; Rendahl, A.K.; Rao, S.P.; Washabau, R.J.; Sriramarao, P. Assessment of Eosinophil Peroxidase as a Potential Diagnostic and Prognostic Marker in Dogs with Inflammatory Bowel Disease. Am. J. Vet. Res. 2017, 78, 36–41. [Google Scholar] [CrossRef]
- Allenspach, K.A.; Mochel, J.P.; Du, Y.; Priestnall, S.L.; Moore, F.; Slayter, M.; Rodrigues, A.; Ackermann, M.; Krockenberger, M.; Mansell, J.; et al. Correlating Gastrointestinal Histopathologic Changes to Clinical Disease Activity in Dogs with Idiopathic Inflammatory Bowel Disease. Vet. Pathol. 2019, 56, 435–443. [Google Scholar] [CrossRef]
- Mansfield, C.S.; James, F.E.; Craven, M.; Davies, D.R.; O’Hara, A.J.; Nicholls, P.K.; Dogan, B.; MacDonough, S.P.; Simpson, K.W. Remission of Histiocytic Ulcerative Colitis in Boxer Dogs Correlates with Eradication of Invasive Intramucosal Escherichia Coli. J. Vet. Intern. Med. 2009, 23, 964–969. [Google Scholar] [CrossRef] [Green Version]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. Stride-Ii: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (Stride) Initiative of the International Organization for the Study of Ibd (Ioibd): Determining Therapeutic Goals for Treat-to-Target Strategies in Ibd. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Jergens, A.E.; Simpson, K.W. Inflammatory Bowel Disease in Veterinary Medicine. Front. Biosci. Elite Ed. 2012, 4, 1404–1419. [Google Scholar] [CrossRef]
- Annese, V.; Duricova, D.; Gower-Rousseau, C.; Jess, T.; Langholz, E. Impact of New Treatments on Hospitalisation, Surgery, Infection, and Mortality in Ibd: A Focus Paper by the Epidemiology Committee of Ecco. J. Crohn’s Colitis 2015, 10, 216–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suchodolski, J.S.; Markel, M.E.; Garcia-Mazcorro, J.F.; Unterer, S.; Heilmann, R.M.; Dowd, S.E.; Kachroo, P.; Ivanov, I.; Minamoto, Y.; Dillman, E.M.; et al. The Fecal Microbiome in Dogs with Acute Diarrhea and Idiopathic Inflammatory Bowel Disease. PLoS ONE 2012, 7, e51907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Martins, R.; Sullivan, M.C.; Friedman, E.S.; Misic, A.M.; El-Fahmawi, A.; De Martinis, E.C.P.; O’Brien, K.; Chen, Y.; Bradley, C.; et al. Diet-Induced Remission in Chronic Enteropathy Is Associated with Altered Microbial Community Structure and Synthesis of Secondary Bile Acids. Microbiome 2019, 7, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Pan, Z.; Yang, R.; Bi, Y.; Xiong, X. The Canine Gastrointestinal Microbiota: Early Studies and Research Frontiers. Gut Microbes 2020, 11, 635–654. [Google Scholar] [CrossRef] [PubMed]
- AlShawaqfeh, M.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J.; Steiner, J.; Serpedin, E.; Suchodolski, J. A Dysbiosis Index to Assess Microbial Changes in Fecal Samples of Dogs with Chronic Inflammatory Enteropathy. FEMS Microbiol. Ecol. 2017, 93, 431–457. [Google Scholar] [CrossRef] [Green Version]
- Suchodolski, J.S. Intestinal Microbiota of Dogs and Cats: A Bigger World Than We Thought. Vet. Clin. Small Anim. Pract. 2011, 41, 261–272. [Google Scholar] [CrossRef]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.; et al. The Mucus and Mucins of the Goblet Cells and Enterocytes Provide the First Defense Line of the Gastrointestinal Tract and Interact with the Immune System. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef] [Green Version]
- Corfield, A.P. Mucins: A Biologically Relevant Glycan Barrier in Mucosal Protection. Biochim. Biophys. Acta BBA Gen. Subj. 2015, 1850, 236–252. [Google Scholar] [CrossRef]
- Hattrup, C.L.; Gendler, S.J. Structure and Function of the Cell Surface (Tethered) Mucins. Annu. Rev. Physiol. 2008, 70, 431–457. [Google Scholar] [CrossRef]
- Johansson, M.E.; Hansson, G.C. Immunological Aspects of Intestinal Mucus and Mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef]
- Rosen, S.D. Ligands for L-Selectin: Homing, Inflammation, and Beyond. Annu. Rev. Immunol. 2004, 22, 129–156. [Google Scholar] [CrossRef]
- Balimane, P.V.; Chong, S.; Morrison, R.A. Current Methodologies Used for Evaluation of Intestinal Permeability and Absorption. J. Pharmacol. Toxicol. Methods 2000, 44, 301–312. [Google Scholar] [CrossRef]
- Farquhar, M.J.; McCluskey, E.; Staunton, R.; Hughes, K.R.; Coltherd, J.C. Characterisation of a Canine Epithelial Cell Line for Modelling the Intestinal Barrier. Altern. Lab. Anim. 2018, 46, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Halpern, M.D.; Denning, P.W. The Role of Intestinal Epithelial Barrier Function in the Development of Nec. Tissue Barriers 2015, 3, e1000707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollander, D. Intestinal Permeability, Leaky Gut, and Intestinal Disorders. Curr. Gastroenterol. Rep. 1999, 1, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J.; Hughes, K.R. Tnf-A-Induced Intestinal Epithelial Cell Shedding: Implications for Intestinal Barrier Function. Ann. N. Y. Acad. Sci. 2012, 1258, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms Regulating Intestinal Barrier Integrity and Its Pathological Implications. Exp. Mol. Med. 2018, 50, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eissa, N.; Kittana, H.; Gomes-Neto, J.C.; Hussein, H. Mucosal Immunity and Gut Microbiota in Dogs with Chronic Enteropathy. Res. Vet. Sci. 2019, 122, 156–164. [Google Scholar] [CrossRef]
- Shaoul, R.; Okada, Y.; Cutz, E.; Marcon, M.A. Colonic Expression of Muc2, Muc5ac, and Tff1 in Inflammatory Bowel Disease in Children. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 488–493. [Google Scholar] [CrossRef]
- Wright, N.A.; Poulsom, R.; Stamp, G.; Van Noorden, S.; Sarraf, C.; Elia, G.; Ahnen, D.; Jeffery, R.; Longcroft, J.; Pike, C.; et al. Trefoil Peptide Gene Expression in Gastrointestinal Epithelial Cells in Inflammatory Bowel Disease. Gastroenterology 1993, 104, 12–20. [Google Scholar] [CrossRef]
- Schmitz, S.; Hill, S.; Werling, D.; Allenspach, K. Expression of Trefoil Factor Genes in the Duodenum and Colon of Dogs with Inflammatory Bowel Disease and Healthy Dogs. Vet. Immunol. Immunopathol. 2013, 151, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Van der Heyden, S.; Vercauteren, G.; Daminet, S.; Paepe, D.; Chiers, K.; Polis, I.; Waelbers, T.; Hesta, M.; Schauvliege, S.; Wegge, B.; et al. Expression of P-Glycoprotein in the Intestinal Epithelium of Dogs with Lymphoplasmacytic Enteritis. J. Comp. Pathol. 2011, 145, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, K.; Bergman, P.J.; Sauter, S.; Gröne, A.; Doherr, M.G.; Gaschen, F. P-Glycoprotein Expression in Lamina Propria Lymphocytes of Duodenal Biopsy Samples in Dogs with Chronic Idiopathic Enteropathies. J. Comp. Pathol. 2006, 134, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Fiocchi, C. Endothelial Cell-Immune Cell Interaction in Ibd. Dig. Dis. 2016, 34, 43–50. [Google Scholar] [CrossRef]
- Rodrigues, S.F.; Granger, D.N. Blood Cells and Endothelial Barrier Function. Tissue Barriers 2015, 3, e978720. [Google Scholar] [CrossRef] [Green Version]
- Sands, B.E.; Kaplan, G.G. The Role of Tnfα in Ulcerative Colitis. J. Clin. Pharmacol. 2007, 47, 930–941. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil Recruitment and Function in Health and Inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Langer, H.F.; Chavakis, T. Leukocyte—Endothelial Interactions in Inflammation. J. Cell. Mol. Med. 2009, 13, 1211–1220. [Google Scholar] [CrossRef]
- Petri, W.A., Jr.; Miller, M.; Binder, H.J.; Levine, M.M.; Dillingham, R.; Guerrant, R.L. Enteric Infections, Diarrhea, and Their Impact on Function and Development. J. Clin. Investig. 2008, 118, 1277–1290. [Google Scholar] [CrossRef]
- Kathrani, A.; Schmitz, S.; Priestnall, S.L.; Smith, K.C.; Werling, D.; Garden, O.A.; Allenspach, K. Cd11c+ Cells Are Significantly Decreased in the Duodenum, Ileum and Colon of Dogs with Inflammatory Bowel Disease. J. Comp. Pathol. 2011, 145, 359–366. [Google Scholar] [CrossRef]
- German, A.J.; Helps, C.R.; Hall, E.J.; Day, M.J. Cytokine Mrna Expression in Mucosal Biopsies from German Shepherd Dogs with Small Intestinal Enteropathies. Dig. Dis. Sci. 2000, 45, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Jergens, A.E.; Sonea, I.M.; O’Connor, A.M.; Kauffman, L.K.; Grozdanic, S.D.; Ackermann, M.R.; Evans, R.B. Intestinal Cytokine Mrna Expression in Canine Inflammatory Bowel Disease: A Meta-Analysis with Critical Appraisal. Comp. Med. 2009, 59, 153–162. [Google Scholar] [PubMed]
- Osada, H.; Ogawa, M.; Hasegawa, A.; Nagai, M.; Shirai, J.; Sasaki, K.; Shimoda, M.; Itoh, H.; Kondo, H.; Ohmori, K. Expression of Epithelial Cell-Derived Cytokine Genes in the Duodenal and Colonic Mucosae of Dogs with Chronic Enteropathy. J. Vet. Med. Sci. 2017, 79, 393–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Sullivan, S.; Gilmer, J.F.; Medina, C. Matrix Metalloproteinases in Inflammatory Bowel Disease: An Update. Mediat. Inflamm. 2015, 2015, 964131. [Google Scholar] [CrossRef]
- Gao, Q.; Meijer, M.J.; Kubben, F.J.; Sier, C.F.; Kruidenier, L.; van Duijn, W.; van den Berg, M.; van Hogezand, R.A.; Lamers, C.B.; Verspaget, H.W. Expression of Matrix Metalloproteinases-2 and -9 in Intestinal Tissue of Patients with Inflammatory Bowel Diseases. Dig. Liver Dis. 2005, 37, 584–592. [Google Scholar] [CrossRef]
- Kirkegaard, T.; Hansen, A.; Bruun, E.; Brynskov, J. Expression and Localisation of Matrix Metalloproteinases and Their Natural Inhibitors in Fistulae of Patients with Crohn’s Disease. Gut 2004, 53, 701–709. [Google Scholar] [CrossRef] [Green Version]
- Garg, P.; Vijay-Kumar, M.; Wang, L.; Gewirtz, A.T.; Merlin, D.; Sitaraman, S.V. Matrix Metalloproteinase-9-Mediated Tissue Injury Overrides the Protective Effect of Matrix Metalloproteinase-2 During Colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G175–G184. [Google Scholar] [CrossRef] [Green Version]
- Hogan, S.P. Functional Role of Eosinophils in Gastrointestinal Inflammation. Immunol. Allergy Clin. N. Am. 2009, 29, 129–140. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, S.Y.; Bak, S.M.; Suh, I.B.; Lee, S.Y.; Shin, C.; Shim, J.J.; In, K.H.; Kang, K.H.; Yoo, S.H. Effects of Matrix Metalloproteinase Inhibitor on Lps-Induced Goblet Cell Metaplasia. Am. J. Physiol. Cell. Mol. Physiol. 2004, 287, L127–L133. [Google Scholar] [CrossRef]
- Lubbe, W.J.; Zhou, Z.Y.; Fu, W.; Zuzga, D.; Schulz, S.; Fridman, R.; Muschel, R.J.; Waldman, S.A.; Pitari, G.M. Tumor Epithelial Cell Matrix Metalloproteinase 9 Is a Target for Antimetastatic Therapy in Colorectal Cancer. Clin. Cancer Res. 2006, 12, 1876–1882. [Google Scholar] [CrossRef] [Green Version]
- Hanifeh, M.; Rajamäki, M.M.; Syrjä, P.; Mäkitalo, L.; Kilpinen, S.; Spillmann, T. Identification of Matrix Metalloproteinase-2 and -9 Activities within the Intestinal Mucosa of Dogs with Chronic Enteropathies. Acta Vet. Scand. 2018, 60, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancho, C.; Sainz, Á.; García-Sancho, M.; Villaescusa, A.; Rodríguez-Franco, F. Evaluation of Perinuclear Antineutrophilic Cytoplasmic Antibodies in Sera from Dogs with Inflammatory Bowel Disease or Intestinal Lymphoma. Am. J. Vet. Res. 2011, 72, 1333–1337. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, E.; Pierini, A.; Gori, E.; Lucarelli, C.; Lubas, G.; Marchetti, V. Neutrophil-to-Lymphocyte Ratio (Nlr) in Canine Inflammatory Bowel Disease (Ibd). Vet. Sci. 2020, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Becher, A.; Suchodolski, J.S.; Steiner, J.M.; Heilmann, R.M. Blood Neutrophil-to-Lymphocyte Ratio (Nlr) as a Diagnostic Marker in Dogs with Chronic Enteropathy. J. Vet. Diagn. Investig. 2021, 33, 516–527. [Google Scholar] [CrossRef]
- Hanifeh, M.; Sankari, S.; Rajamäki, M.M.; Syrjä, P.; Kilpinen, S.; Suchodolski, J.S.; Heilmann, R.M.; Guadiano, P.; Lidbury, J.; Steiner, J.M.; et al. S100a12 Concentrations and Myeloperoxidase Activities Are Increased in the Intestinal Mucosa of Dogs with Chronic Enteropathies. BMC Vet. Res. 2018, 14, 125. [Google Scholar] [CrossRef]
- Heilmann, R.M.; Nestler, J.; Schwarz, J.; Grützner, N.; Ambrus, A.; Seeger, J.; Suchodolski, J.S.; Steiner, J.M.; Gurtner, C. Mucosal Expression of S100a12 (Calgranulin C) and S100a8/A9 (Calprotectin) and Correlation with Serum and Fecal Concentrations in Dogs with Chronic Inflammatory Enteropathy. Vet. Immunol. Immunopathol. 2019, 211, 64–74. [Google Scholar] [CrossRef]
- Khaki-Khatibi, F.; Qujeq, D.; Kashifard, M.; Moein, S.; Maniati, M.; Vaghari-Tabari, M. Calprotectin in Inflammatory Bowel Disease. Clin. Chim. Acta 2020, 510, 556–565. [Google Scholar] [CrossRef]
- Walsham, N.E.; Sherwood, R.A. Fecal Calprotectin in Inflammatory Bowel Disease. Clin. Exp. Gastroenterol. 2016, 9, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Petryszyn, P.; Staniak, A.; Wolosianska, A.; Ekk-Cierniakowski, P. Faecal Calprotectin as a Diagnostic Marker of Inflammatory Bowel Disease in Patients with Gastrointestinal Symptoms: Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1306–1312. [Google Scholar] [CrossRef]
- Heilmann, R.M.; Jergens, A.E.; Ackermann, M.R.; Barr, J.W.; Suchodolski, J.S.; Steiner, J.M. Serum Calprotectin Concentrations in Dogs with Idiopathic Inflammatory Bowel Disease. Am. J. Vet. Res. 2012, 73, 1900–1907. [Google Scholar] [CrossRef]
- Heilmann, R.M.; Berghoff, N.; Mansell, J.; Grützner, N.; Parnell, N.K.; Gurtner, C.; Suchodolski, J.S.; Steiner, J.M. Association of Fecal Calprotectin Concentrations with Disease Severity, Response to Treatment, and Other Biomarkers in Dogs with Chronic Inflammatory Enteropathies. J. Vet. Intern. Med. 2018, 32, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Félix, A.P.; Souza, C.M.M.; de Oliveira, S.G. Biomarkers of Gastrointestinal Functionality in Dogs: A Systematic Review and Meta-Analysis. Anim. Feed. Sci. Technol. 2022, 283, 115183. [Google Scholar] [CrossRef]
- Okabe, Y.; Medzhitov, R. Tissue Biology Perspective on Macrophages. Nat. Immunol. 2016, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage Plasticity and Polarization in Tissue Repair and Remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- Bain, C.C.; Bravo-Blas, A.; Scott, C.L.; Perdiguero, E.G.; Geissmann, F.; Henri, S.; Malissen, B.; Osborne, L.C.; Artis, D.; Mowat, A.M. Constant Replenishment from Circulating Monocytes Maintains the Macrophage Pool in the Intestine of Adult Mice. Nat. Immunol. 2014, 15, 929–937. [Google Scholar] [CrossRef] [Green Version]
- Hume, D.A. Macrophages as Apc and the Dendritic Cell Myth. J. Immunol. 2008, 181, 5829–5835. [Google Scholar] [CrossRef] [Green Version]
- Mowat, A.M.; Bain, C.C. Mucosal Macrophages in Intestinal Homeostasis and Inflammation. J. Innate Immun. 2011, 3, 550–564. [Google Scholar] [CrossRef]
- Zhou, Z.; Ding, M.; Huang, L.; Gilkeson, G.; Lang, R.; Jiang, W. Toll-Like Receptor-Mediated Immune Responses in Intestinal Macrophages; Implications for Mucosal Immunity and Autoimmune Diseases. Clin. Immunol. 2016, 173, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Bain, C.C.; Schridde, A. Origin, Differentiation, and Function of Intestinal Macrophages. Front. Immunol. 2018, 9, 2733. [Google Scholar] [CrossRef]
- Kamada, N.; Hisamatsu, T.; Okamoto, S.; Sato, T.; Matsuoka, K.; Arai, K.; Nakai, T.; Hasegawa, A.; Inoue, N.; Watanabe, N.; et al. Abnormally Differentiated Subsets of Intestinal Macrophage Play a Key Role in Th1-Dominant Chronic Colitis through Excess Production of Il-12 and Il-23 in Response to Bacteria. J. Immunol. 2005, 175, 6900. [Google Scholar] [CrossRef] [Green Version]
- Xavier, R.J.; Podolsky, D.K. Unravelling the Pathogenesis of Inflammatory Bowel Disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Nolte, A.; Junginger, J.; Baum, B.; Hewicker-Trautwein, M. Heterogeneity of Macrophages in Canine Histiocytic Ulcerative Colitis. Innate Immun. 2017, 23, 228–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- German, A.J.; Hall, E.J.; Day, M.J. Immune Cell Populations within the Duodenal Mucosa of Dogs with Enteropathies. J. Vet. Intern. Med. 2001, 15, 14–25. [Google Scholar] [CrossRef] [PubMed]
- German, A.J.; Hall, E.J.; Kelly, D.F.; Watson, A.D.J.; Day, M.J. An Immunohistochemical Study of Histiocytic Ulcerative Colitis in Boxer Dogs. J. Comp. Pathol. 2000, 122, 163–175. [Google Scholar] [CrossRef]
- Manchester, A.C.; Hill, S.; Sabatino, B.; Armentano, R.; Carroll, M.; Kessler, B.; Miller, M.; Dogan, B.; McDonough, S.P.; Simpson, K.W. Association between Granulomatous Colitis in French Bulldogs and Invasive Escherichia Coli and Response to Fluoroquinolone Antimicrobials. J. Vet. Intern. Med. 2013, 27, 56–61. [Google Scholar] [CrossRef]
- Wagner, A.; Junginger, J.; Lemensieck, F.; Hewicker-Trautwein, M. Immunohistochemical Characterization of Gastrointestinal Macrophages/Phagocytes in Dogs with Inflammatory Bowel Disease (Ibd) and Non-Ibd Dogs. Vet. Immunol. Immunopathol. 2018, 197, 49–57. [Google Scholar] [CrossRef]
- Dandrieux, J.R.; Martinez Lopez, L.M.; Stent, A.; Jergens, A.; Allenspach, K.; Nowell, C.J.; Firestone, S.M.; Kimpton, W.; Mansfield, C.S. Changes in Duodenal Cd163-Positive Cells in Dogs with Chronic Enteropathy after Successful Treatment. Innate Immun. 2018, 24, 400–410. [Google Scholar] [CrossRef] [Green Version]
- Luckschander, N.; Hall, J.A.; Gaschen, F.; Forster, U.; Wenzlow, N.; Hermann, P.; Allenspach, K.; Dobbelaere, D.; Burgener, I.A.; Welle, M. Activation of Nuclear Factor-Κb in Dogs with Chronic Enteropathies. Vet. Immunol. Immunopathol. 2010, 133, 228–236. [Google Scholar] [CrossRef]
- Eissa, S.; Abdulkarim, H.; Dasouki, M.; Al Mousa, H.; Arnout, R.; Al Saud, B.; Rahman, A.A.; Zourob, M. Multiplexed Detection of Dock8, Pgm3 and Stat3 Proteins for the Diagnosis of Hyper-Immunoglobulin E Syndrome Using Gold Nanoparticles-Based Immunosensor Array Platform. Biosens. Bioelectron. 2018, 117, 613–619. [Google Scholar] [CrossRef]
- Filippone, R.T.; Sahakian, L.; Apostolopoulos, V.; Nurgali, K. Eosinophils in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1140–1151. [Google Scholar] [CrossRef]
- Acharya, K.R.; Ackerman, S.J. Eosinophil Granule Proteins: Form and Function. J. Biol. Chem. 2014, 289, 17406–17415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Impellizzeri, G.; Marasco, G.; Eusebi, L.H.; Salfi, N.; Bazzoli, F.; Zagari, R.M. Eosinophilic Colitis: A Clinical Review. Dig. Liver Dis. 2019, 51, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Cerquetella, M.; Spaterna, A.; Laus, F.; Tesei, B.; Rossi, G.; Antonelli, E.; Villanacci, V.; Bassotti, G. Inflammatory Bowel Disease in the Dog: Differences and Similarities with Humans. World J. Gastroenterol. 2010, 16, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Junginger, J.; Schwittlick, U.; Lemensieck, F.; Nolte, I.; Hewicker-Trautwein, M. Immunohistochemical Investigation of Foxp3 Expression in the Intestine in Healthy and Diseased Dogs. Vet. Res. 2012, 43, 23. [Google Scholar] [CrossRef] [Green Version]
- Sattasathuchana, P.; Steiner, J.M. Canine Eosinophilic Gastrointestinal Disorders. Anim. Health Res. Rev. 2014, 15, 76–86. [Google Scholar] [CrossRef]
- Dainese, R.; Galliani, E.A.; De Lazzari, F.; D’Incà, R.; Mariné-Barjoan, E.; Vivinus-Nebot, M.H.; Hébuterne, X.; Sturniolo, G.C.; Piche, T. Role of Serological Markers of Activated Eosinophils in Inflammatory Bowel Diseases. Eur. J. Gastroenterol. Hepatol. 2012, 24, 393–397. [Google Scholar] [CrossRef]
- Sattasathuchana, P.; Allenspach, K.; Lopes, R.; Suchodolski, J.S.; Steiner, J.M. Evaluation of Serum 3-Bromotyrosine Concentrations in Dogs with Steroid-Responsive Diarrhea and Food-Responsive Diarrhea. J. Vet. Intern. Med. 2017, 31, 1056–1061. [Google Scholar] [CrossRef]
- Bastan, I.; Ge, X.N.; Dileepan, M.; Greenberg, Y.G.; Guedes, A.G.; Hwang, S.H.; Hammock, B.D.; Washabau, R.J.; Rao, S.P.; Sriramarao, P. Inhibition of Soluble Epoxide Hydrolase Attenuates Eosinophil Recruitment and Food Allergen-Induced Gastrointestinal Inflammation. J. Leukoc. Biol. 2018, 104, 109–122. [Google Scholar] [CrossRef]
- Bastan, I.; Rendahl, A.K.; Seelig, D.; Day, M.J.; Hall, E.J.; Rao, S.P.; Washabau, R.J.; Sriramarao, P. Assessment of Eosinophils in Gastrointestinal Inflammatory Disease of Dogs. J. Vet. Intern. Med. 2018, 32, 1911–1917. [Google Scholar] [CrossRef]
- Bischoff, S.C. Mast Cells in Gastrointestinal Disorders. Eur. J. Pharmacol. 2016, 778, 139–145. [Google Scholar] [CrossRef]
- De Zuani, M.; Dal Secco, C.; Frossi, B. Mast Cells at the Crossroads of Microbiota and Ibd. Eur. J. Immunol. 2018, 48, 1929–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locher, C.; Tipold, A.; Welle, M.; Busato, A.; Zurbriggen, A.; Griot-Wenk, M.E. Quantitative Assessment of Mast Cells and Expression of Ige Protein and Mrna for Ige and Interleukin 4 in the Gastrointestinal Tract of Healthy Dogs and Dogs with Inflammatory Bowel Disease. Am. J. Vet. Res. 2001, 62, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmidt, S.; Meneses, F.; Nolte, I.; Hewicker-Trautwein, M. Distribution of Mast Cell Subtypes and Immune Cell Populations in Canine Intestines: Evidence for Age-Related Decline in T Cells and Macrophages and Increase of Iga-Positive Plasma Cells. Res. Vet. Sci. 2008, 84, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmidt, S.; Meneses, F.; Nolte, I.; Hewicker-Trautwein, M. Characterization of Mast Cell Numbers and Subtypes in Biopsies from the Gastrointestinal Tract of Dogs with Lymphocytic-Plasmacytic or Eosinophilic Gastroenterocolitis. Vet. Immunol. Immunopathol. 2007, 120, 80–92. [Google Scholar] [CrossRef]
- Berghoff, N.; Steiner, J.M. Laboratory Tests for the Diagnosis and Management of Chronic Canine and Feline Enteropathies. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 311–328. [Google Scholar] [CrossRef]
- Berghoff, N.; Hill, S.; Parnell, N.K.; Mansell, J.; Suchodolski, J.S.; Steiner, J.M. Fecal and Urinary N-Methylhistamine Concentrations in Dogs with Chronic Gastrointestinal Disease. Vet. J. 2014, 201, 289–294. [Google Scholar] [CrossRef]
- Bouma, G.; Strober, W. The Immunological and Genetic Basis of Inflammatory Bowel Disease. Nat. Rev. Immunol. 2003, 3, 521–533. [Google Scholar] [CrossRef]
- Smyth, M.J.; Godfrey, D.I. Nkt Cells and Tumor Immunity—A Double-Edged Sword. Nat. Immunol. 2000, 1, 459–460. [Google Scholar] [CrossRef]
- Tanaka, J.; Saga, K.; Kido, M.; Nishiura, H.; Akamatsu, T.; Chiba, T.; Watanabe, N. Proinflammatory Th2 Cytokines Induce Production of Thymic Stromal Lymphopoietin in Human Colonic Epithelial Cells. Dig. Dis. Sci. 2010, 55, 1896–1904. [Google Scholar] [CrossRef]
- Wilson, S.B.; Delovitch, T.L. Janus-Like Role of Regulatory Inkt Cells in Autoimmune Disease and Tumour Immunity. Nat. Rev. Immunol. 2003, 3, 211–222. [Google Scholar] [CrossRef]
- Saubermann, L.J.; Beck, P.; De Jong, Y.P.; Pitman, R.S.; Ryan, M.S.; Kim, H.S.; Exley, M.; Snapper, S.; Balk, S.P.; Hagen, S.J.; et al. Activation of Natural Killer T Cells by Alpha-Galactosylceramide in the Presence of Cd1d Provides Protection against Colitis in Mice. Gastroenterology 2000, 119, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Heller, F.; Fuss, I.J.; Nieuwenhuis, E.E.; Blumberg, R.S.; Strober, W. Oxazolone Colitis, a Th2 Colitis Model Resembling Ulcerative Colitis, Is Mediated by Il-13-Producing Nk-T Cells. Immunity 2002, 17, 629–638. [Google Scholar] [CrossRef] [Green Version]
- Steel, A.W.; Mela, C.M.; Lindsay, J.O.; Gazzard, B.G.; Goodier, M.R. Increased Proportion of Cd16+ Nk Cells in the Colonic Lamina Propria of Inflammatory Bowel Disease Patients, but Not after Azathioprine Treatment. Aliment. Pharmacol. Ther. 2011, 33, 115–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takayama, T.; Kamada, N.; Chinen, H.; Okamoto, S.; Kitazume, M.T.; Chang, J.; Matuzaki, Y.; Suzuki, S.; Sugita, A.; Koganei, K.; et al. Imbalance of Nkp44(+)Nkp46(−) and Nkp44(−)Nkp46(+) Natural Killer Cells in the Intestinal Mucosa of Patients with Crohn’s Disease. Gastroenterology 2010, 139, 1995–2004.e15. [Google Scholar] [CrossRef]
- Fathollahi, A.; Aslani, S.; Mostafaei, S.; Rezaei, N.; Mahmoudi, M. The Role of Killer-Cell Immunoglobulin-Like Receptor (Kir) Genes in Susceptibility to Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. Inflamm. Res. 2018, 67, 727–736. [Google Scholar] [CrossRef]
- Vadstrup, K.; Bendtsen, F. Anti-Nkg2d Mab: A New Treatment for Crohn’s Disease? Int. J. Mol. Sci. 2017, 18, 1997. [Google Scholar] [CrossRef]
- Baumgarth, N.; Tung, J.W.; Herzenberg, L.A. Inherent Specificities in Natural Antibodies: A Key to Immune Defense against Pathogen Invasion. Springer Semin. Immunopathol. 2005, 26, 347–362. [Google Scholar] [CrossRef]
- de Veer, M.J.; Kemp, J.M.; Meeusen, E.N. The Innate Host Defence against Nematode Parasites. Parasite Immunol. 2007, 29, 1–9. [Google Scholar] [CrossRef]
- Bunker, J.J.; Erickson, S.A.; Flynn, T.M.; Henry, C.; Koval, J.C.; Meisel, M.; Jabri, B.; Antonopoulos, D.A.; Wilson, P.C.; Bendelac, A. Natural Polyreactive Iga Antibodies Coat the Intestinal Microbiota. Science 2017, 358, eaan6619. [Google Scholar] [CrossRef] [Green Version]
- Pietzsch, J.; Hoppmann, S. Human S100a12: A Novel Key Player in Inflammation? Amino Acids 2009, 36, 381–389. [Google Scholar] [CrossRef]
- Foell, D.; Wittkowski, H.; Vogl, T.; Roth, J. S100 Proteins Expressed in Phagocytes: A Novel Group of Damage-Associated Molecular Pattern Molecules. J. Leukoc. Biol. 2007, 81, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, R.M.; Grellet, A.; Allenspach, K.; Lecoindre, P.; Day, M.J.; Priestnall, S.L.; Toresson, L.; Procoli, F.; Grützner, N.; Suchodolski, J.S.; et al. Association between Fecal S100a12 Concentration and Histologic, Endoscopic, and Clinical Disease Severity in Dogs with Idiopathic Inflammatory Bowel Disease. Vet. Immunol. Immunopathol. 2014, 158, 156–166. [Google Scholar] [CrossRef]
- Cabrera-García, A.I.; Protschka, M.; Alber, G.; Kather, S.; Dengler, F.; Müller, U.; Steiner, J.M.; Heilmann, R.M. Dysregulation of Gastrointestinal Rage (Receptor for Advanced Glycation End Products) Expression in Dogs with Chronic Inflammatory Enteropathy. Vet. Immunol. Immunopathol. 2021, 234, 110216. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-García, A.I.; Suchodolski, J.S.; Steiner, J.M.; Heilmann, R.M. Association between Serum Soluble Receptor for Advanced Glycation End-Products (Rage) Deficiency and Severity of Clinicopathologic Evidence of Canine Chronic Inflammatory Enteropathy. J. Vet. Diagn. Investig. 2020, 32, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-García, A.I.; Protschka, M.; Kather, S.; Dengler, F.; Alber, G.; Müller, U.; Steiner, J.; Heilmann, R. Dysregulation of Gastrointestinal Rage (Receptor for Advanced Glycation End Products) Expression in a Spontaneous Animal Model of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2021, 27, S3. [Google Scholar] [CrossRef]
- Allenspach, K.; Mochel, J.P. Current Diagnostics for Chronic Enteropathies in Dogs. Vet. Clin. Pathol. 2022, 50, 18–28. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.J. How Toll-like Receptors Signal: What We Know and What We Don’t Know. Curr. Opin. Immunol. 2006, 18, 3–9. [Google Scholar] [CrossRef]
- Fritz, J.H.; Ferrero, R.L.; Philpott, D.J.; Girardin, S.E. Nod-Like Proteins in Immunity, Inflammation and Disease. Nat. Immunol. 2006, 7, 1250–1257. [Google Scholar] [CrossRef]
- McMahon, L.A.; House, A.K.; Catchpole, B.; Elson-Riggins, J.; Riddle, A.; Smith, K.; Werling, D.; Burgener, I.A.; Allenspach, K. Expression of Toll-Like Receptor 2 in Duodenal Biopsies from Dogs with Inflammatory Bowel Disease Is Associated with Severity of Disease. Vet. Immunol. Immunopathol. 2010, 135, 158–163. [Google Scholar] [CrossRef]
- Burgener, I.A.; König, A.; Allenspach, K.; Sauter, S.N.; Boisclair, J.; Doherr, M.G.; Jungi, T.W. Upregulation of Toll-Like Receptors in Chronic Enteropathies in Dogs. J. Vet. Intern. Med. 2008, 22, 553–560. [Google Scholar] [CrossRef]
- Kaser, A.; Pasaniuc, B. Ibd Genetics: Focus on (Dys) Regulation in Immune Cells and the Epithelium. Gastroenterology 2014, 146, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Kathrani, A.; House, A.; Catchpole, B.; Murphy, A.; German, A.; Werling, D.; Allenspach, K. Polymorphisms in the Tlr4 and Tlr5 Gene Are Significantly Associated with Inflammatory Bowel Disease in German Shepherd Dogs. PLoS ONE 2010, 5, e15740. [Google Scholar] [CrossRef] [PubMed]
- Kathrani, A.; Lee, H.; White, C.; Catchpole, B.; Murphy, A.; German, A.; Werling, D.; Allenspach, K. Association between Nucleotide Oligomerisation Domain Two (Nod2) Gene Polymorphisms and Canine Inflammatory Bowel Disease. Vet. Immunol. Immunopathol. 2014, 161, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Kathrani, A.; Werling, D.; Allenspach, K. Canine Breeds at High Risk of Developing Inflammatory Bowel Disease in the South-Eastern Uk. Vet. Rec. 2011, 169, 635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kathrani, A.; Holder, A.; Catchpole, B.; Alvarez, L.; Simpson, K.; Werling, D.; Allenspach, K. Tlr5 Risk-Associated Haplotype for Canine Inflammatory Bowel Disease Confers Hyper-Responsiveness to Flagellin. PLoS ONE 2012, 7, e30117. [Google Scholar] [CrossRef] [PubMed]
- Cheroutre, H.; Lambolez, F.; Mucida, D. The Light and Dark Sides of Intestinal Intraepithelial Lymphocytes. Nat. Rev. Immunol. 2011, 11, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhu, L.; Qin, S. Gut Microbiota Modulation on Intestinal Mucosal Adaptive Immunity. J. Immunol. Res. 2019, 2019, 4735040. [Google Scholar] [CrossRef]
- Tatiya-aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Immune Response and Inflammatory Pathway of Ulcerative Colitis. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 1–10. [Google Scholar] [CrossRef]
- Degasperi, G.R. Mucosal Immunology in the Inflammatory Bowel Diseases. In Biological Therapy for Inflammatory Bowel Disease; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Heilmann, R.M.; Suchodolski, J.S. Is Inflammatory Bowel Disease in Dogs and Cats Associated with a Th1 or Th2 Polarization? Vet. Immunol. Immunopathol. 2015, 168, 131–134. [Google Scholar] [CrossRef]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. Il-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef]
- Choy, M.C.; Visvanathan, K.; De Cruz, P. An Overview of the Innate and Adaptive Immune System in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Maillard, M.H.; Snapper, S.B. Cytokines and chemokines in mucosal homeostasis. In Inflammatory Bowel Disease—Translating Basic Science Bowel Disease—Translating Basic Science into Clinical Practice; Targan, S.R., Shanahan, F., Karp, L.C., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 119–156. [Google Scholar] [CrossRef]
- Marafini, I.; Sedda, S.; Dinallo, V.; Monteleone, G. Inflammatory Cytokines: From Discoveries to Therapies in Ibd. Expert Opin. Biol. Ther. 2019, 19, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Finotto, S.; Glimcher, L.H. The Role of Th1/Th2 Polarization in Mucosal Immunity. Nat. Med. 2002, 8, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Ridyard, A.E.; Nuttall, T.J.; Else, R.W.; Simpson, J.W.; Miller, H.R.P. Evaluation of Th1, Th2 and Immunosuppressive Cytokine Mrna Expression within the Colonic Mucosa of Dogs with Idiopathic Lymphocytic–Plasmacytic Colitis. Vet. Immunol. Immunopathol. 2002, 86, 205–214. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Boden, E.K.; Lord, J.D. Cd4 T Cells in Ibd: Crossing the Line? Dig. Dis. Sci. 2017, 62, 2208–2210. [Google Scholar] [CrossRef] [Green Version]
- Ohta, H.; Takada, K.; Sunden, Y.; Tamura, Y.; Osuga, T.; Lim, S.Y.; Murakami, M.; Sasaki, N.; Wickramasekara Rajapakshage, B.K.; Nakamura, K.; et al. Cd4⁺ T Cell Cytokine Gene and Protein Expression in Duodenal Mucosa of Dogs with Inflammatory Bowel Disease. J. Vet. Med. Sci. 2014, 76, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, S.; Garden, O.A.; Werling, D.; Allenspach, K. Gene Expression of Selected Signature Cytokines of T Cell Subsets in Duodenal Tissues of Dogs with and without Inflammatory Bowel Disease. Vet. Immunol. Immunopathol. 2012, 146, 87–91. [Google Scholar] [CrossRef]
- Kinjo, T.; Azuma, Y.; Nishiyama, K.; Fujimoto, Y.; Miki, M.; Kuramoto, N. Intestinal Il-17 Expression in Canine Inflammatory Bowel Disease. Int. J. Vet. Health Sci. Res. 2017, 5, 171–175. [Google Scholar] [CrossRef]
- Ohta, H.; Takada, K.; Torisu, S.; Yuki, M.; Tamura, Y.; Yokoyama, N.; Osuga, T.; Lim, S.Y.; Murakami, M.; Sasaki, N.; et al. Expression of Cd4+ T Cell Cytokine Genes in the Colorectal Mucosa of Inflammatory Colorectal Polyps in Miniature Dachshunds. Vet. Immunol. Immunopathol. 2013, 155, 259–263. [Google Scholar] [CrossRef]
- Maeda, S.; Ohno, K.; Fujiwara-Igarashi, A.; Uchida, K.; Tsujimoto, H. Changes in Foxp3-Positive Regulatory T Cell Number in the Intestine of Dogs with Idiopathic Inflammatory Bowel Disease and Intestinal Lymphoma. Vet. Pathol. 2016, 53, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Ohno, K.; Uchida, K.; Nakashima, K.; Fukushima, K.; Tsukamoto, A.; Nakajima, M.; Fujino, Y.; Tsujimoto, H. Decreased Immunoglobulin a Concentrations in Feces, Duodenum, and Peripheral Blood Mononuclear Cells of Dogs with Inflammatory Bowel Disease. J. Vet. Intern. Med. 2013, 27, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejska-Sawerska, A.; Rychlik, A.; Depta, A.; Wdowiak, M.; Nowicki, M.; Kander, M. Cytokines in Canine Inflammatory Bowel Disease. Pol. J. Vet. Sci. 2013, 16, 165–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.O.; Panopoulos, A.D.; Nurieva, R.; Chang, S.H.; Wang, D.; Watowich, S.S.; Dong, C. Stat3 Regulates Cytokine-Mediated Generation of Inflammatory Helper T Cells. J. Biol. Chem. 2007, 282, 9358–9363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimoto, K. Role of Stat3 in Inflammatory Bowel Disease. World J. Gastroenterol. 2008, 14, 5110–5114. [Google Scholar] [CrossRef] [Green Version]
- Neufert, C.; Pickert, G.; Zheng, Y.; Wittkopf, N.; Warntjen, M.; Nikolae, A.; Ouyang, W.; Neurath, M.F.; Becker, C. Activation of Epithelial Stat3 Regulates Intestinal Homeostasis. Cell Cycle 2010, 9, 652–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manz, A.; Allenspach, K.; Kummer, S.; Richter, B.; Walter, I.; Macho-Maschler, S.; Tichy, A.; Burgener, I.A.; Luckschander-Zeller, N. Upregulation of Signal Transducer and Activator of Transcription 3 in Dogs with Chronic Inflammatory Enteropathies. J. Vet. Intern. Med. 2021, 35, 1288–1296. [Google Scholar] [CrossRef]
- Madakamutil, L.T.; Christen, U.; Lena, C.J.; Wang-Zhu, Y.; Attinger, A.; Sundarrajan, M.; Ellmeier, W.; von Herrath, M.G.; Jensen, P.; Littman, D.R.; et al. Cd8αα-Mediated Survival and Differentiation of Cd8 Memory T Cell Precursors. Science 2004, 304, 590. [Google Scholar] [CrossRef]
- van Wijk, F.; Cheroutre, H. Mucosal T Cells in Gut Homeostasis and Inflammation. Expert Rev. Clin. Immunol. 2010, 6, 559–566. [Google Scholar] [CrossRef] [Green Version]
- McVay, L.D.; Li, B.; Biancaniello, R.; Creighton, M.A.; Bachwich, D.; Lichtenstein, G.; Rombeau, J.L.; Carding, S.R. Changes in Human Mucosal Γδ T Cell Repertoire and Function Associated with the Disease Process in Inflammatory Bowel Disease. Mol. Med. 1997, 3, 183–203. [Google Scholar] [CrossRef] [Green Version]
- Haas, E.; Rütgen, B.C.; Gerner, W.; Richter, B.; Tichy, A.; Galler, A.; Bilek, A.; Thalhammer, J.G.; Saalmüller, A.; Luckschander-Zeller, N. Phenotypic Characterization of Canine Intestinal Intraepithelial Lymphocytes in Dogs with Inflammatory Bowel Disease. J. Vet. Intern. Med. 2014, 28, 1708–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubinak, J.L.; Petersen, C.; Stephens, W.Z.; Soto, R.; Bake, E.; O’Connell, R.M.; Round, J.L. Myd88 Signaling in T Cells Directs Iga-Mediated Control of the Microbiota to Promote Health. Cell Host Microbe 2015, 17, 153–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizoguchi, A.; Bhan, A.K. Immunobiology of B Cells in Inflammatory Bowel Disease. In Crohn’s Disease and Ulcerative Colitis: From Epidemiology and Immunobiology to a Rational Diagnostic and Therapeutic Approach; Baumgart, D.C., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 111–117. [Google Scholar] [CrossRef]
- Scott, M.G.; Nahm, M.H.; Macke, K.; Nash, G.S.; Bertovich, M.J.; MacDermott, R.P. Spontaneous Secretion of Igg Subclasses by Intestinal Mononuclear Cells: Differences between Ulcerative Colitis, Crohn’s Disease, and Controls. Clin. Exp. Immunol. 1986, 66, 209–215. [Google Scholar]
- Silva, F.A.R.; Rodrigues, B.L.; Ayrizono, M.d.L.S.; Leal, R.F. The Immunological Basis of Inflammatory Bowel Disease. Gastroenterol. Res. Pract. 2016, 2016, 2097274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahluwalia, B.; Moraes, L.; Magnusson, M.K.; Öhman, L. Immunopathogenesis of Inflammatory Bowel Disease and Mechanisms of Biological Therapies. Scand. J. Gastroenterol. 2018, 53, 379–389. [Google Scholar] [CrossRef]
- Hevia, A.; López, P.; Suárez, A.; Jacquot, C.; Urdaci, M.C.; Margolles, A.; Sánchez, B. Association of Levels of Antibodies from Patients with Inflammatory Bowel Disease with Extracellular Proteins of Food and Probiotic Bacteria. BioMed Res. Int. 2014, 2014, 351204. [Google Scholar] [CrossRef] [Green Version]
- Macpherson, A.; Khoo, U.Y.; Forgacs, I.; Philpott-Howard, J.; Bjarnason, I. Mucosal Antibodies in Inflammatory Bowel Disease Are Directed against Intestinal Bacteria. Gut 1996, 38, 365. [Google Scholar] [CrossRef] [Green Version]
- Galler, A.; Rütgen, B.C.; Haas, E.; Saalmüller, A.; Hirt, R.A.; Gerner, W.; Schwendenwein, I.; Richter, B.; Thalhammer, J.G.; Luckschander-Zeller, N. Immunophenotype of Peripheral Blood Lymphocytes in Dogs with Inflammatory Bowel Disease. J. Vet. Intern. Med. 2017, 31, 1730–1739. [Google Scholar] [CrossRef] [Green Version]
- Stonehewer, J.; Simpson, J.W.; Else, R.W.; MacIntyre, N. Evaluation of B and T Lymphocytes and Plasma Cells in Colonic Mucosa from Healthy Dogs and from Dogs with Inflammatory Bowel Disease. Res. Vet. Sci. 1998, 65, 59–63. [Google Scholar] [CrossRef]
- Planer, J.D.; Peng, Y.; Kau, A.L.; Blanton, L.V.; Ndao, I.M.; Tarr, P.I.; Warner, B.B.; Gordon, J.I. Development of the Gut Microbiota and Mucosal Iga Responses in Twins and Gnotobiotic Mice. Nature 2016, 534, 263–266. [Google Scholar] [CrossRef] [Green Version]
- Reboldi, A.; Arnon, T.I.; Rodda, L.B.; Atakilit, A.; Sheppard, D.; Cyster, J.G. Iga Production Requires B Cell Interaction with Subepithelial Dendritic Cells in Peyer’s Patches. Science 2016, 352, aaf4822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, S.; Ohno, K.; Fujiwara-Igarashi, A.; Tomiyasu, H.; Fujino, Y.; Tsujimoto, H. Methylation of Tnfrsf13b and Tnfrsf13c in Duodenal Mucosa in Canine Inflammatory Bowel Disease and Its Association with Decreased Mucosal Iga Expression. Vet. Immunol. Immunopathol. 2014, 160, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Castigli, E.; Wilson, S.A.; Scott, S.; Dedeoglu, F.; Xu, S.; Lam, K.-P.; Bram, R.J.; Jabara, H.; Geha, R.S. Taci and Baff-R Mediate Isotype Switching in B Cells. J. Exp. Med. 2005, 201, 35–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estruch, J.J.; Barken, D.; Bennett, N.; Krawiec, D.K.; Ogilvie, G.K.; Powers, B.E.; Polansky, B.J.; Sueda, M.T. Evaluation of Novel Serological Markers and Autoantibodies in Dogs with Inflammatory Bowel Disease. J. Vet. Intern. Med. 2020, 34, 1177–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soontararak, S.; Chow, L.; Johnson, V.; Coy, J.; Webb, C.; Wennogle, S.; Dow, S. Humoral Immune Responses against Gut Bacteria in Dogs with Inflammatory Bowel Disease. PLoS ONE 2019, 14, e0220522. [Google Scholar] [CrossRef] [Green Version]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining Trained Immunity and Its Role in Health and Disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [Green Version]
- Konstantinidis, A.O.; Pardali, D.; Adamama-Moraitou, K.K.; Gazouli, M.; Dovas, C.I.; Legaki, E.; Brellou, G.D.; Savvas, I.; Jergens, A.E.; Rallis, T.S.; et al. Colonic Mucosal and Serum Expression of Micrornas in Canine Large Intestinal Inflammatory Bowel Disease. BMC Vet. Res. 2020, 16, 69. [Google Scholar] [CrossRef]
- Tamura, Y.; Ohta, H.; Yokoyama, N.; Lim, S.Y.; Osuga, T.; Morishita, K.; Nakamura, K.; Yamasaki, M.; Takiguchi, M. Evaluation of Selected Cytokine Gene Expression in Colonic Mucosa from Dogs with Idiopathic Lymphocytic-Plasmacytic Colitis. J. Vet. Med. Sci. 2014, 76, 1407–1410. [Google Scholar] [CrossRef] [Green Version]
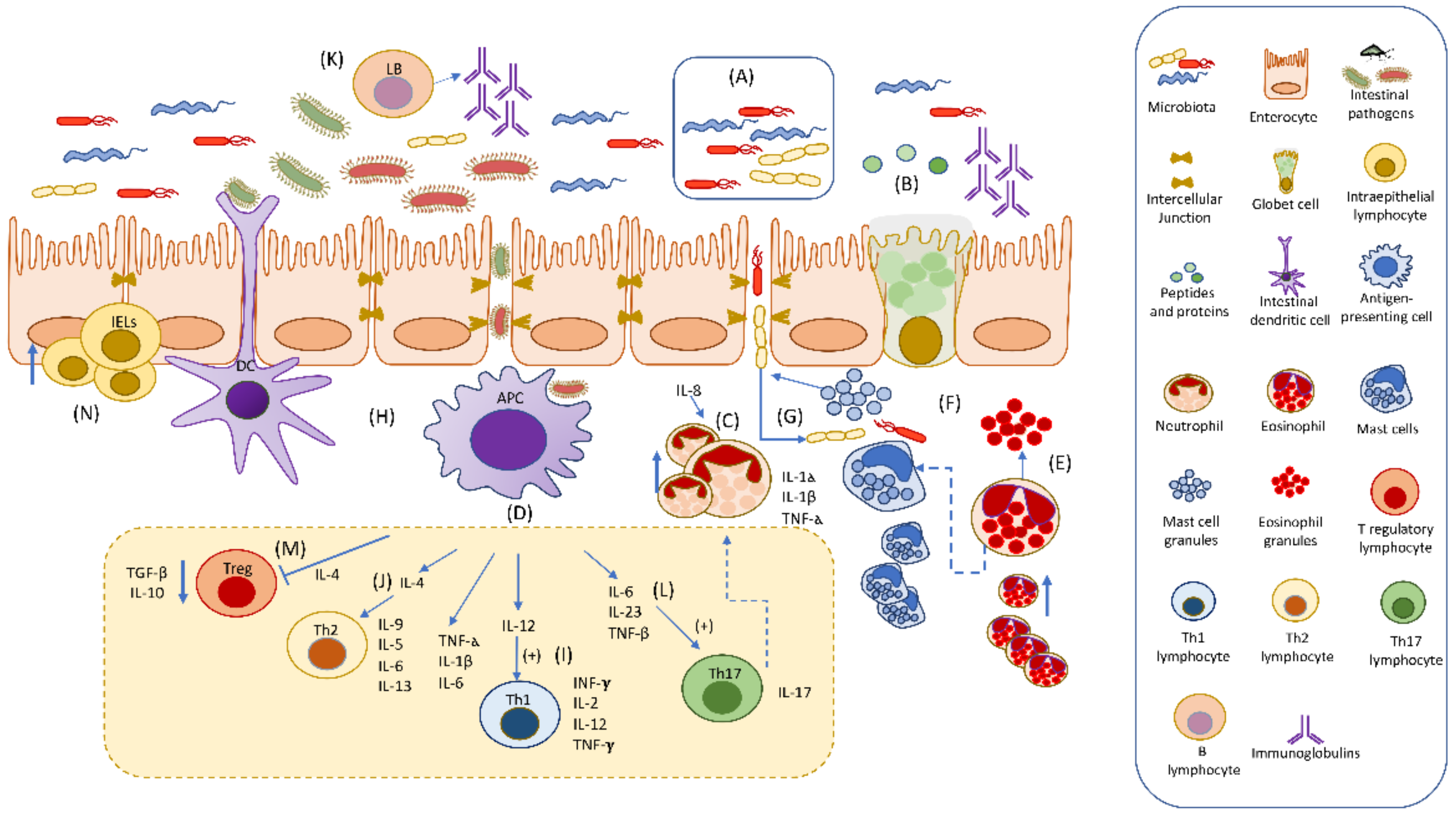
| Innate Immune Response | References |
|---|---|
| Microbiota | |
| A decrease in proportion of Clostridia and increase in proportion of Proteobacteria in the duodenum | [12] |
| A decrease in Faecalibacterium spp and Fusobacteria | [12] |
| Mucosal epithelial barrier | |
| Pathophysiological or environmental factors could induce loss of the mucosal barrier integrity and immune tolerance against intestinal symbionts | [27,28] |
| Trefoil factor (TFF) 1 expression is elevated in the duodenum, whereas TFF3 expression is down-regulated in the colon, suggesting that it contributes to impaired epithelial barrier function | [31] |
| Abnormal P-glycoprotein (P-gp) expression is observed in dogs with lymphoplasmacytic enteritis (LPE) | [32] |
| Upregulation of P-gp expression in lamina propria lymphocytes after prednisolone treatment | [33] |
| Innate immune cells and derived molecules | |
| A reduced expression of the β-integrin CD11c | [40] |
| An increase in neutrophils as a factor associated with severity | [73] |
| Perinuclear anti-neutrophil cytoplasmic autoantibodies (pANCA) and neutrophil-to-lymphocyte ratio (NLR) as biomarkers of severity | [52,53] |
| Calgranulin-C and myeloperoxidase (MPO) activities are increased in the duodenum and colon of dogs with chronic enteropathies, and myeloperoxidase (MPO) is also increased in the ileum and cecum. Calprotectin is overexpressed and released by activated mononuclear cells in canine CIE | [55,56] |
| Matrix metalloproteinases (MMPs)-2 and -9 are upregulated in dogs with CIE | [51] |
| Increased numbers of macrophages in the duodenal mucosa | [73] |
| An increase in macrophage infiltration in the lamina propria in colonic and noncolonic affected regions, a decrease in Goblet cells, and an increase in MHC class II expression in enterocytes of boxer breed dogs with CIE | [74] |
| An increase in macrophages/mm2 with increased NF-κB pathway activity in the lamina propria | [78] |
| Degranulated eosinophils in the lower region of the lamina propria and degranulated and intact eosinophils in the upper | [6] |
| Increased concentration of Serum 3-BrY (associated with eosinophil activation) in dogs with SRE/IRE compared to those with FRE or healthy control dogs | [87] |
| Increased mast cells in the area of eosinophilic gastroenterocolitis | [85] |
| More IgE-positive cells and mast cells in the mucosa and mesenteric lymph nodes | [92] |
| A decrease in metachromatically stained granules and mast cells in dogs with lymphocytic-plasmacytic or eosinophilic gastroenterocolitis | [94] |
| Increased fecal and/or urinary NMH concentrations in some dogs with CIE | [96] |
| Increased fecal S100A12 concentrations associated with clinical disease activity, the severity of endoscopic lesions, and the severity of colonic inflammation | [112] |
| Decreased serum sRAGE concentrations in canine CIE | [114] |
| Overexpression of epithelial RAGE along the gastrointestinal tract in dogs with CIE | [115] |
| Adaptive Immune Response | |
| T helper lymphocytes (CD4+) | |
| A balance in the expression of proinflammatory and anti-inflammatory cytokines in German shepherd dogs | [42] |
| An increase in IL-2 and TNF-α expression in dogs with colitis | [136] |
| An increase in IL12p40-associated mRNA in dogs with lymphocytic-plasmocytic enteritis and lymphocytic-plasmocytic colitis, when the duodenum is affected. An increase in IL-4 mRNA expression when the colon is affected | [42] |
| An increased expression of IL-17A, IL-23p19, and Il-12p35 | [139,140,141,142] |
| Low number of Treg cell and IL-10 and TGF-β mRNA expression in dogs with lymphocytic-plasmocytic enteritis | [143,144] |
| Intestinal intraepithelial T lymphocytes | |
| Increased numbers of TCRγδ+ cells | [93,153] |
| B lymphocytes | |
| Increased numbers of B lymphocytes in the bloodstream and intestinal mucosa. IgG+, IgG3+, and IgG4+ also increase in plasma cells | [73,161,162] |
| Reduced IgA levels in intestinal mucosa, feces, and peripheral blood | [28,144] |
| High levels of specific IgA against serological markers such as polynuclear leukocytes, bacterial OmpC, calprotectin, gliadins, and bacterial flagellins | [167] |
| An increase in IgG-coated gut bacteria, which induce increased production of TNF-α by macrophages | [168] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siel, D.; Beltrán, C.J.; Martínez, E.; Pino, M.; Vargas, N.; Salinas, A.; Pérez, O.; Pereira, I.; Ramírez-Toloza, G. Elucidating the Role of Innate and Adaptive Immune Responses in the Pathogenesis of Canine Chronic Inflammatory Enteropathy—A Search for Potential Biomarkers. Animals 2022, 12, 1645. https://doi.org/10.3390/ani12131645
Siel D, Beltrán CJ, Martínez E, Pino M, Vargas N, Salinas A, Pérez O, Pereira I, Ramírez-Toloza G. Elucidating the Role of Innate and Adaptive Immune Responses in the Pathogenesis of Canine Chronic Inflammatory Enteropathy—A Search for Potential Biomarkers. Animals. 2022; 12(13):1645. https://doi.org/10.3390/ani12131645
Chicago/Turabian StyleSiel, Daniela, Caroll J. Beltrán, Eduard Martínez, Macarena Pino, Nazla Vargas, Alexandra Salinas, Oliver Pérez, Ismael Pereira, and Galia Ramírez-Toloza. 2022. "Elucidating the Role of Innate and Adaptive Immune Responses in the Pathogenesis of Canine Chronic Inflammatory Enteropathy—A Search for Potential Biomarkers" Animals 12, no. 13: 1645. https://doi.org/10.3390/ani12131645









