Effects of Replacing Fishmeal and Soybean Protein Concentrate with Degossypolized Cottonseed Protein in Diets on Growth Performance, Nutrient Digestibility, Intestinal Morphology, Cecum Microbiome and Fermentation of Weaned Piglets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets and Experimental Design
2.2. Animal Management
2.3. Experimental Sample Collection
2.4. Chemical Analysis
2.5. Measurement of Intestinal Morphology
2.6. Analysis of Microbial Community in Cecum
2.7. Measurement of Volatile Acids and Branch-Chain Fatty Acids
2.8. Statistical Analysis
3. Results
3.1. Chemical Composition
3.2. Growth Performance and Diarrhea Rate
3.3. The Apparent Total Tract Digestibility of Nutrients
3.4. Intestinal Morphology
3.5. Cecum Microbiota
3.6. Fermentation in Cecum
4. Discussion
4.1. Chemical Composition
4.2. Growth Performance and Nutrient Digestibility
4.3. Intestinal Morphology
4.4. Cecum Microbiota
4.5. Fermentation in Cecum
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, X.; Hu, J.; Shang, Q.; Liu, H.; Piao, X. Chemical composition, energy content and amino acid digestibility in cottonseed meals fed to growing pigs. J. Appl. Anim. Res. 2019, 47, 280–288. [Google Scholar] [CrossRef]
- Kim, S.W.; Van Heugten, E.; Ji, F.; Lee, C.H.; Mateo, R.D. Fermented soybean meal as a vegetable protein source for nursery pigs: I. Effects on growth performance of nursery pigs. J. Anim. Sci. 2010, 88, 214–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinn, S.M.; Gibbons, W.R.; Brown, M.L.; DeRouchey, J.M.; Levesque, C.L. Evaluation of microbially enhanced soybean meal as analternative to fshmeal in weaned pig diets. Animal 2016, 11, 784–793. [Google Scholar] [CrossRef]
- Zhang, S.; Piao, X.; Ma, X.; Xu, X.; Zeng, Z.; Tian, Q.; Li, Y. Comparison of spray-dried egg and albumen powder with conventional animal protein sources as feed ingredients in diets fed to weaned pigs. Anim. Sci. J. 2015, 86, 772–781. [Google Scholar] [CrossRef] [PubMed]
- USDA. Oil Crops Outlook. Available online: https://www.ers.usda.gov/webdocs/outlooks/103712/ocs-21I.pdf (accessed on 13 December 2021).
- Stein, H.H.; Lagos, L.V.; Casas, G.A. Nutritional value of feed ingredients of plant origin fed to pigs. Anim. Feed Sci. Technol. 2016, 218, 33–69. [Google Scholar] [CrossRef]
- Knabe, D.A.; Tanksley, T.D.; Hesby, J.H. Effect of Lysine, Crude Fiber and Free Gossypol in Cottonseed Meal on the Performance of Growing Pigs. J. Anim. Sci. 1979, 49, 134–142. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Zhang, G.; Zhao, J.B.; Zhou, X.J.; Dong, W.X.; Liu, L.; Zhang, S. Energy and nutrient digestibility of degossypolized cottonseed protein and its utilization as a protein source in nursery pigs. Livest. Sci. 2019, 223, 53–59. [Google Scholar] [CrossRef]
- Pan, L.; Zhao, P.F.; Ma, X.K.; Shang, Q.H.; Xu, Y.T.; Long, S.F.; Wu, Y.; Yuan, F.M.; Piao, X.S. Probiotic supplementation protects weaned pigs against enterotoxigenic Escherichia coli K88 challenge and improves performance similar to antibiotics. J. Anim. Sci. 2017, 95, 2627–2639. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis, 19th ed.; Association of Official Agricultural Chemists: Arlington, VA, USA, 2012. [Google Scholar]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Wang, J.J.; Chen, L.X.; Li, P. Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J. Nutr. 2008, 138, 1025–1031. [Google Scholar] [CrossRef] [Green Version]
- RDP Rekeases. Available online: http://rdp.cme.msu.edu/ (accessed on 30 September 2016).
- He, B.B.; Bai, Y.; Jiang, L.L.; Wang, W.; Li, T.T.; Liu, P.; Tao, S.Y.; Zhao, J.B.; Han, D.D.; Wang, J.J. Effects of oat bran on nutrient digestibility, intestinal microbiota, and inflammatory responses in the hindgut of growing pigs. Int. J. Mol. Sci. 2018, 19, 2407. [Google Scholar] [CrossRef] [Green Version]
- Pelitire, S.M.; Dowd, M.K.; Cheng, H.N. Acidic solvent extraction of gossypol from cottonseed meal. Anim. Feed Sci. Technol. 2014, 195, 120–128. [Google Scholar] [CrossRef]
- Ma, D.L.; Ma, X.K.; Liu, L.; Zhang, S. Chemical composition, energy and amino acid digestibility in seven cottonseed co-products fed to growing pigs. J. Anim. Sci. 2018, 96, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- Hale, F.; Lyman, C.M. Tolerance in growing-fattening pigs effect of protein level in the ration on gossypol. J. Anim. Sci. 1957, 16, 364–369. [Google Scholar] [CrossRef]
- Alves, D.R.S.; de Oliveira, S.R.; Luczinski, T.G. Palatability of protein hydrolysates from industrial byproducts for nile tilapia juveniles. Animals 2019, 9, 311. [Google Scholar] [CrossRef] [Green Version]
- Lentle, R.G.; Janssen, P.W. Physical characteristics of digesta and their influence on flow and mixing in the mammalian intestine: A review. J. Comp. Physiol. B 2008, 178, 673–690. [Google Scholar] [CrossRef]
- Knabe, D.A.; LaRue, D.C.; Gregg, E.J. Apparent digestibility of nitrogen and amino acids in protein feedstuffs by growing pigs. J. Anim. Sci. 1989, 67, 441–458. [Google Scholar] [CrossRef]
- Han, M.; Song, P.; Huang, C.; Rezaei, A.; Farrar, S.; Brown, M.A.; Ma, X. Dietary grape seed proanthocyanidins (GSPs) improve weaned intestinal microbiota and mucosal barrier using a piglet model. Oncotarget 2016, 7, 80313. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Sun, J.; Chen, X.; Nie, C.; Zhao, J.; Guan, W.; Ma, X. Combination of Clostridium butyricum and corn bran optimized intestinal microbial fermentation using a weaned pig model. Front. Microbiol. 2018, 9, 3091. [Google Scholar] [CrossRef]
- Shang, Q.; Liu, H.; Liu, S.; He, T.; Piao, X. Effects of dietary fiber sources during late gestation and lactation on sow performance, milk quality, and intestinal health in piglets. J. Anim. Sci. 2019, 97, 4922–4933. [Google Scholar] [CrossRef]
- Li, D.F.; Nelssen, J.L.; Reddy, P.G.; Blecha, F.; Hancock, J.D.; Allee, G.L.; Goodband, R.D.; Klemm, R.D. Transient hypersensitivity to soybean meal in the early-weaned pig. J. Anim. Sci. 1990, 68, 1790–1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marion, J.; Biernat, M.; Thomas, F.; Savary, G.; Le Breton, Y.; Zabielski, R.; Le Dividich, J. Small intestine growth and morphometry in piglets weaned at 7 days of age. Effects of level of energy intake. Effects of level of energy intake. Reprod. Nutr. Dev. 2002, 42, 339–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natividad, J.M.; Verdu, E.F. Modulation of intestinal barrier by intestinal microbiota: Pathological and therapeutic implications. Pharmacol. Res. 2013, 69, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, S.; Li, M.; Piao, X. Effects of maternal 25-hydroxycholecalciferol during the last week of gestation and lactation on serum parameters, intestinal morphology and microbiota in suckling piglets. Arch. Anim. Nutr. 2020, 74, 445–461. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, R.R.; O’Gara, F. The impact of phytochemicals present in the diet on microbial signalling in the human gut. J. Funct. Foods. 2015, 14, 684–691. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, P.; Wu, Y.; Guo, P.; Liu, L.; Ma, N.; Ma, X. Dietary fiber increases butyrate-producing bacteria and improves the growth performance of weaned piglets. J. Agric. Food. Chem. 2018, 66, 7995–8004. [Google Scholar] [CrossRef]
- Marotti, I.; Bregola, V.; Aloisio, I.; Di Gioia, D.; Bosi, S.; Di Silvestro, R.; Quinn, R.; Dinelli, G. Prebiotic effect of soluble fibres from modern and old durum-type wheat varieties on Lactobacillus and Bifidobacterium strains. J. Sci. Food. Agric. 2012, 92, 2133–2140. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Chen, W.; Wang, G.; Rong, T.; Liu, Z.; Ma, X. Hermetia illucens larvae as a Fishmeal replacement alters intestinal specific bacterial populations and immune homeostasis in weanling piglets. J. Anim. Sci. 2020, 98, skz395. [Google Scholar] [CrossRef]
- Hossen, I.; Hua, W.; Ting, L.; Mehmood, A.; Jingyi, S.; Duoxia, X.; Junsong, X. Phytochemicals and inflammatory bowel disease: A review. Crit. Rev. Food Sci. 2022, 60, 1321–1345. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, K.; Chen, H.; Su, Y.; Zhu, W. Caecal infusion of the short-chain fatty acid propionate affects the microbiota and expression of inflammatory cytokines in the colon in a fistula pig model. Microb. Biotechnol. 2018, 11, 859–868. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Xu, X.W.; Meng, F.X.; Huo, Y.Y.; Oren, A.; Yang, J.Y.; Wang, C.S. Halomonas zincidurans sp. nov., a heavy-metal-tolerant bacterium isolated from the deep-sea environment. Int. J. Syst. Evol. Micr. 2013, 63, 4230–4236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Mu, C.; Zhang, C.; Yang, Y.; Su, Y.; Zhu, W. Marked response in microbial community and metabolism in the ileum and cecum of suckling piglets after early antibiotics exposure. Front. Microbiol. 2018, 9, 1166. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Yang, Y.; Yu, K.; Yu, M.; Zhang, C.; Su, Y.; Zhu, W. Alteration of metabolomic markers of amino-acid metabolism in piglets with in-feed antibiotics. Amino Acids. 2017, 49, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Liu, P.; Zhao, J.; Sun, J.; Guan, W. Dietary Clostridium butyricum induces a phased shift in fecal microbiota structure and increases the acetic acid-producing bacteria in a weaned piglet model. J. Agric. Food Chem. 2018, 66, 5157–5166. [Google Scholar] [CrossRef]
- Berni Canani, R.; Sangwan, N.; Stefka, A.T.; Nocerino, R.; Paparo, L.; Aitoro, R. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2016, 10, 742–750. [Google Scholar] [CrossRef]
- Blachier, F.; Mariotti, F.; Huneau, J.F.; Tomé, D. Effects of amino acidderived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids. 2007, 33, 547–562. [Google Scholar] [CrossRef]

| Items 1 | DCP | FM | SPC |
|---|---|---|---|
| DM, % | 90.43 | 93.84 | 93.14 |
| GE, MJ/kg | 18.24 | 18.67 | 19.27 |
| CP, % | 65.48 | 64.8 | 65.7 |
| Ether extract, % | 0.46 | 9.80 | 1.05 |
| Neutral detergent fiber, % | 20.42 | - | 8.60 |
| Acid detergent fiber, % | 5.51 | - | 5.44 |
| Free gossypol, mg/kg | 224.60 | - | - |
| Indispensable amino acids | |||
| Arginine, % | 8.11 | 4.05 | 4.35 |
| Histidine, % | 2.17 | 1.18 | 1.68 |
| Isoleucine, % | 1.86 | 2.58 | 3.01 |
| Leucine, % | 3.71 | 4.73 | 5.04 |
| Lysine, % | 2.68 | 4.57 | 4.15 |
| Methionine, % | 3.30 | 1.69 | 0.88 |
| Threonine, % | 2.15 | 2.42 | 2.50 |
| Tryptophan, % | 0.79 | 0.86 | 0.81 |
| Valine, % | 2.87 | 2.85 | 3.17 |
| Items 1, % | CON | FMr | SPCr |
|---|---|---|---|
| Corn | 53.67 | 52.68 | 53.53 |
| Soybean meal | 16 | 16 | 16 |
| SPC | 6 | 6 | 0 |
| FM | 6 | 0 | 6 |
| DCP | 0 | 6 | 6 |
| Whey powder | 10 | 10 | 10 |
| Soybean oil | 2.9 | 2.9 | 2.9 |
| Sucrose | 2 | 2 | 2 |
| Limestone | 0.7 | 1 | 0.7 |
| Dicalcium phosphate | 0.92 | 1.4 | 0.9 |
| Salt | 0.25 | 0.25 | 0.25 |
| L-Lysine·HCl | 0.47 | 0.62 | 0.6 |
| L-Threonine | 0.17 | 0.19 | 0.19 |
| L-Tryptophan | 0.03 | 0.03 | 0.04 |
| DL-Methionine | 0.09 | 0.13 | 0.09 |
| Chromic oxide | 0.3 | 0.3 | 0.3 |
| Premix | 0.5 | 0.5 | 0.5 |
| Calculated nutrient level | |||
| ME, kcal/kg | 3466 | 3455 | 3466 |
| Crude protein | 20.81 | 21.09 | 21.02 |
| SID Lys | 1.36 | 1.36 | 1.36 |
| SID Thr | 0.8 | 0.8 | 0.8 |
| SID Met | 0.4 | 0.4 | 0.4 |
| SID Trp | 0.22 | 0.22 | 0.22 |
| Ca | 0.81 | 0.8 | 0.8 |
| Digestible phosphorus | 0.41 | 0.4 | 0.4 |
| Measured nutrient level | |||
| GE, kcal/kg | 4005 | 3951 | 3975 |
| Crude protein | 19.56 | 19.8 | 19.78 |
| Ether extract | 5.61 | 5.01 | 5.56 |
| Neutral detergent fiber | 9.28 | 10.28 | 9.56 |
| Acid detergent fiber | 2.61 | 3.11 | 2.74 |
| Item 1 | CON | FMr | SPCr | SEM | p-Value |
|---|---|---|---|---|---|
| Initial BW, kg | 8.04 | 8.06 | 8.07 | 0.26 | 0.96 |
| Day 14 BW, kg | 12.96 | 13.34 | 13.33 | 0.44 | 0.75 |
| Day 28 BW, kg | 21.81 | 22.05 | 21.65 | 0.71 | 0.70 |
| Day 1 to 14 | |||||
| ADG, g/d | 351 | 377 | 376 | 17.21 | 0.50 |
| ADFI, g/d | 479 | 490 | 510 | 19.91 | 0.55 |
| F:G | 1.41 | 1.29 | 1.32 | 0.07 | 0.58 |
| diarrhea rate, % | 4.64 | 3.93 | 7.26 | 0.87 | 0.06 |
| Day 15 to 28 | |||||
| ADG, g/d | 632 | 622 | 594 | 26.88 | 0.61 |
| ADFI, g/d | 962a | 892b | 874b | 15.76 | < 0.01 |
| F:G | 1.54 | 1.43 | 1.47 | 0.06 | 0.43 |
| diarrhea rate, % | 0.72 | 1.67 | 1.19 | 0.28 | 0.11 |
| Day 1 to 28 | |||||
| ADG, g/d | 492 | 500 | 485 | 17.67 | 0.85 |
| ADFI, g/d | 720 | 684 | 689 | 12.02 | 0.13 |
| F:G | 1.49 | 1.36 | 1.42 | 0.05 | 0.25 |
| diarrhea rate, % | 2.68 | 2.80 | 4.23 | 0.53 | 0.13 |
| Items 1 | CON | FMr | SPCr | SEM | p-Value |
|---|---|---|---|---|---|
| Day 14 | |||||
| CP, % | 74.33 a | 69.46 b | 69.32 b | 0.95 | <0.01 |
| GE, % | 82.17 a | 78.3 b | 77.19 b | 0.71 | <0.01 |
| DM, % | 81.63 a | 77.35 b | 76.78 b | 0.72 | <0.01 |
| OM, % | 84.67 a | 81.21 b | 80.51 b | 0.60 | <0.01 |
| Day 28 | |||||
| CP, % | 72.9 | 70.57 | 73.79 | 0.83 | 0.06 |
| GE, % | 80.18 | 78.67 | 80.03 | 0.72 | 0.32 |
| DM, % | 80.03 | 78.65 | 78.78 | 0.67 | 0.33 |
| OM, % | 82.96 | 82.09 | 82.27 | 0.51 | 0.49 |
| Item 1 | CON | FMr | SPCr | SEM | p-Value |
|---|---|---|---|---|---|
| Jejunum | |||||
| Villus height, μm | 419 | 399 | 393 | 5.99 | 0.08 |
| Crypt depth, μm | 198 b | 224 a | 202 b | 3.64 | <0.01 |
| Villus height:crypt depth | 2.13 a | 1.78 b | 1.96 ab | 0.05 | 0.03 |
| Ileum | |||||
| Villus height, μm | 346 | 326 | 336 | 8.78 | 0.35 |
| Crypt depth, μm | 161 | 161 | 160 | 6.73 | 0.98 |
| Villus height:crypt depth | 2.15 | 2.02 | 2.12 | 0.12 | 0.75 |
| Items 1 | CON | FMr | SPCr | p-Value |
|---|---|---|---|---|
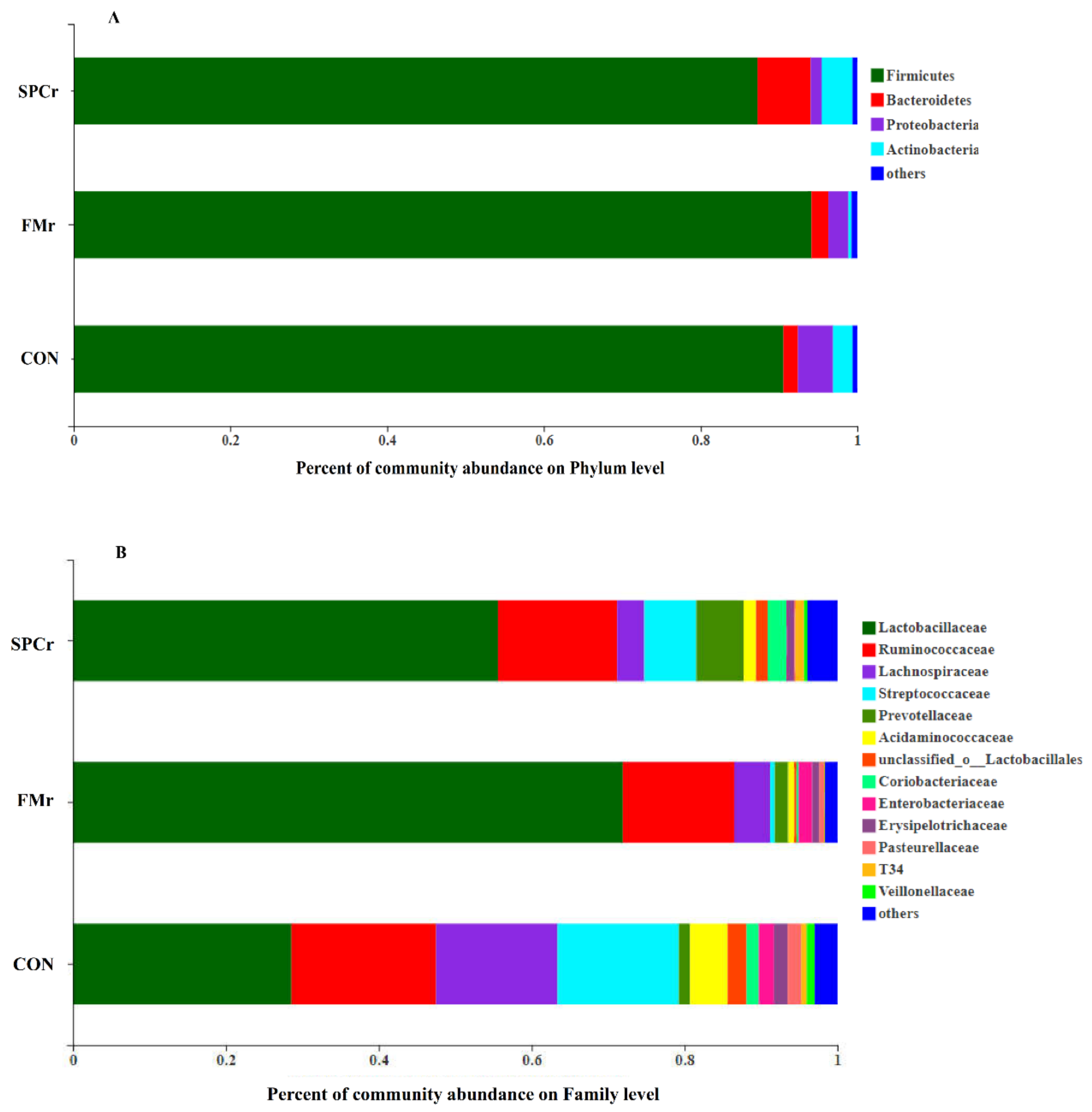
| Firmicutes, % | 90.53 ± 1.71 | 95.26 ± 2.54 | 86.13 ± 9.24 | 0.18 |
| Bacteroidetes, % | 1.86 ± 0.89 | 2.30 ± 1.45 | 6.64 ± 8.51 | 0.88 |
| Proteobacteria, % | 4.48 ± 3.55 | 0.72 ± 0.99 | 3.24 ± 3.58 | 0.30 |
| Actinobacteria, % | 2.51 ± 1.63 | 0.99 ± 0.99 | 3.31 ± 2.79 | 0.43 |
| Items 1, 2 | CON | FMr | SPCr | p-Value |
|---|---|---|---|---|
| Lactobacillaceae, % | 28.56 b ± 7.78 | 83.74 a ± 1.14 | 43.75 ab ± 16.09 | 0.04 |
| Ruminococcaceae, % | 18.93 ± 6.27 | 5.43 ± 1.58 | 24.75 ± 15.29 | 0.06 |
| Lachnospiraceae, % | 15.91 a ± 8.14 | 3.32 b ± 1.49 | 4.91 b ± 1.20 | 0.04 |
| Streptococcaceae, % | 15.85 a ± 6.00 | 0.41 b ± 0.42 | 7.02 ab ± 7.08 | 0.04 |
| Prevotellaceae, % | 1.48 ± 1.88 | 1.88 ± 1.21 | 6.09 ± 7.99 | 0.96 |
| Acidaminococcaceae, % | 4.89 ± 0.97 | 0.97 ± 0.66 | 1.38 ± 1.33 | 0.96 |
| unclassified_o_Lactobacillales, % | 2.48 ± 0.22 | 0.22 ± 0.23 | 1.67 ± 2.24 | 0.20 |
| Coriobacteriaceae, % | 1.63 ± 1.13 | 0.67 ± 0.69 | 2.07 ± 1.67 | 0.43 |
| Enterobacteriaceae, % | 1.96 ± 3.30 | 0.01 ± 0.02 | 1.80 ± 3.08 | 0.25 |
| Erysipelotrichaceae, % | 1.83 ± 0.57 | 0.32 ± 0.02 | 1.56 ± 0.90 | 0.06 |
| Pasteurellaceae, % | 1.77 ± 0.01 | 0.01 ± 0.01 | 0.81 ± 0.99 | 0.06 |
| T34, % | 0.71 ± 1.22 | 0.66 ± 0.99 | 0.50 ± 0.44 | 0.83 |
| Veillonellaceae, % | 1.06 ± 1.64 | 0.10 ± 0.11 | 0.38 ± 0.36 | 0.56 |
| Items 1 | CON | FMr | SPCr | SEM | p-Value |
|---|---|---|---|---|---|
| VFAs | |||||
| Acetate | 95.913 a | 88.601 b | 92.326 ab | 1.273 | 0.02 |
| Propionate | 61.080 b | 66.377 a | 64.940 ab | 0.940 | 0.02 |
| Butyrate | 11.166 c | 13.726 a | 12.523 b | 0.170 | <0.01 |
| Total VFAs | 168.159 | 168.704 | 169.789 | 0.159 | 0.89 |
| BCFAs | |||||
| Isobutyrate | 2.301 | 2.101 | 2.35 | 0.072 | 0.11 |
| Valerate | 5.015 | 5.023 | 5.155 | 0.109 | 0.62 |
| Isovalerate | 3.600 | 3.425 | 3.556 | 0.070 | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Gao, W.; Shi, H.; Hu, Q.; Lai, C. Effects of Replacing Fishmeal and Soybean Protein Concentrate with Degossypolized Cottonseed Protein in Diets on Growth Performance, Nutrient Digestibility, Intestinal Morphology, Cecum Microbiome and Fermentation of Weaned Piglets. Animals 2022, 12, 1667. https://doi.org/10.3390/ani12131667
Wang L, Gao W, Shi H, Hu Q, Lai C. Effects of Replacing Fishmeal and Soybean Protein Concentrate with Degossypolized Cottonseed Protein in Diets on Growth Performance, Nutrient Digestibility, Intestinal Morphology, Cecum Microbiome and Fermentation of Weaned Piglets. Animals. 2022; 12(13):1667. https://doi.org/10.3390/ani12131667
Chicago/Turabian StyleWang, Li, Wenjun Gao, Huangwei Shi, Qile Hu, and Changhua Lai. 2022. "Effects of Replacing Fishmeal and Soybean Protein Concentrate with Degossypolized Cottonseed Protein in Diets on Growth Performance, Nutrient Digestibility, Intestinal Morphology, Cecum Microbiome and Fermentation of Weaned Piglets" Animals 12, no. 13: 1667. https://doi.org/10.3390/ani12131667





