Distinct Amphibian Elevational and Seasonal Phylogenetic Structures Are Determined by Microhabitat Variables in Temperate Montane Streams
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Amphibian Sampling
2.3. Microhabitat Variables
2.4. Phylogenetic Tree
2.5. Statistical Analyses
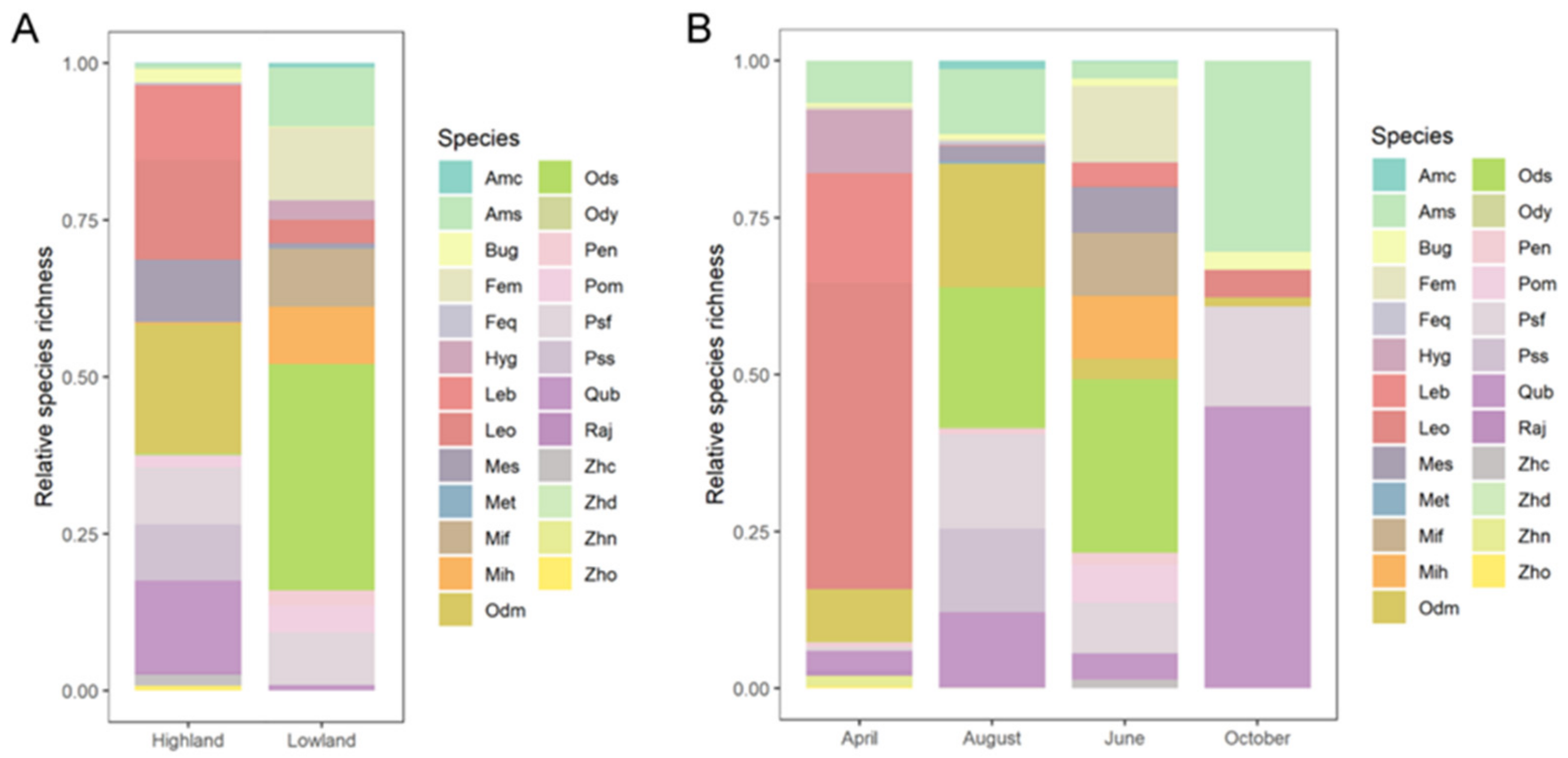
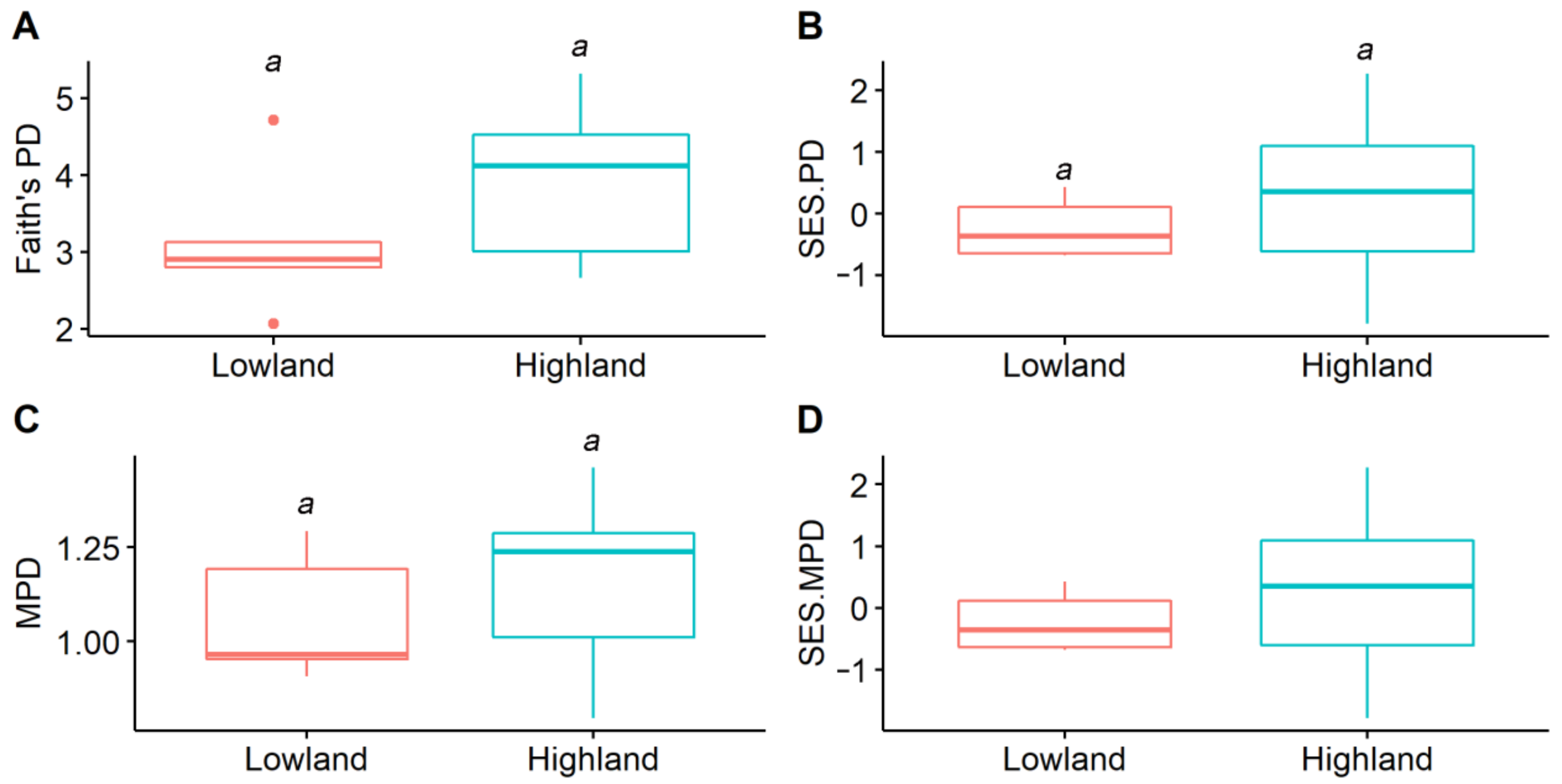
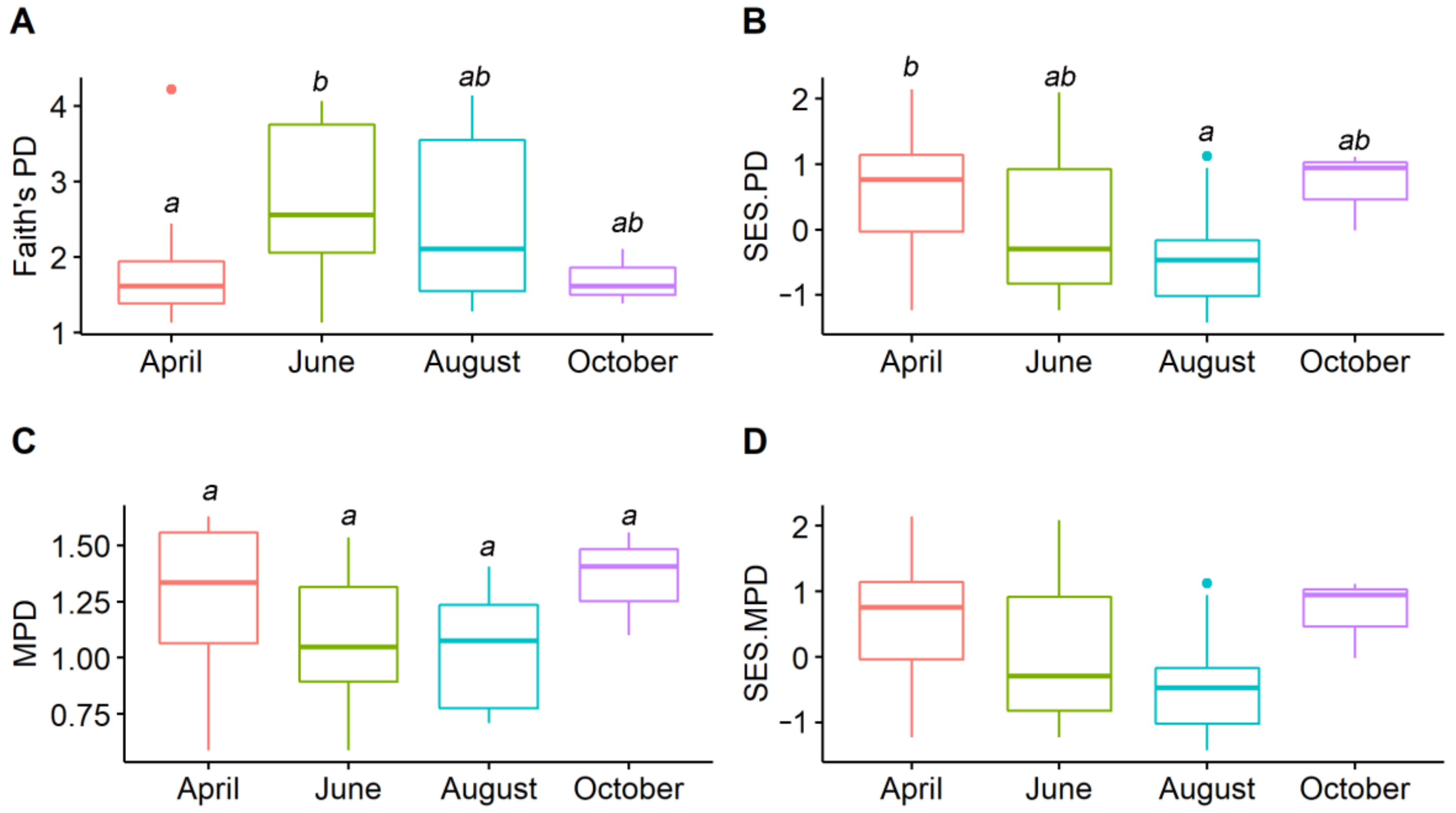
3. Results
4. Discussion
4.1. Elevational Spatial Difference in Amphibian Phylogenetic Structures
4.2. Seasonal Temporal Difference in Amphibian Phylogenetic Structures
4.3. Microhabitat Determinants of Amphibian Phylogenetic Structures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity Loss and Its Impact on Humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Lücking, R. Three Challenges to Contemporaneous Taxonomy from a Licheno-Mycological Perspective. Megataxa 2020, 1, 78–103. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Wang, B.; Shu, G.; Li, C.; Jiang, J. Amphibian Species Contribute Similarly to Taxonomic, but Not Functional and Phylogenetic Diversity: Inferences from Amphibian Biodiversity on Emei Mountain. Asian Herpetol. Res. 2018, 9, 110–118. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Jonathan Davies, T.; Regetz, J.; Kembel, S.W.; Cleland, E.; Oakley, T.H. Phylogenetic Diversity Metrics for Ecological Communities: Integrating Species Richness, Abundance and Evolutionary History. Ecol. Lett. 2010, 13, 96–105. [Google Scholar] [CrossRef]
- Faith, D.P. Conservation Evaluation and Phylogenetic Diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Redding, D.W.; Mooers, A.Ø. Incorporating Evolutionary Measures into Conservation Prioritization. Conserv. Biol. 2006, 20, 1670–1678. [Google Scholar] [CrossRef]
- Forest, F.; Grenyer, R.; Rouget, M.; Davies, T.J.; Cowling, R.M.; Faith, D.P.; Balmford, A.; Manning, J.C.; Procheş, Ş.; van der Bank, M.; et al. Preserving the Evolutionary Potential of Floras in Biodiversity Hotspots. Nature 2007, 445, 757–760. [Google Scholar] [CrossRef]
- Isaac, N.J.B.; Turvey, S.T.; Collen, B.; Waterman, C.; Baillie, J.E.M. Mammals on the EDGE: Conservation Priorities Based on Threat and Phylogeny. PLoS ONE 2007, 2, e296. [Google Scholar] [CrossRef] [Green Version]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and Community Ecology. Annu. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef] [Green Version]
- Webb, C.O.; Losos, J.B.; Agrawal, A.A. Integrating Phylogenies into Community Ecology. Ecology 2006, 87, S1–S2. [Google Scholar] [CrossRef]
- Silvertown, J.; Dodd, M.; Gowing, D.; Lawson, C.; McConway, K. Phylogeny and the Hierarchical Organization of Plant Diversity. Ecology 2006, 87, S39–S49. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Zhong, M.-J.; Zhang, J.; Si, X.-F.; Yang, S.-N.; Jiang, J.-P.; Hu, J.-H. Multidimensional Amphibian Diversity and Community Structure along a 2600 m Elevational Gradient on the Eastern Margin of the Qinghai-Tibetan Plateau. Zool. Res. 2022, 43, 40–51. [Google Scholar] [CrossRef]
- Hu, Y.; Fan, H.; Chen, Y.; Chang, J.; Zhan, X.; Wu, H.; Zhang, B.; Wang, M.; Zhang, W.; Yang, L.; et al. Spatial Patterns and Conservation of Genetic and Phylogenetic Diversity of Wildlife in China. Sci. Adv. 2021, 7, eabd5725. [Google Scholar] [CrossRef]
- Roelants, K.; Gower, D.J.; Wilkinson, M.; Loader, S.P.; Biju, S.D.; Guillaume, K.; Moriau, L.; Bossuyt, F. Global Patterns of Diversification in the History of Modern Amphibians. Proc. Natl. Acad. Sci. USA 2007, 104, 887–892. [Google Scholar] [CrossRef] [Green Version]
- Strauß, A.; Guilhaumon, F.; Randrianiaina, R.D.; Wollenberg Valero, K.C.; Vences, M.; Glos, J. Opposing Patterns of Seasonal Change in Functional and Phylogenetic Diversity of Tadpole Assemblages. PLoS ONE 2016, 11, e0151744. [Google Scholar] [CrossRef]
- Zhu, W.-B.; Zhao, C.-L.; Liao, C.-L.; Zou, Z.; Xu, D.; Zhu, W.; Zhao, T.; Jiang, J. Spatial and Temporal Patterns of Amphibian Species Richness on Tianping Mountain, Hunan Province, China. Zool. Res. 2020, 41, 182–187. [Google Scholar] [CrossRef]
- Da Silva, F.R.; Almeida-Neto, M.; do Prado, V.H.M.; Haddad, C.F.B.; de Cerqueira Rossa-Feres, D. Humidity Levels Drive Reproductive Modes and Phylogenetic Diversity of Amphibians in the Brazilian Atlantic Forest. J. Biogeogr. 2012, 39, 1720–1732. [Google Scholar] [CrossRef]
- Pyron, R.A.; Wiens, J.J. Large-Scale Phylogenetic Analyses Reveal the Causes of High Tropical Amphibian Diversity. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131622. [Google Scholar] [CrossRef] [Green Version]
- Khatiwada, J.R.; Zhao, T.; Chen, Y.; Wang, B.; Xie, F.; Cannatella, D.C.; Jiang, J. Amphibian Community Structure along Elevation Gradients in Eastern Nepal Himalaya. BMC Ecol. 2019, 19, 19. [Google Scholar] [CrossRef] [Green Version]
- Xiong, E.; Zhu, K.; You, L.; Wang, Y.; Fu, P.; Gu, R.; Xiao, Z. Investigation on Butterflies in Sangzhi County and Tianping Mountain Natural Preservation Region. J. Hunan Agric. Univ. 1999, 25, 312–317. [Google Scholar]
- Sun, Z.-J.; Zhu, W.; Zhu, W.-B.; Zhao, C.-L.; Liao, C.-L.; Zou, B.; Xu, D.; Fan, W.-B.; Su, S.-Q.; Jiang, J.-P.; et al. Spatiotemporal Patterns of Anuran Functional Diversity in Temperate Montane Forests. Zool. Res. 2021, 42, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Grundel, R.; Beamer, D.A.; Glowacki, G.A.; Frohnapple, K.J.; Pavlovic, N.B. Opposing Responses to Ecological Gradients Structure Amphibian and Reptile Communities across a Temperate Grassland–Savanna–Forest Landscape. Biodivers. Conserv. 2015, 24, 1089–1108. [Google Scholar] [CrossRef]
- Khatiwada, J.R.; Shu, G.; Wang, B.; Zhao, T.; Xie, F.; Jiang, J. Description of a New Species of Amolops Cope, 1865 (Amphibia: Ranidae) from Nepal and Nomenclatural Validation of Amolops Nepalicus Yang, 1991. Asian Herpetol. Res. 2020, 11, 71–94. [Google Scholar] [CrossRef]
- Ding, Z.; Hu, H.; Cadotte, M.W.; Liang, J.; Hu, Y.; Si, X. Elevational Patterns of Bird Functional and Phylogenetic Structure in the Central Himalaya. Ecography 2021, 44, 1403–1417. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, G.; Ci, X.; Swenson, N.G.; Cao, M.; Sha, L.; Li, J.; Baskin, C.C.; Slik, J.W.F.; Lin, L. Functional and Phylogenetic Assembly in a Chinese Tropical Tree Community across Size Classes, Spatial Scales and Habitats. Funct. Ecol. 2014, 28, 520–529. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R.; Huyvaert, K.P. AIC Model Selection and Multimodel Inference in Behavioral Ecology: Some Background, Observations, and Comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-Project.Org/ (accessed on 12 January 2022).
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New Multidimensional Functional Diversity Indices for a Multifaceted Framework in Functional Ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [Green Version]
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research [R Package Psych Version 2.2.5]. 2022. Available online: https://cran.r-project.org/web/packages/psych/index.html (accessed on 1 May 2022).
- Pohlert, T. The Pairwise Multiple Comparison of Mean Ranks Package (PMCMR) [R Package PMCMR Version 4.4]. 2021. Available online: https://cran.r-project.org/web/packages/PMCMR/index.html (accessed on 1 May 2022).
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Walsh, C.J.; Papas, P.J.; Crowther, D.; Sim, P.T.; Yoo, J. Stormwater Drainage Pipes as a Threat to a Stream-Dwelling Amphipod of Conservation Significance, Austrogammarus Australis, in Southeastern Australia. Biodivers. Conserv. 2004, 13, 781–793. [Google Scholar] [CrossRef]
- Khatiwada, J.R.; Zhao, T.; Jiang, J. Variation of Body Temperature of Active Amphibians along Elevation Gradients in Eastern Nepal Himalaya. J. Therm. Biol. 2020, 92, 102653. [Google Scholar] [CrossRef]
- Dodd, C.K. Amphibian Ecology and Conservation: A Handbook of Techniques; Oxford University Press: New York, NY, USA, 2010. [Google Scholar]
- Fei, L.; Hu, S.; Ye, C.; Tian, W.; Jiang, J.; Wu, G.; Li, J.; Wang, Y. Fauna Sinica, Amphibia, Vol.2, Anura; Science Press: Beijing, China, 2009. [Google Scholar]
- Fei, L.; Ye, C.; Jiang, J. Colored Atlas of Chinese Amphibians and Their Distributions; Sichuan Publishing House of Science & Technology: Chengdu, China, 2012. [Google Scholar]
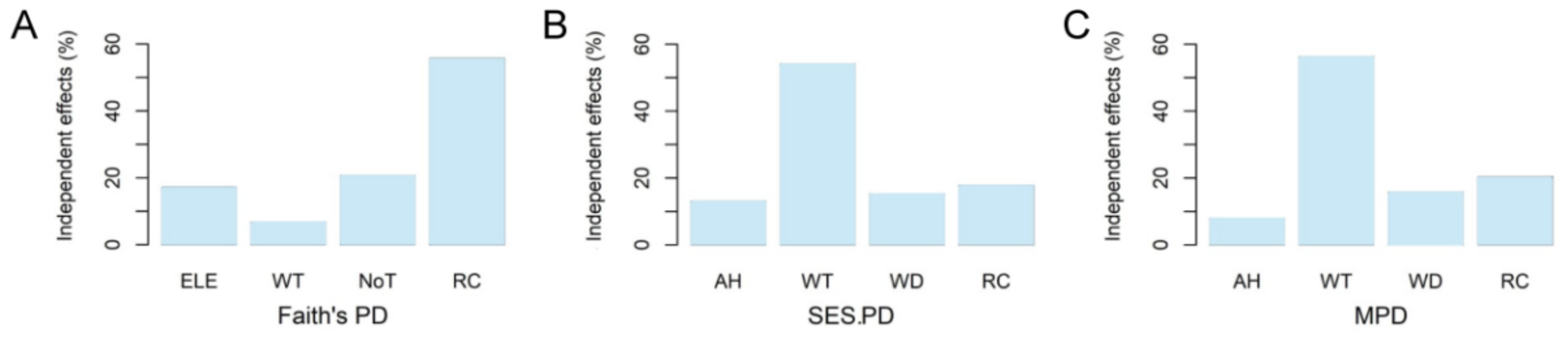
| Indices | Intercept | ELE | AT | AH | WT | pH | WD | NoT | LLC | RC | CV | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Faith’s PD | Estimate | −0.029 | <0.001 | / | / | 0.065 | / | / | 0.003 | / | 0.019 | / |
| Std.Error | 1.037 | <0.001 | / | / | 0.046 | / | / | 0.002 | / | 0.005 | / | |
| p | 0.978 | 0.125 | / | / | 0.165 | / | / | 0.155 | / | <0.001 | / | |
| SES.PD | Estimate | 4.293 | / | / | −0.019 | −0.148 | / | −0.009 | / | / | 0.007 | / |
| Std.Error | 0.980 | / | / | 0.010 | 0.031 | / | 0.003 | / | / | 0.004 | / | |
| p | <0.001 | / | / | 0.052 | <0.001 | / | 0.007 | / | / | 0.139 | / | |
| MPD | Estimate | 2.182 | / | / | −0.003 | −0.043 | / | −0.003 | / | / | 0.002 | / |
| Std.Error | 0.277 | / | / | 0.003 | 0.009 | / | <0.001 | / | / | 0.002 | / | |
| p | <0.001 | / | / | 0.178 | <0.001 | / | 0.005 | / | / | 0.071 | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.-W.; Lan, J.; Sun, Z.-J.; Zhu, W.-B.; Zhao, T. Distinct Amphibian Elevational and Seasonal Phylogenetic Structures Are Determined by Microhabitat Variables in Temperate Montane Streams. Animals 2022, 12, 1673. https://doi.org/10.3390/ani12131673
Peng X-W, Lan J, Sun Z-J, Zhu W-B, Zhao T. Distinct Amphibian Elevational and Seasonal Phylogenetic Structures Are Determined by Microhabitat Variables in Temperate Montane Streams. Animals. 2022; 12(13):1673. https://doi.org/10.3390/ani12131673
Chicago/Turabian StylePeng, Xi-Wen, Jing Lan, Zi-Jian Sun, Wen-Bo Zhu, and Tian Zhao. 2022. "Distinct Amphibian Elevational and Seasonal Phylogenetic Structures Are Determined by Microhabitat Variables in Temperate Montane Streams" Animals 12, no. 13: 1673. https://doi.org/10.3390/ani12131673






