Comparative Transcriptome Analysis Provides Novel Insights into the Effect of Lipid Metabolism on Laying of Geese
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Histological Observation
2.3. RNA-Seq and Bioinformatics Analysis
2.4. Quantitative Real-Time PCR Validation
2.5. Statistical Analysis
3. Results
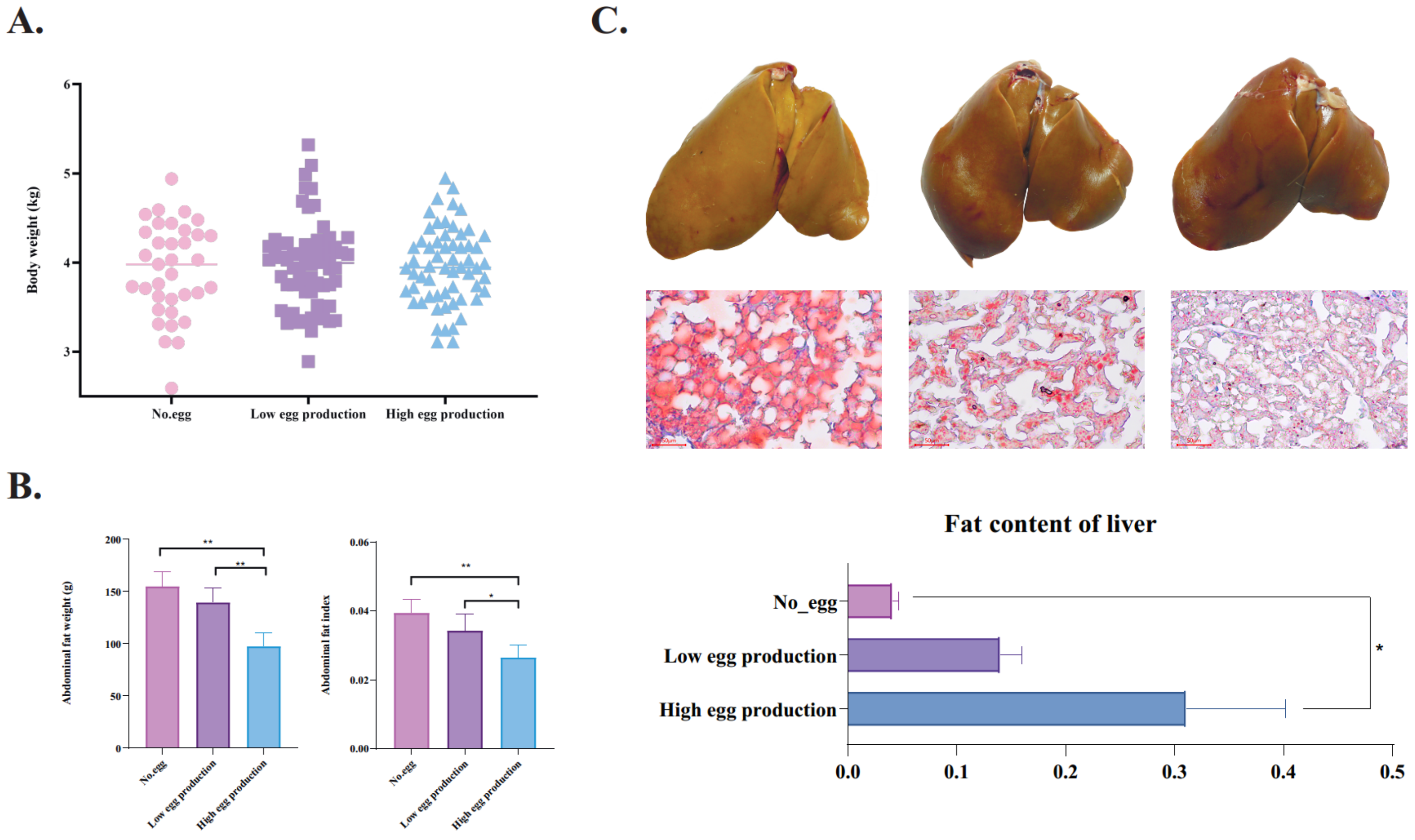
3.1. The Lipid Deposition Patterns of Geese with Different Laying Performance
3.2. Overview of the mRNA Transcriptome with Different Egg Production Performance Geese
3.3. Functional Analysis of DEGs among Different Egg Production Performances in Abdominal Fat, Liver, and Ovarian Stroma
3.4. Network Construction of Liver, Abdominal Fat, and Ovarian Stroma Regulating Laying Performance in Geese
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, S.; Gan, X.; He, H.; Hu, S.; Deng, Y.; Chen, X.; Li, L.; Hu, J.; Li, L.; Wang, J. Dynamic characteristics of lipid metabolism in cultured granulosa cells from geese follicles at different developmental stages. Biosci. Rep. 2019, 39, BSR20192188. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hu, S.; Wang, Y.; Deng, Y.; Yang, S.; Hu, J.; Li, L.; Wang, J. mRNA and miRNA Transcriptome Profiling of Granulosa and Theca Layers From Geese Ovarian Follicles Reveals the Crucial Pathways and Interaction Networks for Regulation of Follicle Selection. Front. Genet. 2019, 10, 988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, R.; Gan, X.; Hu, S.; Gao, S.; Deng, Y.; Qiu, J.; Sun, W.; Li, L.; Han, C.; Hu, J.; et al. Evidence for the existence of de novo lipogenesis in goose granulosa cells. Poult. Sci. 2019, 98, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Hermier, D. Lipoprotein metabolism and fattening in poultry. J. Nutr. 1997, 127, 805s–808s. [Google Scholar] [CrossRef]
- Wang, G.; Jin, L.; Li, Y.; Tang, Q.; Hu, S.; Xu, H.; Gill, C.A.; Li, M.; Wang, J. Transcriptomic analysis between Normal and high-intake feeding geese provides insight into adipose deposition and susceptibility to fatty liver in migratory birds. BMC Genom. 2019, 20, 372. [Google Scholar] [CrossRef] [Green Version]
- Milisits, G.; Szentirmai, E.; Donko, T.; Budai, Z.; Sütő, Z. Effect of starting body fat content and genotype of laying hens on the changes in their live weight, body fat content, egg production and egg composition during the first egg-laying period. Br. Poult. Sci. 2015, 56, 666–672. [Google Scholar] [CrossRef]
- Bornstein, S.; Plavnik, I.; Lev, Y. Body weight and/or fatness as potential determinants of the onset of egg production in broiler breeder hens. Br. Poult. Sci. 1984, 25, 323–341. [Google Scholar] [CrossRef]
- Wei, Z.; Li, P.; Huang, S.; Lkhagvagarav, P.; Zhu, M.; Liang, C.; Jia, C. Identification of key genes and molecular mechanisms associated with low egg production of broiler breeder hens in ad libitum. BMC Genom. 2019, 20, 408. [Google Scholar] [CrossRef]
- Ouyang, Q.; Hu, S.; Wang, G.; Hu, J.; Zhang, J.; Li, L.; Hu, B.; He, H.; Liu, H.; Xia, L.; et al. Comparative Transcriptome Analysis Suggests Key Roles for 5-Hydroxytryptamlne Receptors in Control of Goose Egg Production. Genes 2020, 11, 455. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [Green Version]
- Liang, M.J.; Wang, Z.P.; Xu, L.; Leng, L.; Li, H. Estimating the genetic parameters for liver fat traits in broiler lines divergently selected for abdominal fat. Genet. Mol. Res. 2015, 14, 9646–9654. [Google Scholar] [CrossRef]
- Leng, L.; Zhang, H.; Dong, J.Q.; Wang, Z.P.; Zhang, X.Y.; Wang, S.Z.; Cao, Z.P.; Li, Y.M.; Li, H. Selection against abdominal fat percentage may increase intramuscular fat content in broilers. J. Anim. Breed. Genet. 2016, 135, 422–428. [Google Scholar] [CrossRef]
- Lu, L.; Chen, Y.; Wang, Z.; Li, X.; Chen, W.; Tao, Z.; Shen, J.; Tian, Y.; Wang, D.; Li, G. The goose genome sequence leads to insights into the evolution of waterfowl and susceptibility to fatty liver. Genome Biol. 2015, 16, 89. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.Y.; Wu, M.Q.; Wang, S.Z.; Zhang, H.; Du, Z.Q.; Li, Y.M.; Cao, Z.P.; Luan, P.; Leng, L.; Li, H. Genetic selection on abdominal fat content alters the reproductive performance of broilers. Anim. Int. J. Anim. Biosci. 2017, 12, 1232–1241. [Google Scholar] [CrossRef]
- Pan, Y.E.; Liu, Z.C.; Chang, C.J.; Huang, Y.F.; Lai, C.Y.; Walzem, R.L.; Chen, S.E. Feed restriction ameliorates metabolic dysregulation and improves reproductive performance of meat-type country chickens. Anim. Reprod. Sci. 2014, 151, 229–236. [Google Scholar] [CrossRef]
- Sauerwein, H.; Bendixen, E.; Restelli, L.; Ceciliani, F. The adipose tissue in farm animals: A proteomic approach. Curr. Protein Pept. Sci. 2014, 15, 146–155. [Google Scholar] [CrossRef]
- Gloux, A.; Duclos, M.J.; Brionne, A.; Bourin, M.; Nys, Y.; Réhault-Godbert, S. Integrative analysis of transcriptomic data related to the liver of laying hens: From physiological basics to newly identified functions. BMC Genom. 2019, 20, 821. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Kim, W.K.; Cline, M.A.; Gilbert, E.R. Factors affecting adipose tissue development in chickens: A review. Poult. Sci. 2017, 96, 3687–3699. [Google Scholar] [CrossRef]
- Zaefarian, F.; Abdollahi, M.R.; Cowieson, A.; Ravindran, V. Avian Liver: The Forgotten Organ. Animals 2019, 9, 63. [Google Scholar] [CrossRef] [Green Version]
- Loh, D.H.; Kuljis, D.A.; Azuma, L.; Wu, Y.; Truong, D.; Wang, H.B.; Colwell, C.S. Disrupted reproduction, estrous cycle, and circadian rhythms in female mice deficient in vasoactive intestinal peptide. J. Biol. Rhythm. 2014, 29, 355–369. [Google Scholar] [CrossRef] [Green Version]
- Di Cara, F.; King-Jones, K. The Circadian Clock Is a Key Driver of Steroid Hormone Production in Drosophila. Curr. Biol. 2016, 26, 2469–2477. [Google Scholar] [CrossRef] [Green Version]
- Tao, Z.; Song, W.; Zhu, C.; Xu, W.; Liu, H.; Zhang, S.; Huifang, L. Comparative transcriptomic analysis of high and low egg-producing duck ovaries. Poult. Sci. 2017, 96, 4378–4388. [Google Scholar] [CrossRef]
- Panda, S.; Antoch, M.P.; Miller, B.H.; Su, A.I.; Schook, A.B.; Straume, M.; Schultz, P.G.; Kay, S.A.; Takahashi, J.S.; Hogenesch, J.B. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 2002, 109, 307–320. [Google Scholar] [CrossRef] [Green Version]
- Storch, K.F.; Lipan, O.; Leykin, I.; Viswanathan, N.; Davis, F.C.; Wong, W.H.; Weitz, C.J. Extensive and divergent circadian gene expression in liver and heart. Nature 2002, 417, 78–83. [Google Scholar] [CrossRef]
- Reinke, H.; Asher, G. Circadian Clock Control of Liver Metabolic Functions. Gastroenterology 2016, 150, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.M.; Zhang, Y.; Tsuchiya, H.; Smalling, R.; Jetten, A.M.; Wang, L. Small heterodimer partner/neuronal PAS domain protein 2 axis regulates the oscillation of liver lipid metabolism. Hepatology 2015, 61, 497–505. [Google Scholar] [CrossRef] [Green Version]
- Dudley, C.A.; Erbel-Sieler, C.; Estill, S.J.; Reick, M.; Franken, P.; Pitts, S.; McKnight, S.L. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science 2003, 301, 379–383. [Google Scholar] [CrossRef]
- Hojo, H.; Enya, S.; Arai, M.; Suzuki, Y.; Nojiri, T.; Kangawa, K.; Koyama, S.; Kawaoka, S. Remote reprogramming of hepatic circadian transcriptome by breast cancer. Oncotarget 2017, 8, 34128–34140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Veen, D.R.; Laing, E.E.; Bae, S.E.; Johnston, J.D.; Dijk, D.J.; Archer, S.N. A Topological Cluster of Differentially Regulated Genes in Mice Lacking PER3. Front. Mol. Neurosci. 2020, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Crumbley, C.; Wang, Y.; Kojetin, D.J.; Burris, T.P. Characterization of the core mammalian clock component, NPAS2, as a REV-ERBalpha/RORalpha target gene. J. Biol. Chem. 2010, 285, 35386–35392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izaola, O.; de Luis, D.; Sajoux, I.; Domingo, J.C.; Vidal, M. Inflammation and obesity (lipoinflammation). Nutr. Hosp. 2015, 31, 2352–2358. [Google Scholar] [CrossRef] [Green Version]
- Karczewski, J.; Śledzińska, E.; Baturo, A.; Jończyk, I.; Maleszko, A.; Samborski, P.; Begier-Krasińska, B.; Dobrowolska, A. Obesity and inflammation. Eur. Cytokine Netw. 2018, 29, 83–94. [Google Scholar] [CrossRef]
- Mattina, G.F.; van Lieshout, R.J.; Steiner, M. Inflammation, depression and cardiovascular disease in women: The role of the immune system across critical reproductive events. Ther. Adv. Cardiovasc. Dis. 2019, 13, 1753944719851950. [Google Scholar] [CrossRef]
- Frigolet, M.E.; Gutiérrez-Aguilar, R. The colors of adipose tissue. Gac. Med. Mex. 2020, 156, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Lou, Y.; Zhao, A. Transcriptome analysis of follicles reveals the importance of autophagy and hormones in regulating broodiness of Zhedong white goose. Sci. Rep. 2016, 6, 36877. [Google Scholar] [CrossRef] [Green Version]
- Brady, K.; Liu, H.C.; Hicks, J.A.; Long, J.A.; Porter, T.E. Transcriptome Analysis During Follicle Development in Turkey Hens with Low and High Egg Production. Front. Genet. 2021, 12, 619196. [Google Scholar] [CrossRef]
- Zhu, G.; Mao, Y.; Zhou, W.; Jiang, Y. Dynamic Changes in the Follicular Transcriptome and Promoter DNA Methylation Pattern of Steroidogenic Genes in Chicken Follicles throughout the Ovulation Cycle. PLoS ONE 2015, 10, e0146028. [Google Scholar] [CrossRef]



| Primer Name | Sequence (5′–3′) | Product Length (bp) |
|---|---|---|
| GAPDH-F | GCTGATGCTCCCATGTTCGTGAT | 86 |
| GAPDH-R | GTGGTGCAAGAGGCATTGCTGAC | |
| β-ACTIN-F | CAACGAGCGGTTCAGGTGT | 92 |
| β-ACTIN-R | TGGAGTTGAAGGTGGTCTCGT | |
| RORG-F | TGTGCCAGAACGACCAGAT | 102 |
| RORG-R | AGAGGACGGTCCGGTTGT | |
| PER3-F | GAGCAGTGCCTTTGTTGGGT | 276 |
| PER3-R | TCAGAGGGCTTGTTCGGACT | |
| NPAS2-F | TCACAGAGCACCACCGATTA | 148 |
| NPAS2-R | ATAGCAACACGACTTCCCCT | |
| NR1H4-F | GCCTCAGATTTCATCGCCAC | 228 |
| NR1H4-R | GCTTTGTCACCACAGACCACG | |
| LCAT-F | CAGCGTGTCTTCCTCATTGC | 187 |
| LCAT-R | ACATAAGTGGGATGCCCTGAT | |
| DGAT1-F | GCCTACCCCGACAACCTCAC | 180 |
| DGAT1-R | CACCATCCACTGCTGGATCA | |
| IL8-F | CCTGGTAAGGATGGGAAACG | 168 |
| IL8-R | GGGTCCAAGCACACCTCTCT | |
| CCL4-F | ATGAAGGTCTCTGTGGCTGC | 119 |
| CCL4-R | TCCCGTTGGATGTAGGTGAA | |
| DGAT2-F | ACCCACAATCTGCTGACCAC | 239 |
| DGAT2-R | GATAAGATGTAGTCTATGCTGTCGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, Q.; Hu, S.; Tang, B.; Hu, B.; Hu, J.; He, H.; Li, L.; Wang, J. Comparative Transcriptome Analysis Provides Novel Insights into the Effect of Lipid Metabolism on Laying of Geese. Animals 2022, 12, 1775. https://doi.org/10.3390/ani12141775
Ouyang Q, Hu S, Tang B, Hu B, Hu J, He H, Li L, Wang J. Comparative Transcriptome Analysis Provides Novel Insights into the Effect of Lipid Metabolism on Laying of Geese. Animals. 2022; 12(14):1775. https://doi.org/10.3390/ani12141775
Chicago/Turabian StyleOuyang, Qingyuan, Shenqiang Hu, Bincheng Tang, Bo Hu, Jiwei Hu, Hua He, Liang Li, and Jiwen Wang. 2022. "Comparative Transcriptome Analysis Provides Novel Insights into the Effect of Lipid Metabolism on Laying of Geese" Animals 12, no. 14: 1775. https://doi.org/10.3390/ani12141775





