Visual Responses to Moving and Flashed Stimuli of Neurons in Domestic Pigeon (Columba livia domestica) Optic Tectum
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
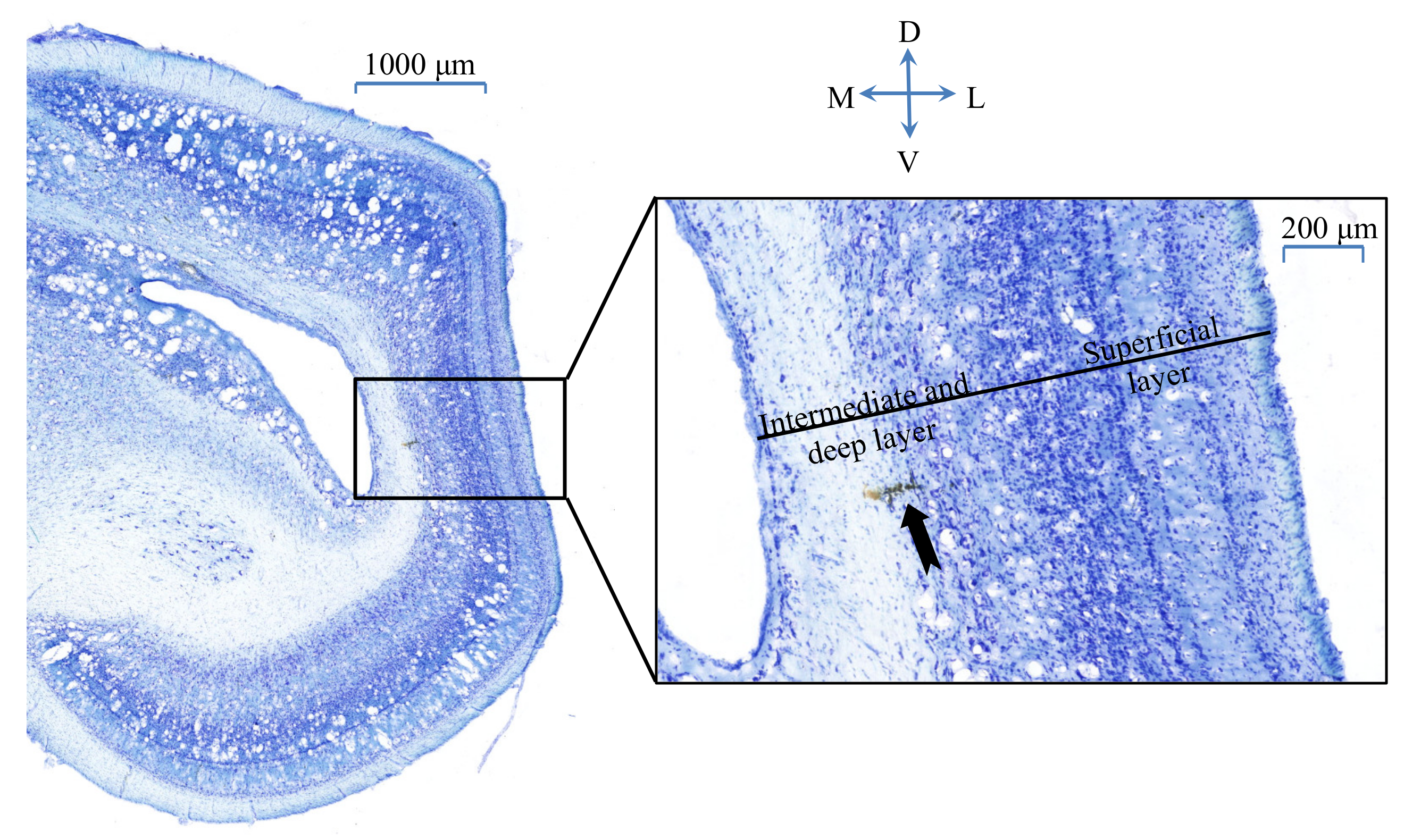
2.1. Animal Preparation
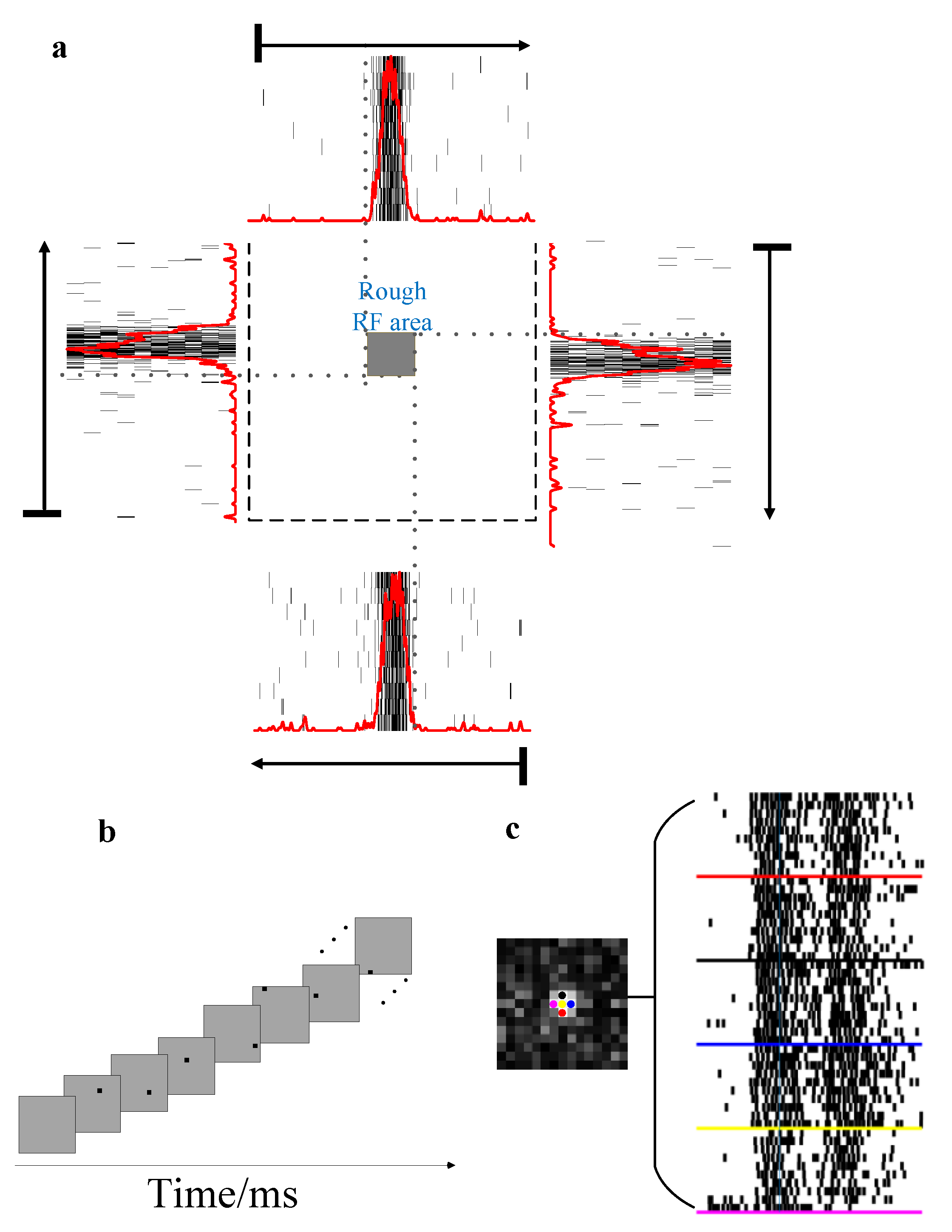
2.2. Visual Stimulation and Electrophysiological Recordings
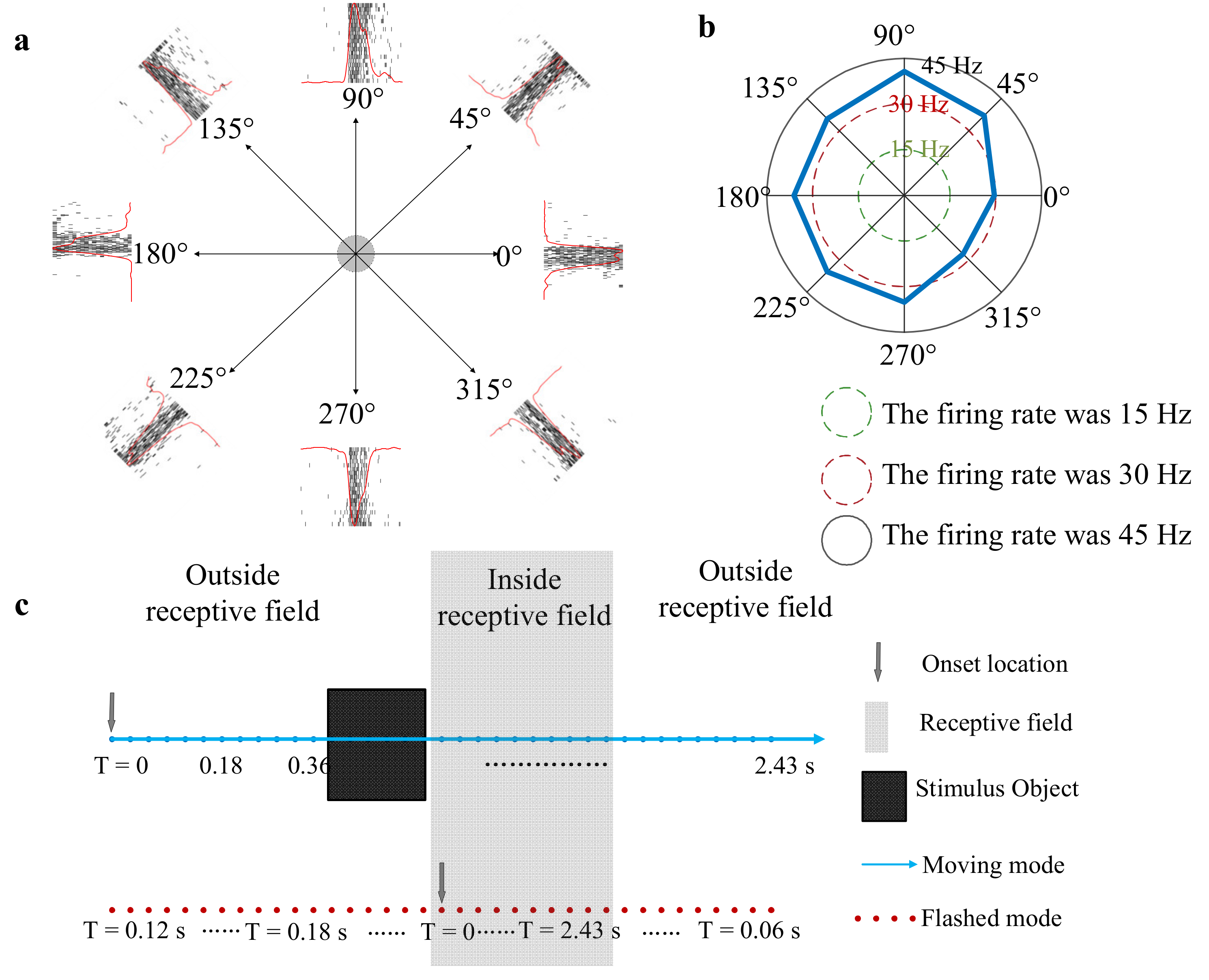
- Stimulus A: A square (square: 0.1 cd/m2, background: 40 cd/m2) moved from one side to the other side across the RF area at 10°/s in eight directions (spaced by 45° with nasal 0°, Figure 4a) in a pseudo-random sequence (20 times/direction) to determine the preferred direction of a given unit. The length of the square side was equal to the radius of the measured RF and the inter-trial interval was 80 ms.
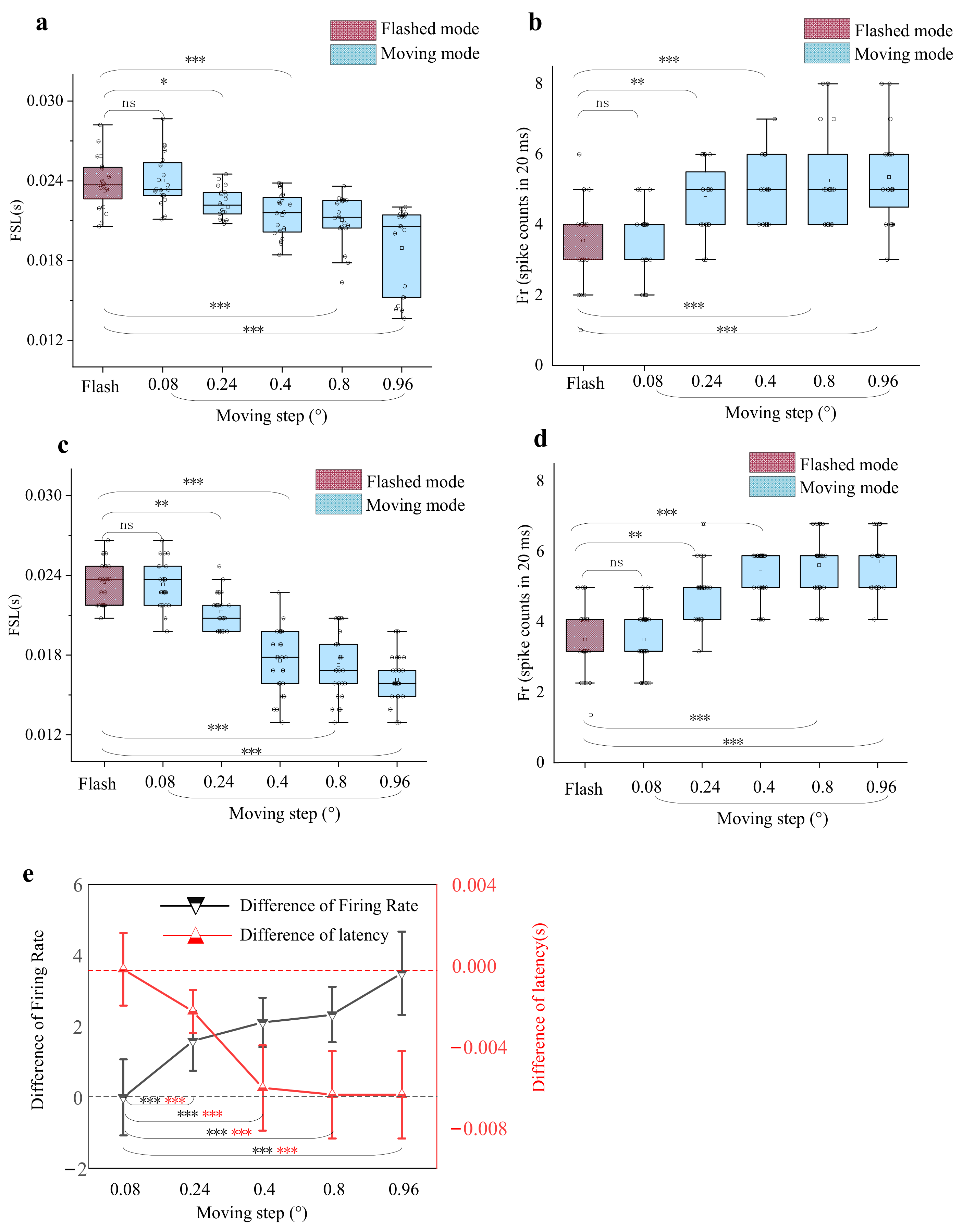
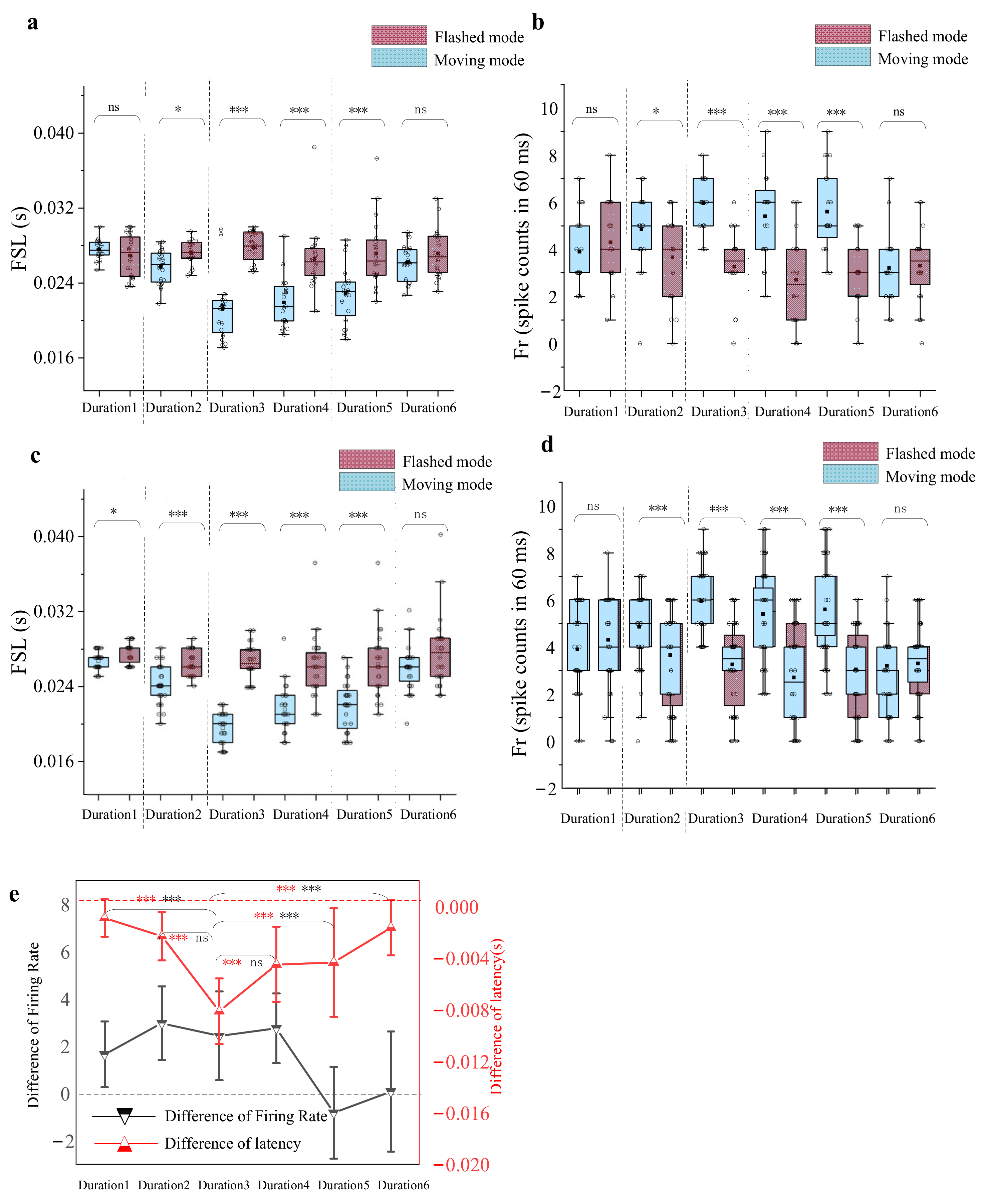
- Stimulus B: A moving square (the same size as the square in stimulus (A) was presented on a gray background (40 cd/m2) (a line of blue dots in Figure 4c). The square luminance was 0.1 cd/m2. The length of the path (the length is three times as long as the length of the measured RF) remains consistent in the experiment. In the moving condition, a square was presented at random speeds in a specific direction (direction was determined by stimulus (A)). After one motion period finished, there was a 100 ms gray blank (40 cd/m2) followed to avoid adaptation [25]. The midpoint of the moving square’s trajectory was at the receptive field center. Furthermore, the moving speed was manipulated by the moving step and stimulus durations (time length of a single stimulus staying at one location). The moving step manipulation experiment and stimulus duration manipulation experiment were designed as follows. In the moving step manipulation experiment, the square was presented for two video frames (stimulus duration at one location: 20 ms) at each location, but the moving step length (the distance between adjacent blue dots in Figure 4c) was set to 0.08°, 0.24°, 0.4°, 0.8° and 0.96° (corresponding to speeds of 4°/s, 12°/s, 20°/s, 40°/s and 48°/s, respectively). Since the length of the total path remains constant, the duration of one motion period decreased as the speed increases. In the stimulus duration manipulation experiment, the moving step length was fixed at 0.24°, and the time length of stimulus presentation on each location (hereinafter referred to as the stimulus duration) was set to 10 ms, 20 ms, 30 ms, 40 ms, 50 ms and 60 ms, corresponding to speeds of 24°/s, 12°/s, 8°/s, 6°/s, 4.8°/s and 4°/s, respectively.
- Stimulus C: A flashed square, which was the same size as the moving square, was presented in the same position in a pseudo-random sequence (20 times/position) that matched the moving square’s trajectory (a line of the red dot in Figure 4c). The time of a single flash at one position is constant with the time of a single square at one motion location. In the above motion paradigm, since motion is a continuous process, the square sequentially crossed different parts of the RF without any gray blank, which was always used to avoid adaptation, between adjacent locations. To keep stimulus conditions constant, there was also no gray blank between each two adjacent flashing squares in the flashed paradigm.
2.3. Data Analysis
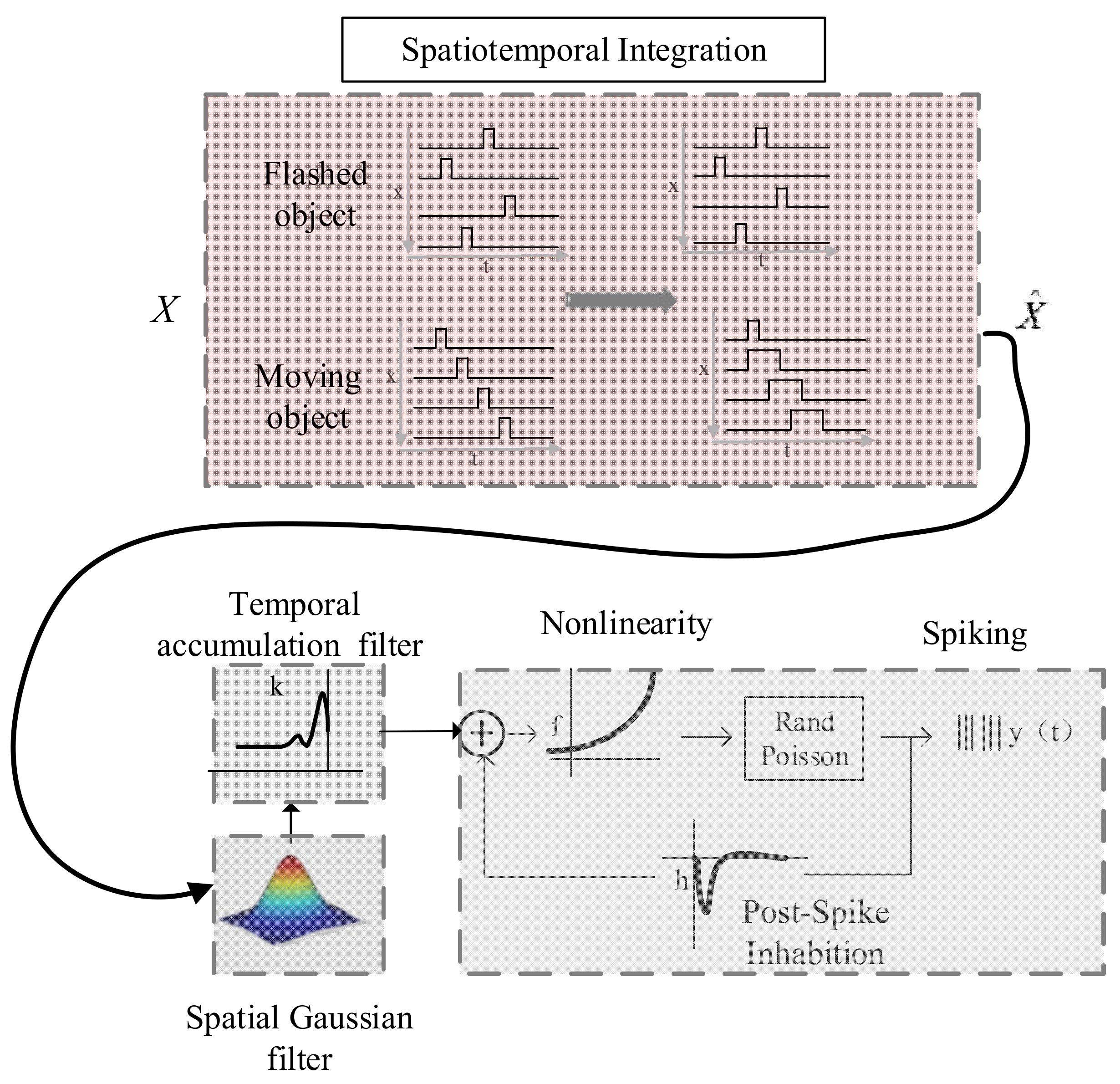
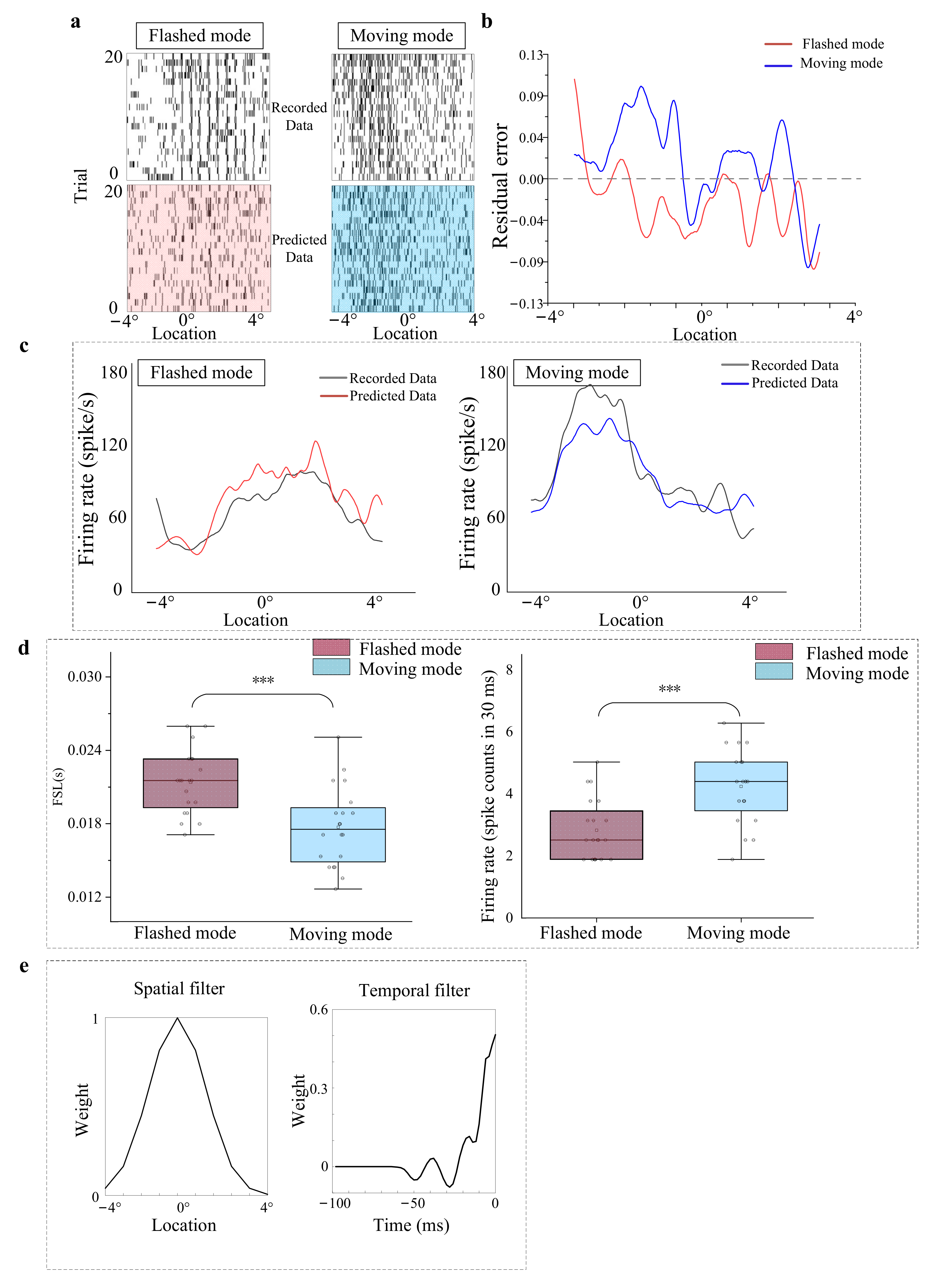
2.4. Modeling
3. Results
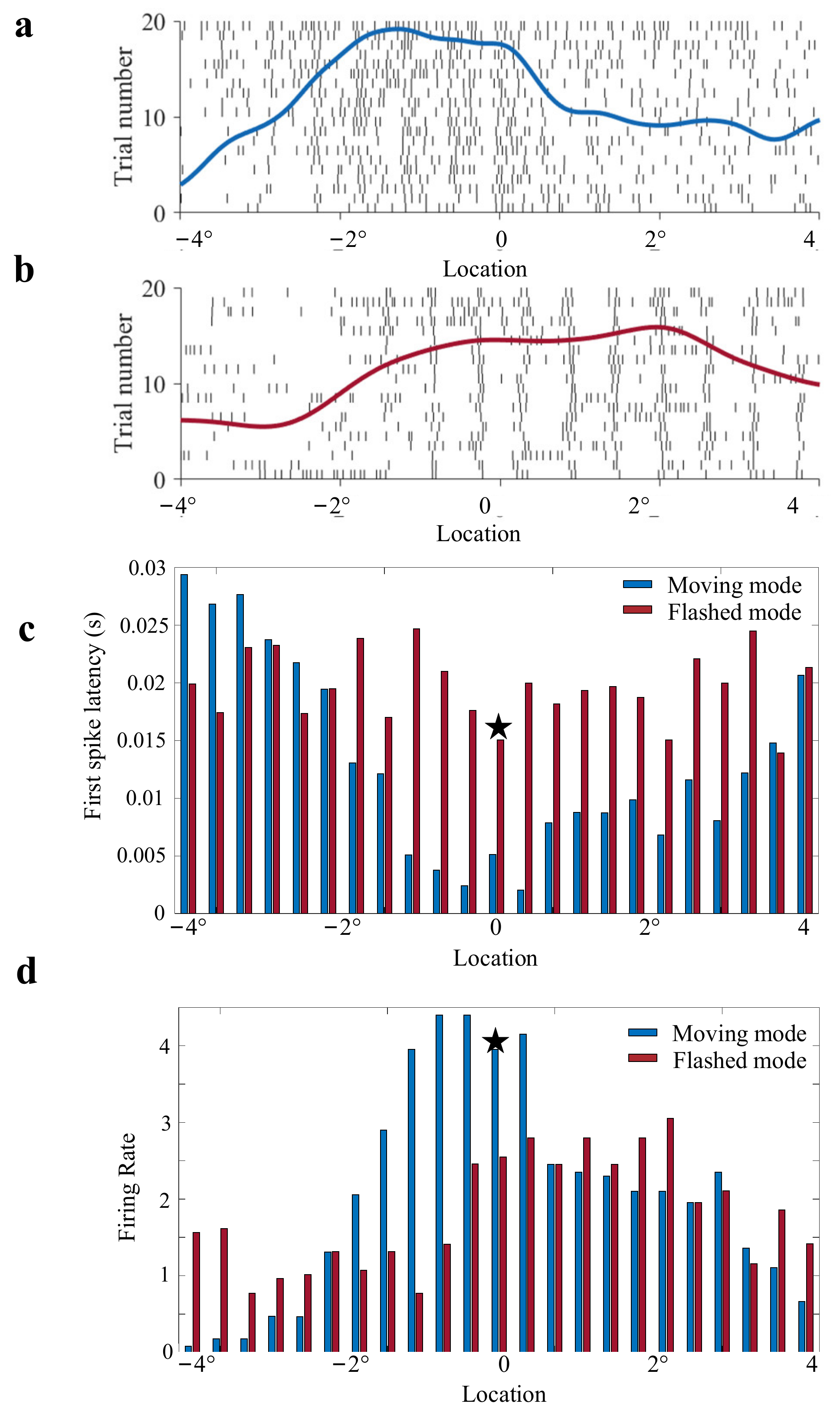
3.1. Response of OT Neurons (Unit) to Moving and Flashed Stimuli
3.2. Dependence of Response Differences on the Moving Step Lengths
3.3. Dependence of the Response Difference on Stimulus Duration
3.4. Modeling of Spatiotemporal Integration Mechanisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potier, S.; Lieuvin, M.; Pfaff, M.; Kelber, A. How fast can raptors see? J. Exp. Biol. 2019, 223, jeb.209031. [Google Scholar] [CrossRef] [PubMed]
- Wylie, D.; Gutiérrez-Ibáñez, C.; Iwaniuk, A. Integrating Brain, Behaviour and Phylogeny to understand the Evolution of Sensory Systems in Birds. Front. Neurosci. 2015, 9, 281. [Google Scholar] [CrossRef] [Green Version]
- Jancke, D.; Erlhagen, W.; Schöner, G.; Dinse, H. Shorter latencies for motion trajectories than for flashes in population responses of cat primary visual cortex. J. Physiol. 2004, 556, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.J.; Brivanlou, I.H.; Jordan, T.A.; Meister, M. Anticipation of moving stimuli by the retina. Nature 1999, 398, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyan, M.; Ecker, A.; Patel, S.; Cotton, J.; Bethge, M.; Pitkow, X.; Berens, P.; Tolias, A. Faster processing of moving compared to flashed bars in awake macaque V1 provides a neural correlate of the flash lag illusion. J. Neurophysiol. 2018, 120, 2430–2452. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.P.; Pierce, K.E.; Ward, D. Avian Vision: A Review of Form and Function with Special Consideration to Birds of Prey. J. Exot. Pet Med. 2007, 16, 69–87. [Google Scholar] [CrossRef]
- Orban, G.A.; Hoffmann, K.P.; Duysens, J. Velocity selectivity in the cat visual system. I. Responses of LGN cells to moving bar stimuli: A comparison with cortical areas 17 and 18. J. Neurophysiol. 1985, 54, 1026–1049. [Google Scholar] [CrossRef]
- Nijhawan, R. Motion Extrapolation in Catching. Nature 1994, 370, 256–257. [Google Scholar] [CrossRef]
- Whitney, D.; Murakami, I.; Cavanagh, P. Illusory spatial offset of a flash relative to a moving stimulus is caused by differential latencies for moving and flashed stimuli. Vis. Res. 2000, 40, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Arnold, D.H.; Durant, S.; Johnston, A. Latency differences and the flash-lag effect. Vis. Res. 2003, 43, 1829–1835. [Google Scholar] [CrossRef] [Green Version]
- Schneider, K. The Flash-Lag, Fröhlich and Related Motion Illusions Are Natural Consequences of Discrete Sampling in the Visual System. Front. Psychol. 2018, 9, 1227. [Google Scholar] [CrossRef] [Green Version]
- Hubel, D. Cortical Unit Responses to Visual Stimuli in Nonanesthetized Cats. Am. J. Ophthalmol. 1958, 46, 110–121. [Google Scholar] [CrossRef]
- Hubel, D.H.; Wiesel, T.N. Receptive Fields of Single Neurons in the Cat’s Striate Cortex. J. Physiol. 1959, 148, 574–591. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.; DiFranco, D. Motion characteristics of single units in the pigeon optic tectum. Vis. Res. 1976, 16, 1229–1234. [Google Scholar] [CrossRef]
- Luksch, H.; Khanbabaie, R.; Wessel, R. Synaptic dynamics mediate sensitivity to motion independent of stimulus details. Nat. Neurosci. 2004, 7, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Luksch, H.; Karten, H.J.; Kleinfeld, D.; Wessel, R. Chattering and differential signal processing in identified motion-sensitive neurons of parallel visual pathways in the chick tectum. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 6440–6446. [Google Scholar] [CrossRef]
- Verhaal, J.; Luksch, H. Neuronal responses to motion and apparent motion in the optic tectum of chickens. Brain Res. 2016, 1635, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Verhaal, J.; Luksch, H. Processing of motion stimuli by cells in the optic tectum of chickens. Neuroreport 2015, 26, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, Q.; Qian, L.; Zhao, M.; Wang, Z.; Shi, L. Encoding Model for Continuous Motion-sensitive Neurons in the Intermediate and Deep Layers of the Pigeon Optic Tectum. Neuroscience 2022, 484, 1–15. [Google Scholar] [CrossRef]
- Shuman, H.; Niu, X.; Shi, L. An Accumulated Energy Encoding Model of the Pigeon Optic Tectum: Accounting for the Difference of Response to Moving and Flashed Stimulus. Int. J. Psychophysiol. 2021, 168, S185. [Google Scholar] [CrossRef]
- Latimer, K.W.; Fairhall, A.L. Capturing Multiple Timescales of Adaptation to Second-Order Statistics with Generalized Linear Models: Gain Scaling and Fractional Differentiation. Front. Syst. Neurosci. 2020, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.I.; Pillow, J.W. Capturing the Dynamical Repertoire of Single Neurons with Generalized Linear Models. Neural Comput. 2017, 29, 3260–3289. [Google Scholar] [CrossRef]
- Yates, J.L.; Park, I.M.; Katz, L.N.; Pillow, J.W.; Huk, A.C. Functional dissection of signal and noise in MT and LIP during decision-making. Nat. Neurosci. 2017, 20, 1285–1292. [Google Scholar] [CrossRef]
- Niu, X.; Huang, S.; Yang, S.; Wang, Z.; Li, Z.; Shi, L. Comparison of pop-out responses to luminance and motion contrasting stimuli of tectal neurons in pigeons. Brain Res. 2020, 1747, 147068. [Google Scholar] [CrossRef]
- Wang, J.; Niu, X.; Wang, S.; Wang, Z.; Shi, L. Entrainment within neuronal response in optic tectum of pigeon to video displays. J. Comp. Physiol. A 2020, 206, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, M.; Wang, Z.; Shi, L. First spike latency of ON/OFF neurons in the optic tectum of pigeons. Integr. Zool. 2019, 14, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Huang, S.; Zhu, M.; Wang, Z.; Shi, L. Surround Modulation Properties of Tectal Neurons in Pigeons Characterized by Moving and Flashed Stimuli. Animals 2022, 12, 475. [Google Scholar] [CrossRef] [PubMed]
- Letelier, J.C.; Marin, G.; Sentis, E.; Tenreiro, A.; Fredes, F.; Mpodozis, J. The mapping of the visual field onto the dorso-lateral tectum of the pigeon (Columba livia) and its relations with retinal specializations. J. Neurosci. Methods 2004, 132, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.-Q.; Xiao, Q.; Liu, R.-F.; Wu, L.-Q.; Wang, S.-R. Response characteristics of the pigeon’s pretectal neurons to illusory contours and motion. J. Physiol. 2007, 577, 805–813. [Google Scholar] [CrossRef]
- Quiroga, R.Q.; Nadasdy, Z.; Ben-Shaul, Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput. 2004, 16, 1661–1687. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Niu, X.; Wan, H.; Shang, Z.; Wang, Z. A small-world-based population encoding model of the primary visual cortex. Biol. Cybern. 2015, 109, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Truccolo, W.; Eden, U.T.; Fellows, M.R.; Donoghue, J.P.; Brown, E.N. A Point Process Framework for Relating Neural Spiking Activity to Spiking History, Neural Ensemble, and Extrinsic Covariate Effects. J. Neurophysiol. 2005, 93, 1074–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, P.K.; Borgan, Ø.; Gill, R.D.; Keiding, N. Statistical Models Based on Counting Processes; Springer: New York, NY, USA, 1992. [Google Scholar]
- Tamura, H.; Tanaka, K. Visual Response Properties of Cells in the Ventral and Dorsal Parts of the Macaque Inferotemporal Cortex. Cereb. Cortex 2001, 11, 384–399. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Conde, S.; Macknik, S.; Hubel, D. Microsaccadic eye movements and firing of single cells in the striate cortex of macaque monkeys. Nat. Neurosci. 2000, 3, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyan, M.; Ecker, A.S.; Berens, P.; Tolias, A.S. Macaque monkeys perceive the flash lag illusion. PLoS ONE 2013, 8, e58788. [Google Scholar] [CrossRef]
- Mpodozis, J.; Letelier, J.C.; Concha, M.L.; Maturana, H. Conduction velocity groups in the retino-tectal and retino-thalamic visual pathways of the pigeon (Columbia livia). Int. J. Neurosci. 1995, 81, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, E.I. Evolution of neural processing for visual perception in vertebrates. J. Comp. Neurol. 2020, 528, 2888–2901. [Google Scholar] [CrossRef] [Green Version]
- Knudsen, E.I. Control from below: The role of a midbrain network in spatial attention. Eur. J. Neurosci. 2011, 33, 1961–1972. [Google Scholar] [CrossRef]
- Lai, D.; Brandt, S.; Luksch, H.; Wessel, R. Recurrent antitopographic inhibition mediates competitive stimulus selection in an attention network. J. Neurophysiol. 2011, 105, 793–805. [Google Scholar] [CrossRef] [Green Version]
- Weigel, S.; Kuenzel, T.; Lischka, K.; Huang, G.; Luksch, H. Morphology and dendrite-specific synaptic properties of midbrain neurons shape multimodal integration. J. Neurosci. Off. J. Soc. Neurosci. 2022, 42, 2614–2630. [Google Scholar] [CrossRef]
- Marin, G.J.; Duran, E.; Morales, C.; Gonzalez-Cabrera, C.; Sentis, E.; Mpodozis, J.; Letelier, J.C. Attentional Capture? Synchronized Feedback Signals from the Isthmi Boost Retinal Signals to Higher Visual Areas. J. Neurosci. 2012, 32, 1110–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busch, N.; Debener, S.; Kranczioch, C.; Engel, A.; Herrmann, C. Size matters: Effects of stimulus size, duration and eccentricity on the visual gamma-band response. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2004, 115, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Plewan, T.; Weidner, R.; Fink, G. The influence of stimulus duration on visual illusions and simple reaction time. Exp. Brain Res. Exp. Hirnforschung. Expérimentation Cérébrale 2012, 223, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Lee, C. Stimulus duration and vestibular sensory evoked potentials (VsEPs). Hear. Res. 2021, 408, 108293. [Google Scholar] [CrossRef] [PubMed]
- Kuruppath, P.; Belluscio, L. The influence of stimulus duration on olfactory perception. PLoS ONE 2021, 16, e0252931. [Google Scholar] [CrossRef]
- Bagheri, Z.M.; Cazzolato, B.S.; Grainger, S.; O’Carroll, D.C.; Wiederman, S.D. An autonomous robot inspired by insect neurophysiology pursues moving features in natural environments. J. Neural Eng. 2017, 14, 046030. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Wang, S.R. Excitatory and inhibitory receptive fields of tectal cells are differentially modified by magnocellular and parvocellular divisions of the pigeon nucleus isthmi. J. Comp. Physiol. A 2000, 186, 505–511. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Wang, S.-R. Receptive field organization and response properties of visual neurons in the pigeon nucleus semilunaris. Neurosci. Lett. 2002, 331, 179–182. [Google Scholar] [CrossRef]
- Sceniak, M.; Maciver, B. Cellular Actions of Urethane on Rat Visual Cortical Neurons In Vitro. J. Neurophysiol. 2006, 95, 3865–3874. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, J.; Schneider, D.; Woolley, S. Anesthetic state modulates excitability but not spectral tuning or neural discrimination in single auditory midbrain neurons. J. Neurophysiol. 2011, 106, 500–514. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Cao, P.; Yang, Y.; Wang, S.-R. Corollary discharge circuits for saccadic modulation of the pigeon visual system. Nat. Neurosci. 2008, 11, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, N.; Mysore, S. Combinatorial Neural Inhibition for Stimulus Selection across Space. Cell Rep. 2018, 25, 1158–1170.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Niu, X.; Wang, J.; Wang, Z.; Xu, H.; Shi, L. Visual Responses to Moving and Flashed Stimuli of Neurons in Domestic Pigeon (Columba livia domestica) Optic Tectum. Animals 2022, 12, 1798. https://doi.org/10.3390/ani12141798
Huang S, Niu X, Wang J, Wang Z, Xu H, Shi L. Visual Responses to Moving and Flashed Stimuli of Neurons in Domestic Pigeon (Columba livia domestica) Optic Tectum. Animals. 2022; 12(14):1798. https://doi.org/10.3390/ani12141798
Chicago/Turabian StyleHuang, Shuman, Xiaoke Niu, Jiangtao Wang, Zhizhong Wang, Huaxing Xu, and Li Shi. 2022. "Visual Responses to Moving and Flashed Stimuli of Neurons in Domestic Pigeon (Columba livia domestica) Optic Tectum" Animals 12, no. 14: 1798. https://doi.org/10.3390/ani12141798
APA StyleHuang, S., Niu, X., Wang, J., Wang, Z., Xu, H., & Shi, L. (2022). Visual Responses to Moving and Flashed Stimuli of Neurons in Domestic Pigeon (Columba livia domestica) Optic Tectum. Animals, 12(14), 1798. https://doi.org/10.3390/ani12141798






