Seasonal Prevalence of Gastrointestinal Parasites in Macaques (Macaca thibetana) at Mount Emei Scenic Area in China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Areas and Examined Monkeys
- (1)
- Yellow macaques group (n = 31): the range of their activity was from Qingyinge to the ecological area. The group was characterized by yellow hair color and had more young monkeys in the population.
- (2)
- Black macaques group (n = 19): the range of their activity was from near the trestle and cable bridge in the ecological area. The group was characterized by their dark brown color.
- (3)
- New macaques group (n = 57): the range of their activity was from the ecological area to the upper section of Hongchunping. These macaques migrated to the ecological area near Jiulaodong in hongchunping. Their population was characterized by light brown hair color.
- (4)
- Leidongping macaques group (n = 57): the range of activity was from the Leishen temple to Jinding ropeway near Leidongping. Their hair color was mostly grayish brown.
- (5)
- Wuxiangang macaques group (n = 4): the range of their activity was from the Wuxiangang station to Qingyin Pavilion. This group was characterized by their unwillingness to be in contact with tourists.
2.2. Sample Collection
2.3. Microscopic Examination of Fecal Parasites
2.4. Calculation of Prevalence Rate
2.5. Statistical Analysis
3. Results
3.1. Population and Daily Contact Frequency of Macaque in Various Seasons
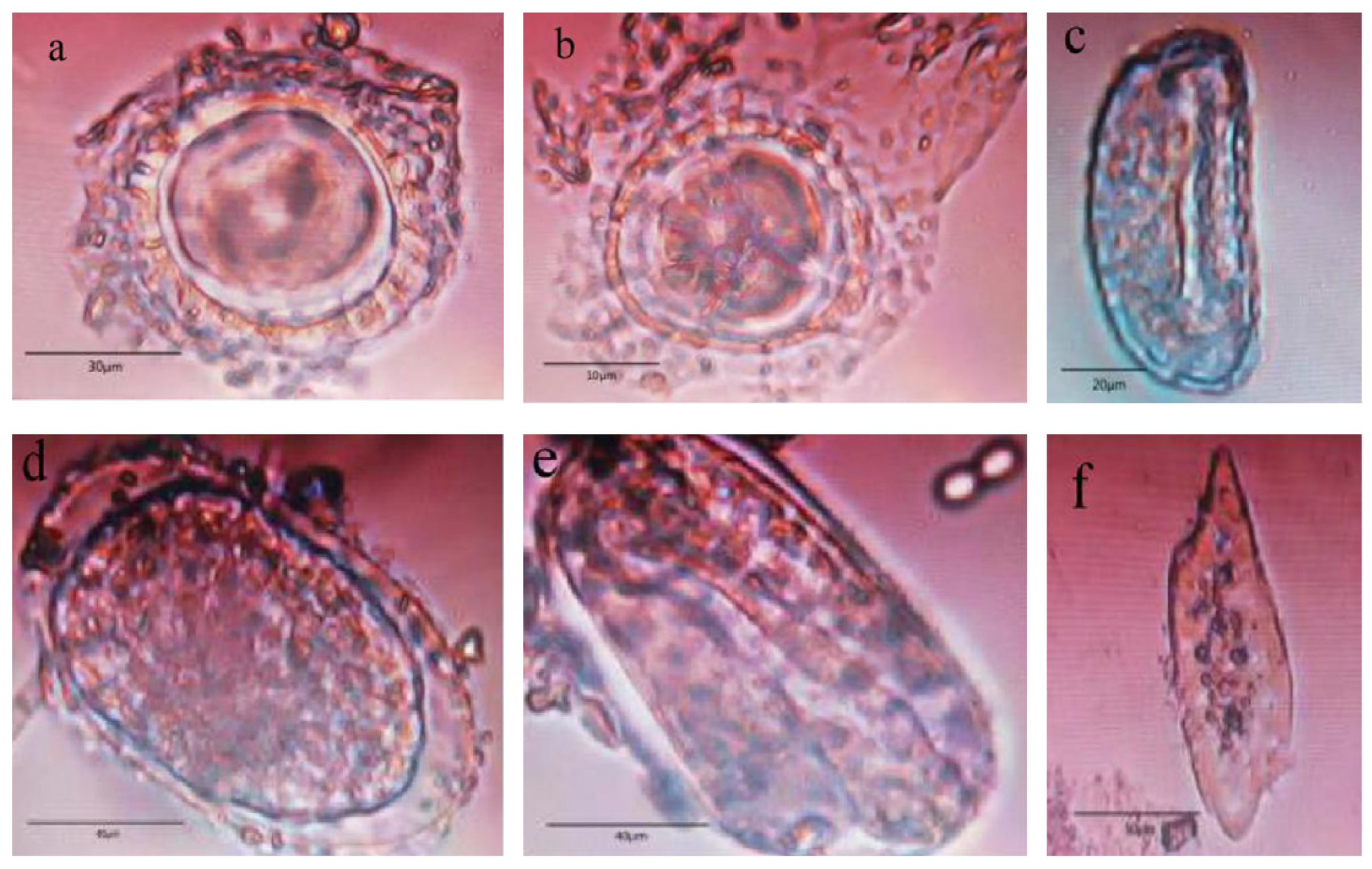
3.2. Microscopic Examination Results and Analysis
3.3. The Prevalence of the Intestinal Parasite in the Macaque Groups
3.4. The Prevalence of the Intestinal Parasite during Different Seasons
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging infectious diseases of wildlife-threats to biodiversity and human health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.A.; Tak, H.; Bhat, B.A.; Dar, J.R.; Ahmed, R. Gastrointestinal helminth parasites of wild ungulates in Hirpora Wildlife Sanctuary, Kashmir, India. J. Parasit. Dis. 2022, 147, 1–7. [Google Scholar] [CrossRef]
- Soulsby, E.J.L. Textbook of Veterinary Clinical Parasitology, Vol I; Helminth Oxford Blackwell Scientific: London, UK, 1982. [Google Scholar]
- Sprenger, L.K.; Yoshitani, U.Y.; Buzatti, A.; Molento, M.B. Occurrence of gastrointestinal parasites in wild animals in State of Paraná, Brazil. An. Acad. Bras. Ciências 2018, 90, 231–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibetannet. Available online: https://en.tibet3.com/photo/2017-02-23/2346.html (accessed on 2 May 2022).
- Salegio, E.A.; Bresnahan, J.C.; Sparrey, C.J.; Camisa, W.; Fischer, J.; Leasure, J.; Buckley, J.; Nout-Lomas, Y.S.; Rosenzweig, E.S.; Moseanko, R.; et al. A unilateral cervical spinal cord contusion injury model in non-human primates (Macaca mulatta). J. Neurotrauma 2016, 33, 439–459. [Google Scholar] [CrossRef] [Green Version]
- Peña, J.C.; Ho, W.Z. Non-human primate models of tuberculosis. Microbiol. Spectr. 2016, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Modolo, L.; Salzburger, W.; Martin, R.D. Phylogeography of Barbary macaques (Macaca sylvanus) and the origin of the Gibraltar colony. Proc. Natl. Acad. Sci. USA 2005, 102, 7392–7397. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.W. Progress and prospects for genetic modification of nonhuman primate models in biomedical research. ILAR J. 2013, 54, 211–223. [Google Scholar] [CrossRef] [Green Version]
- Roodgar, M.; Babveyh, A.; Nguyen, L.H.; Zhou, W.; Sinha, R.; Lee, H.; Hanks, J.B.; Avula, M.; Jiang, L.; Jian, R.; et al. Chromosome-level de novo assembly of the pig-tailed macaque genome using linked-read sequencing and HiC proximity scaffolding. Gigascience 2020, 9, giaa069. [Google Scholar] [CrossRef]
- Li, Z.H.; He, X.P.; Li, H.; He, R.Q.; Hu, X.T. Age-associated changes in amyloid-β and formaldehyde concentrations in cerebrospinal fluid of rhesus monkeys. Zoöl. Res. 2020, 41, 444–448. [Google Scholar] [CrossRef]
- Nogueira, R.; Peltier, N.E.; Anzai, A.; DeAngelis, G.C.; Martínez-Trujillo, J.; Moreno-Bote, R. The effects of population tuning and trial-by-trial variability on information encoding and behavior. J. Neurosci. 2020, 40, 1066–1083. [Google Scholar] [CrossRef]
- Vierboom, M.P.M.; Breedveld, E.; Keehnen, M.; Klomp, R.; Bakker, J. Pain Relief in Nonhuman Primate Models of Arthritis. Methods Mol. Biol. 2017, 1559, 411–417. [Google Scholar] [PubMed]
- Wang, Z. Analysis of zoonosis between wild animals and humans. Spec. Econ. Anim. Plants. 2008, 7, 17–18. [Google Scholar]
- Moudgil, A. Studies on the Prevalence and Management of Parasitic Infections in Zoo Animals. Master’s Dissertation, University of Lisbon, Lisbon, Portugal, 2015. [Google Scholar]
- Islam, S.; Rahman, M.K.; Uddin, M.H.; Rahman, M.M.; Chowdhury, M.N.U.; Hassan, M.M.; Magalhaes, R.S.; Islam, A. Prevalence and diversity of gastrointestinal parasites in free-ranging rhesus macaques (Macaca mulatta) in different land gradients of Bangladesh. Am. J. Primatol. 2022, 84, e23345. [Google Scholar] [CrossRef] [PubMed]
- Adrus, M.; Zainudin, R.; Ahamad, M.; Jayasilan, M.A.; Abdullah, M.T. Gastrointestinal parasites of zoonotic importance observed in the wild, urban, and captive populations of non-human primates in Malaysia. J. Med. Primatol. 2019, 48, 22–31. [Google Scholar] [CrossRef]
- Debenham, J.J.; Tysnes, K.; Khunger, S.; Robertson, L.J. Occurrence of Giardia, Cryptosporidium, and Entamoeba in wild rhesus macaques (Macaca mulatta) living in urban and semi-rural North-West India. Int. J. Parasitol. Parasites Wildl. 2017, 6, 29–34. [Google Scholar] [CrossRef]
- Li, J.; Cui, Z.; Li, X.; Zhang, L. Review of zoonotic amebiasis: Epidemiology, clinical signs, diagnosis, treatment, prevention and control. Res. Vet. Sci. 2021, 136, 174–181. [Google Scholar] [CrossRef]
- Otranto, D.; Deplazes, P. Zoonotic nematodes of wild carnivores. Int. J. Parasitol. Parasites Wildl. 2019, 9, 370–383. [Google Scholar] [CrossRef]
- Samad, M.A. Public health threat caused by zoonotic diseases in Bangladesh. Bangladesh J. Vet. Med. 2013, 9, 95–120. [Google Scholar] [CrossRef] [Green Version]
- Moshfe, A.; Mowlavi, G.; Mobedi, I.; Cheraghzade, R.; Askarian, S.; Mohammadi, R.; Nouripour, S.; Zahabioun, F.; Imani, P.; Mirsepahi, N. Fauna of zoontic parasites of stray dogs in yasouj suburbs in 2008. N. Z. Nurs. J. Kai Tiaki 2011, 75, 14. [Google Scholar]
- Mpofu, T.J.; Nephawe, K.A.; Mtileni, B. Prevalence of gastrointestinal parasites in communal goats from different agro-ecological zones of South Africa. Vet. World 2020, 13, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Wobeser, G. Disease in Wild Animals: Investigation and Management; Spinger: Berlin, Germany, 2007; 393p. [Google Scholar]
- Macpherson, C.N. Human behaviour and the epidemiology of parasitic zoonoses. Int. J. Parasitol. 2005, 35, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Bogale, B.; Chanie, M.; Melaku, A.; Fentahun, T.; Berhanu, A. Occurrence, Intensity and Parasite Composition of Gastrointestinal Helminth Parasites in Walia Ibex (Capra walie) at Semien Mountains National Park, Natural World Heritage Site, Northern Ethiopia. Acta Parasitol. Glob. 2014, 5, 19–25. [Google Scholar]
- Thanasuwan, S.; Piratae, S.; Tankrathok, A. Prevalence of gastrointestinal parasites in cattle in Kalasin Province, Thailand. Vet. World 2021, 14, 2091–2096. [Google Scholar] [CrossRef] [PubMed]
- Learmonth, M.J. Human-Animal Interactions in Zoos: What Can Compassionate Conservation, Conservation Welfare and Duty of Care Tell Us about the Ethics of Interacting, and Avoiding Unintended Consequences? Animals 2020, 10, 2037. [Google Scholar] [CrossRef] [PubMed]
- Hongqian, F.; Lanlan, F. A study on zoonotic taeniasis in ancient and modern times. Agric. Archaeol. 2006, 000, 248–249. [Google Scholar]
- Wei, M.; Feng, M.; Guan, Y.; Zhou, H.; Fu, Y.; Cai, J.; Cheng, X. Study on the genetic diversity of Entamoeba knowlesi strains infected by wild macaques in China. In Proceedings of the 16th National Academic Conference and the 7th International Symposium on Parasitology of the Professional Committee of Zoology Society of China, Jiangxi, China, 18 October 2017. [Google Scholar]
- Antonelli, L.; Foata, J.; Quilichini, Y.; Marchand, B. Influence of season and site location on European cultured sea bass parasites in Corsican fish farms using indicator species analysis (IndVal). Parasitol. Res. 2016, 115, 561–568. [Google Scholar] [CrossRef]
- Setsuda, A.; Varcasia, A.; Scala, A.; Ozawa, S.; Yokoyama, M.; Torii, H.; Suzuki, K.; Kaneshiro, Y.; Corda, A.; Dessì, G.; et al. Gongylonema infection of wild mammals in Japan and Sardinia (Italy). J. Helminthol. 2018, 94, 1–8. [Google Scholar] [CrossRef]
- Setsuda, A.; Da, N.; Hasegawa, H.; Behnke, J.M.; Rana, H.B.; Dhakal, I.P.; Sato, H. Intraspecific and interspecific genetic variation of Gongylonema pulchrum and two rodent Gongylonema spp. (G. aegypti and G. neoplasticum), with the proposal of G. nepalensis n. sp. for the isolate in water buffaloes from Nepal. Parasitol. Res. 2016, 115, 787–795. [Google Scholar] [CrossRef]
- Adkesson, M.J.; Langan, J.N.; Paul, A. Evaluation of control and treatment of Gongylonema spp. infections in callitrichids. J. Zoo Wildl. Med. Off. Publ. Am. Assoc. Zoo Vet. 2007, 38, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, L.; Chadee, K. The immunopathogenesis of Entamoeba histolytica. Exp. Parasitol. 2010, 126, 366–380. [Google Scholar] [CrossRef]
- Wang, N.; Han, M.; Qu, S.; Jiang, X.; Shi, W.; Feng, X.; Dan, J. Research progress of pathogenic amoeba vaccine. J. Jilin Med. Coll. 2019, 40, 4. [Google Scholar]
- Chen, Y.; Chen, B.; Xie, H.; Xie, X.; Jiang, D. Clinical characteristics and prognosis of 6 cases of histolytic amebiasis. J. Trop. Med. 2019, 19, 1285–1287. [Google Scholar]
- Kantor, M.; Abrantes, A.; Estevez, A.; Schiller, A.; Torrent, J.; Gascon, J.; Hernandez, R.; Ochner, C. Entamoeba Histolytica: Updates in Clinical Manifestation, Pathogenesis, and Vaccine Development. Can. J. Gastroenterol. Hepatol. 2018, 2018, 4601420. [Google Scholar] [CrossRef] [Green Version]
- Lejeune, M.; Rybicka, J.M.; Chadee, K. Recent discoveries in the pathogenesis and immune response toward Entamoeba histolytica. Future Microbiol. 2009, 4, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Halliez, M.C.; Buret, A.G. Gastrointestinal Parasites and the Neural Control of Gut Functions. Front. Cell. Neurosci. 2015, 9, 452. [Google Scholar] [CrossRef]
- Heyneman, D. Chapter 89: Medical Microbiology. In Cestodes, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Available online: https://www.ncbi.nlm.nih.gov/books/NBK8399/ (accessed on 13 May 2022).
- Wang, Z.; Liu, P.; Kuang, S.; Shu, H.; Huang, M. A case of diagnosis and prevention of Sphaerozoum fuscum occurred in Lophura nythemera. South China For. Sci. 2015, 43, 64. [Google Scholar] [CrossRef]
- Aziz, M.H.; Ramphul, K. Ancylostoma. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ronquillo, A.C.; Puelles, L.B.; Espinoza, L.P.; Sánchez, V.A.; Luis Pinto Valdivia, J. Ancylostoma duodenale as a cause of upper gastrointestinal bleeding: A case report. Braz. J. Infect. Dis. 2019, 23, 471–473. [Google Scholar] [CrossRef]
- Pearson, R. Hookworm Infection. Medical Topics & Chapters. Available online: https://www.msdmanuals.com/professional/infectious-diseases/nematodes-roundworms/hookworm-infection (accessed on 18 May 2022).
- Hagel, I.; Giusti, T. Ascaris lumbricoides: An overview of therapeutic targets. Infect. Disord. Drug Targets 2010, 10, 349–367. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Guideline: Preventive Chemotherapy to Control Soil-Transmitted Helminth Infections in At-Risk Population Groups; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization. Soil-Transmitted Helminth Infections. Retrieved from World Health Organization Website. Available online: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on 16 November 2020).
- de Melo Hoshino, É.; Tavares-Dias, M. Interannual and Seasonal Variation in Protozoan and Metazoan Parasite Communities of Hemibrycon surinamensis, a Characid Fish Inhabiting the Brazilian Amazon. Acta Parasit. 2019, 64, 479–488. [Google Scholar] [CrossRef]
- Wharton, D.A. Parasites and low temperatures. Parasitology 1999, 119, S7–S17. [Google Scholar] [CrossRef]
- Viljoen, H.; Bennett, N.C.; Ueckermann, E.A.; Lutermann, H. The role of host traits, season and group size on parasite burdens in a cooperative mammal. PLoS ONE 2011, 6, e27003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbahy, N.M.; Elkhtam, A.O.; AbouLaila, M.; Abdelaziz, A.R. Prevalence of different flatworms infecting ruminants in Menoufia Governorate. J. Curr. Vet. Res. 2015, 9, 66–77. [Google Scholar] [CrossRef]
- Thompson, A.; Kutz, S. Introduction to the Special Issue on ‘Emerging Zoonoses and Wildlife’. Int. J. Parasitol. Parasites Wildl. 2019, 9, 322. [Google Scholar] [CrossRef]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef] [PubMed]



| Population Season | Yellow Macaque Group in the Ecological Area | Black Macaque Group in the Ecological Area | New Macaque in the Ecological Area | Leidongping Macaque | Wuxiangang Macaque | Total |
|---|---|---|---|---|---|---|
| Spring | 9 | 4 | 7 | 9 | 2 | 31 |
| Summer | 14 | 9 | 16 | 17 | 2 | 58 |
| Autumn | 5 | 3 | 6 | 9 | 0 | 23 |
| Winter | 3 | 3 | 28 | 22 | 0 | 56 |
| Total | 31 | 19 | 57 | 57 | 4 | 168 |
| Monkeys | Number of Samples | Haunt Time Every Day (h) | Haunt Location | Daily Exposure Time (n) | Contact Frequency (n/h) |
|---|---|---|---|---|---|
| Yellow macaque | 31 | 2.6 | Near Qingyin Pavilion | 16.1 | 6.2 |
| Black macaque | 19 | 2.9 | Suspension bridge in ecological area | 16.4 | 5.7 |
| New macaque | 57 | 3.2 | Sandaoqiao, suspension bridge in ecological area | 14.1 | 4.4 |
| Leidongping macaque | 57 | 6.1 | Leidongping stand | 19.5 | 3.2 |
| Wuxiangang macaque | 4 | 0.6 | Wuxiangang station | 0.3 | 0.5 |
| Population | Yellow Macaque Group in the Ecological Area (n = 31) | Black Macaque Group in the Ecological Area (n = 19) | New Macaque in the Ecological Area (n = 57) | Leidongping Macaque (n = 57) | Wuxiangang Macaque (n = 4) | Total (n = 168) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parasites | DN (n) | PR (%) | DN (n) | PR (%) | DN (n) | PR (%) | DN (n) | PR (%) | DN (n) | PR (%) | DN (n) | PR (%) |
| Ascaris lumbricoides | 2 | 6.45 | 0 | 0 | 2 | 3.51 | 0 | 0 | 0 | 0 | 2 | 1.19 |
| Entamoeba spp. | 12 | 38.71 | 7 | 36.84 | 5 | 8.77 | 7 | 12.28 | 0 | 0 | 31 | 18.45 |
| Blastocysistis spp. | 0 | 0 | 1 | 5.26 | 3 | 5.26 | 0 | 0 | 0 | 0 | 5 | 2.98 |
| Enterobius vermicularis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1.75 | 0 | 0 | 1 | 0.60 |
| Trematoda spp. | 0 | 0 | 1 | 5.26 | 2 | 3.51 | 1 | 1.75 | 0 | 0 | 4 | 2.38 |
| Cestoda spp. | 5 | 16.13 | 0 | 0 | 1 | 1.75 | 2 | 3.51 | 0 | 0 | 7 | 4.17 |
| Gongylonema spp. | 13 | 41.94 | 12 | 63.16 | 6 | 10.56 | 13 | 22.81 | 1 | 25 | 45 | 26.79 |
| Ancylostoma duodenale | 0 | 0 | 1 | 5.26 | 3 | 5.26 | 2 | 3.51 | 0 | 0 | 6 | 3.57 |
| Physaloptera spp. | 0 | 0 | 0 | 0 | 1 | 1.75 | 2 | 3.51 | 0 | 0 | 3 | 1.79 |
| Balantidium coli | 1 | 3.23 | 1 | 5.26 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1.19 |
| Sphaerozoum fuscum | 8 | 25.81 | 1 | 5.26 | 7 | 12.28 | 5 | 8.77 | 1 | 25 | 24 | 14.29 |
| Total | 25 | 80.65 | 17 | 89.47 | 20 | 35.09 | 23 | 40.35 | 1 | 25 | 86 | 51.19 |
| Season/Population Parasites | Spring (n = 31) | Summer (n = 58) | Autumn (n = 23) | Winter (n = 56) | Total (n = 168) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DN (n) | PR (%) | DN (n) | PR (%) | DN (n) | PR (n) | DN (n) | PR (%) | DN (n) | PR (%) | |
| Ascaris lumbricoides | 1 | 3.23 | 1 | 1.72 | 1 | 4.35 | 1 | 1.79 | 4 | 2.38 |
| Entamoeba spp. | 0 | 0 | 31 | 53.45 | 0 | 0 | 0 | 0 | 31 | 18.45 |
| Blastocysistis spp. | 0 | 0 | 4 | 6.90 | 0 | 0 | 0 | 0 | 4 | 2.38 |
| Enterobius vermicularis | 0 | 0 | 0 | 0 | 1 | 4.35 | 0 | 0 | 1 | 0.60 |
| Trematoda spp. | 0 | 0 | 1 | 1.72 | 3 | 13.04 | 0 | 0 | 4 | 4.76 |
| Cestoda spp. | 1 | 3.23 | 4 | 6.90 | 3 | 13.04 | 0 | 0 | 8 | 4.17 |
| Gongylonema spp. | 15 | 48.38 | 18 | 31.03 | 9 | 39.13 | 3 | 5.36 | 45 | 26.79 |
| Ancylostoma duodenale | 0 | 0 | 5 | 8.62 | 1 | 4.35 | 0 | 0 | 6 | 3.57 |
| Physaloptera spp. | 0 | 0 | 3 | 5.17 | 0 | 0 | 0 | 0 | 3 | 1.79 |
| Balantidium coli | 1 | 3.23 | 1 | 1.72 | 0 | 0 | 0 | 0 | 2 | 1.19 |
| Sphaerozoum fuscum | 4 | 12.90 | 16 | 27.59 | 2 | 8.70 | 0 | 0 | 22 | 13.10 |
| Total | 19 | 61.29 | 50 | 86.21 | 13 | 56.52 | 4 | 7.14 | 86 | 51.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Okyere, S.K.; Zheng, J.; Cao, B.; Hu, Y. Seasonal Prevalence of Gastrointestinal Parasites in Macaques (Macaca thibetana) at Mount Emei Scenic Area in China. Animals 2022, 12, 1816. https://doi.org/10.3390/ani12141816
Yang J, Okyere SK, Zheng J, Cao B, Hu Y. Seasonal Prevalence of Gastrointestinal Parasites in Macaques (Macaca thibetana) at Mount Emei Scenic Area in China. Animals. 2022; 12(14):1816. https://doi.org/10.3390/ani12141816
Chicago/Turabian StyleYang, Jiandong, Samuel Kumi Okyere, Jie Zheng, Buyuan Cao, and Yanchun Hu. 2022. "Seasonal Prevalence of Gastrointestinal Parasites in Macaques (Macaca thibetana) at Mount Emei Scenic Area in China" Animals 12, no. 14: 1816. https://doi.org/10.3390/ani12141816






