Mitochondrial Whole D-Loop Variability in Polish Draft Horses of Sztumski Subtype
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. DNA Extraction and Sanger Sequencing
2.4. Statistical Analysis
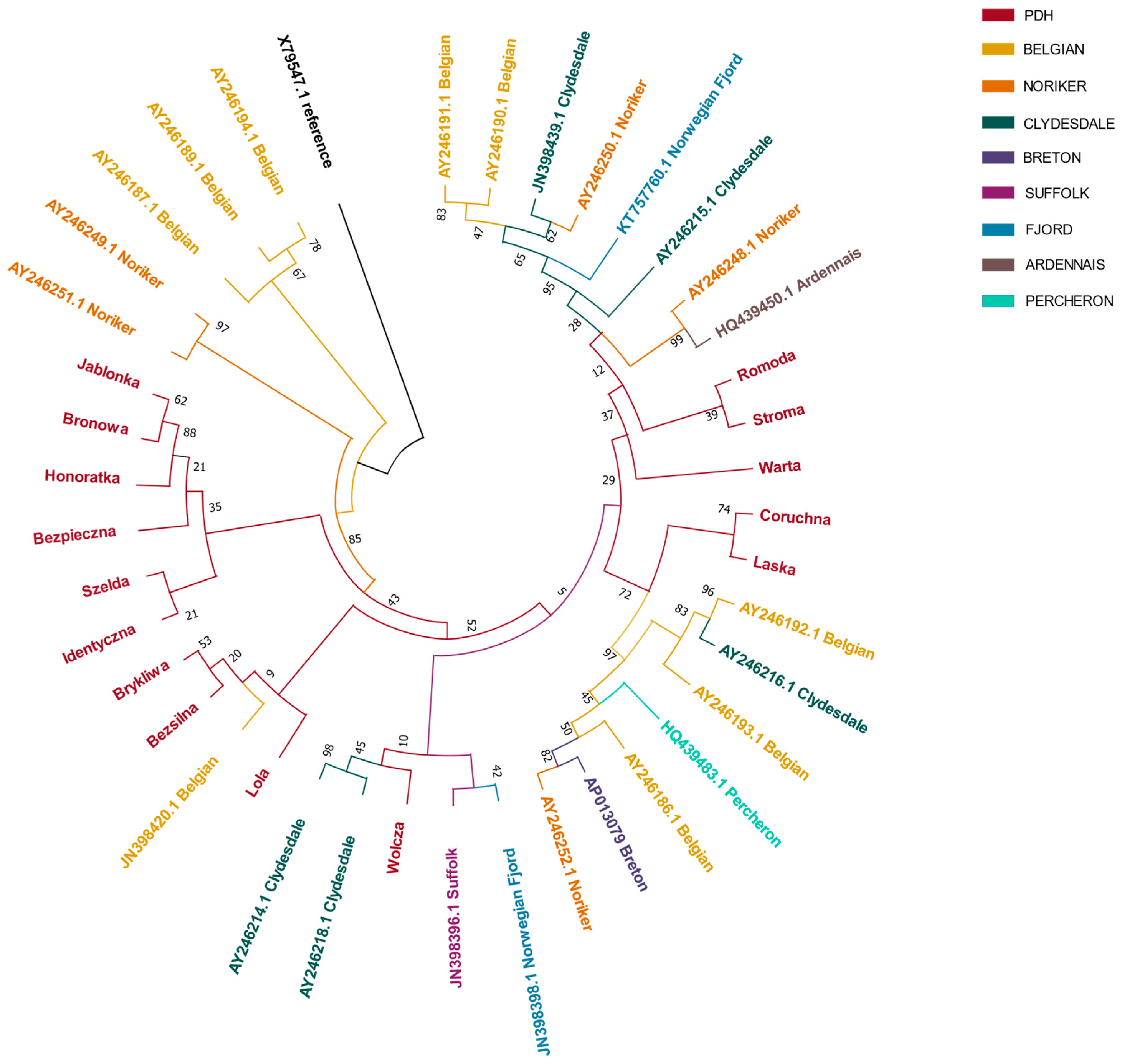
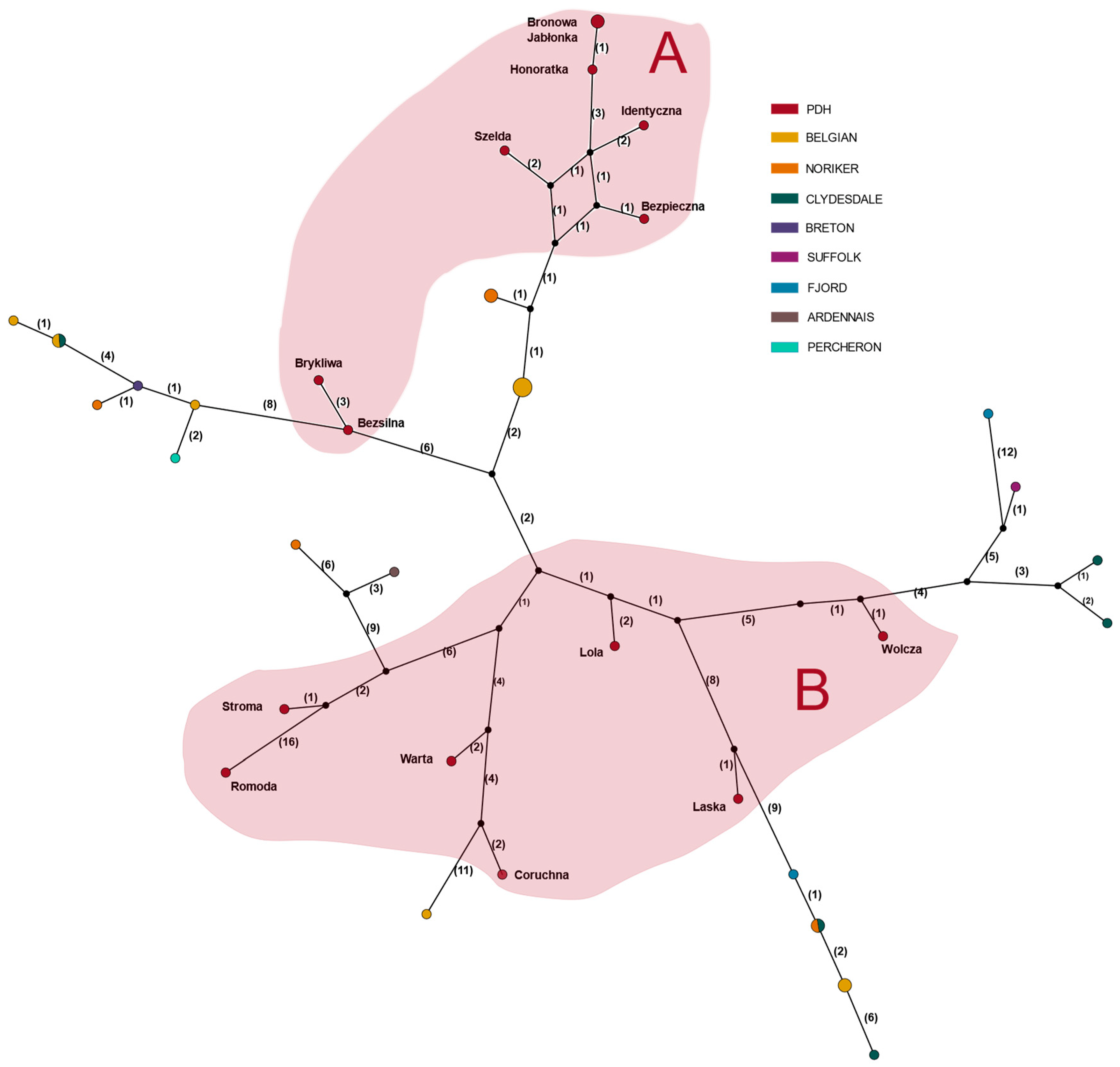
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dashper, K. Listening to Horses Developing Attentive Interspecies Relationships through Sport and Leisure. Soc. Anim. 2017, 25, 207–224. [Google Scholar] [CrossRef]
- Gurgul, A.; Jasielczuk, I.; Semik-Gurgul, E.; Pawlina-Tyszko, K.; Szmatoła, T.; Polak, G.; Bugno-Poniewierska, M. Genetic differentiation of the two types of Polish cold-blooded horses included in the national conservation program. Animals 2020, 10, 542. [Google Scholar] [CrossRef] [Green Version]
- Niewiński, W.; Gawarecki, J.; Kopczyk, A.; Masłowski, M.; Morawiec, M.; Strzelecki, H.; Woźbińska, M.; Jaworski, Z.; Jastrzębska, E.; Polak, G.; et al. Program Hodowli Koni Zimnokrwistych. Available online: https://www.pzhk.pl/wp-content/uploads/pr_hodow_zimn-2018_07_16.pdf (accessed on 11 February 2022).
- Polak, G. Genetic variability of cold-blooded horses participating in genetic resources conservation programs, using pedigree analysis. Ann. Anim. Sci. 2019, 19, 49–60. [Google Scholar] [CrossRef]
- Toro, M.A.; Fernández, J.; Caballero, A. Molecular characterization of breeds and its use in conservation. Livest. Sci. 2009, 120, 174–195. [Google Scholar] [CrossRef]
- Polak, G.; Gurgul, A.; Jasielczuk, I.; Szmatoła, T.; Krupiński, J.; Bugno-Poniewierska, M. Suitability of pedigree information and genomic methods for analyzing inbreeding of Polish cold-blooded horses covered by conservation programs. Genes 2021, 12, 429. [Google Scholar] [CrossRef]
- Yang, L.; Kong, X.; Yang, S.; Dong, X.; Yang, J.; Gou, X.; Zhang, H. Haplotype diversity in mitochondrial DNA reveals the multiple origins of Tibetan horse. PLoS ONE 2018, 13, e0201564. [Google Scholar] [CrossRef] [Green Version]
- Noda, A.; Yonesaka, R.; Sasazaki, S.; Mannen, H. The mtDNA haplogroup P of modern Asian cattle: A genetic legacy of Asian aurochs? PLoS ONE 2018, 13, e0190937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, J.; Xie, X.L.; Wang, D.F.; Zhao, C.; Lv, F.H.; Li, X.; Yang, J.; Yu, J.L.; Shen, M.; Gao, L.; et al. Paternal Origins and Migratory Episodes of Domestic Sheep. Curr. Biol. 2020, 30, 4085–4095.e6. [Google Scholar] [CrossRef]
- Xiufeng, X.; Árnason, Ú. The complete mitochondrial DNA sequence of the horse, Equus caballus: Extensive heteroplasmy of the control region. Gene 1994, 148, 357–362. [Google Scholar] [CrossRef]
- Ishida, N.; Hasegawa, T.; Takeda, K.; Sakagami, M.; Onishi, A.; Inumaru, S.; Komatsu, M.; Mukoyama, H. Polymorphic sequence in the D-loop region of equine mitochondrial DNA. Anim. Genet. 1994, 25, 215–221. [Google Scholar] [CrossRef]
- Lancioni, H.; Cardinali, I.; Giontella, A.; Antognoni, M.T.; Miglio, A. Mitochondrial DNA variation in the Italian Heavy Draught Horse. PeerJ 2020, 2020, e8996. [Google Scholar] [CrossRef]
- Giontella, A.; Sarti, F.M.; Cardinali, I.; Giovannini, S.; Cherchi, R.; Lancioni, H.; Silvestrelli, M.; Pieramati, C. Genetic variability and population structure in the sardinian Anglo-Arab horse. Animals 2020, 10, 1018. [Google Scholar] [CrossRef]
- Kvist, L.; Niskanen, M.; Mannermaa, K.; Wutke, S.; Aspi, J. Genetic variability and history of a native Finnish horse breed. Genet. Sel. Evol. 2019, 51, 35. [Google Scholar] [CrossRef] [Green Version]
- Cieslak, J.; Wodas, L.; Borowska, A.; Cothran, E.G.; Khanshour, A.M.; Mackowski, M. Characterization of the Polish Primitive Horse (Konik) maternal lines using mitochondrial D-loop sequence variation. PeerJ 2017, 2017, e3714. [Google Scholar] [CrossRef]
- Iwańczyk, E.; Juras, R.; Cholewiński, G.; Cothran, E.G. Genetic structure and phylogenetic relationships of the Polish Heavy Horse. J. Appl. Genet. 2006, 47, 353–359. [Google Scholar] [CrossRef]
- Lippold, S.; Matzke, N.J.; Reissmann, M.; Hofreiter, M. Whole mitochondrial genome sequencing of domestic horses reveals incorporation of extensive wild horse diversity during domestication. BMC Evol. Biol. 2011, 11, 328. [Google Scholar] [CrossRef] [Green Version]
- Flannery, A.R.; Cothran, E.G. Mitochondrial DNA Sequence Variation and the Domestication Pattern of the Horse. 2003. Available online: http://www.laclacroixindianpony.com/pdfs/cothranDNAreport.pdf (accessed on 5 June 2022).
- Achilli, A.; Olivieri, A.; Soares, P.; Lancioni, H.; Kashani, B.H.; Perego, U.A.; Nergadze, S.G.; Carossa, V.; Santagostino, M.; Capomaccio, S.; et al. Mitochondrial genomes from modern horses reveal the major haplogroups that underwent domestication. Proc. Natl. Acad. Sci. USA 2012, 109, 2449–2454. [Google Scholar] [CrossRef] [Green Version]
- Wada, K.; Yokohama, M. Whole Mitochondrial Genome Analysis Reveals that Hokkaido Population of the Japanese Native Horse is Composed of Two Divergent Maternal Lineages. 2013. Available online: https://www.webofscience.com/wos/woscc/full-record/WOS:000282221600003 (accessed on 5 June 2022).
- Orlando, L.; Ginolhac, A.; Zhang, G.; Froese, D.; Albrechtsen, A.; Stiller, M.; Schubert, M.; Cappellini, E.; Petersen, B.; Moltke, I.; et al. Recalibrating equus evolution using the genome sequence of an early Middle Pleistocene horse. Nature 2013, 499, 74–78. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3-new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [Green Version]
- Nergadze, S.G.; Lupotto, M.; Pellanda, P.; Santagostino, M.; Vitelli, V.; Giulotto, E. Mitochondrial DNA insertions in the nuclear horse genome. Anim. Genet. 2010, 41, 176–185. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Bandelt, H.-J.; Forster, P.; Rohl, A. Median-Joining Networks for Inferring Intraspecific Phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.W.; Bryant, D. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Vilà, C.; Leonard, J.A.; Götherström, A.; Marklund, S.; Sandberg, K.; Lidén, K.; Wayne, R.K.; Ellegren, H. Widespread origins of domestic horse lineages. Science 2001, 291, 474–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polak, G.; Krupiński, J.; Martyniuk, E.; Calik, J.; Kawęcka, A.; Krawczyk, J.; Majewska, A.; Sikora, J.; Sosin-Bzducha, E.; Szyndler-Nędza, M.; et al. The risk status of Polish local breeds under conservation programmes—New approach. Ann. Anim. Sci. 2021, 21, 125–140. [Google Scholar] [CrossRef]
- Lynch, M.; Ackerman, M.S.; Gout, J.F.; Long, H.; Sung, W.; Thomas, W.K.; Foster, P.L. Genetic drift, selection and the evolution of the mutation rate. Nat. Rev. Genet. 2016, 17, 704–714. [Google Scholar] [CrossRef]
- Druml, T.; Baumung, R.; Sölkner, J. Pedigree analysis in the Austrian Noriker draught horse: Genetic diversity and the impact of breeding for coat colour on population structure. J. Anim. Breed. Genet. 2009, 126, 348–356. [Google Scholar] [CrossRef]
| Primer | Region Amplified | Sequence | Product Length (bp) |
|---|---|---|---|
| HVR1A | 15382–15778 | F: AACGTTTCCTCCCAAGGACT R: GTAGTTGGGAGGGTTGCTGA | 397 |
| HVR1B | 15676–16137 | F: ACCCCATCCAAGTCAAATCA R: CAGGTGCACTTGTTTCCTATG | 461 |
| HVR2 | 16357–16660 | F: ACCTACCCGCGCAGTAAGCAA R: ACGGGGGAAGAAGGGTTGACA | 304 |
| A | Damline Name | Haplotype (Achilli et al. 2012 [19]) | n | 15490 | 15494 | 15495 | 15496 | 15534 | 15538 | 15542 | 15585 * | 15597 * | 15602 | 15604 | 15617 | 15635 | 15649 | 15650 * | 15659 * | 15666 | 15667 | 15700 | 15703 | 15709 | 15720 | 15762 | 15766 |
| C | T | T | A | C | A | C | G | A | C | G | T | C | A | A | T | G | A | C | T | C | G | G | C | ||||
| Haplotypes | Bezpieczna | A | 4 | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ |
| Bezsilna | G | 2 | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | T | ⸱ | G | T | ⸱ | ⸱ | T | ⸱ | ⸱ | C | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | |
| Bronowa | L | 2 | ⸱ | ⸱ | C | ⸱ | ⸱ | ⸱ | ⸱ | A | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | G | ⸱ | A | ⸱ | ⸱ | ⸱ | ⸱ | A | ⸱ | ⸱ | |
| Brykliwa | A | 2 | ⸱ | ⸱ | C | ⸱ | ⸱ | ⸱ | T | ⸱ | G | T | ⸱ | ⸱ | T | ⸱ | G | ⸱ | A | ⸱ | ⸱ | C | ⸱ | A | ⸱ | ⸱ | |
| Córuchna | L | 2 | ⸱ | ⸱ | ⸱ | G | T | ⸱ | ⸱ | A | ⸱ | ⸱ | A | ⸱ | ⸱ | G | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | |
| Honoratka | A | 2 | ⸱ | ⸱ | C | ⸱ | ⸱ | ⸱ | ⸱ | A | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | G | ⸱ | A | ⸱ | ⸱ | ⸱ | ⸱ | A | ⸱ | ⸱ | |
| Identyczna | A | 14 | T | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | |
| Jabłonka | A | 7 | ⸱ | ⸱ | C | ⸱ | ⸱ | ⸱ | ⸱ | A | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | G | ⸱ | A | ⸱ | ⸱ | ⸱ | A | ⸱ | ⸱ | ||
| Lola | B | 10 | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | |
| Łaska | G | 12 | ⸱ | C | ⸱ | G | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | |
| Romoda | B | 2 | ⸱ | ⸱ | C | ⸱ | ⸱ | ⸱ | ⸱ | A | ⸱ | T | A | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | G | ⸱ | C | ⸱ | A | ⸱ | ⸱ | |
| Stroma | B | 3 | ⸱ | ⸱ | C | ⸱ | ⸱ | ⸱ | ⸱ | A | ⸱ | T | ⸱ | C | ⸱ | ⸱ | ⸱ | C | ⸱ | ⸱ | T | C | ⸱ | A | A | T | |
| Szelda | P | 6 | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | G | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | G | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | |
| Warta | M | 2 | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | C | ⸱ | ⸱ | ⸱ | C | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | |
| Wołcza | I | 2 | ⸱ | ⸱ | C | ⸱ | ⸱ | G | ⸱ | A | ⸱ | T | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | T | A | ⸱ | ⸱ | |
| B | Reference X79547.1 | Haplotype (Achilli et al. 2012) | n | 15769 | 15770 | 15771 | 15801 | 15807 | 15810 | 15811 | 15826 | 15827 | 15870 | 15871 | 15956 | 15974 | 15995 | 16007 | 16022 | 16031 | 16055 | 16071 | 16543 | 16546 | 16558 | 16559 | 16629 |
| T | C | C | C | C | A | C | A | A | C | C | A | C | A | T | T | T | A | T | T | T | ins | C | A | ||||
| Haplotypes | Bezpieczna | A | 4 | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | G | ⸱ | G | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | G | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ |
| Bezsilna | G | 2 | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | T | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | C | ⸱ | ⸱ | ⸱ | ⸱ | C | ⸱ | ⸱ | |
| Bronowa | L | 2 | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | G | ⸱ | G | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | G | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | |
| Brykliwa | A | 2 | ⸱ | ⸱ | T | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | G | T | T | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | |
| Córuchna | L | 2 | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | G | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | C | ⸱ | G | |
| Honoratka | A | 2 | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | G | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | G | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | |
| Identyczna | A | 14 | ⸱ | ⸱ | ⸱ | ⸱ | T | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | C | ⸱ | ⸱ | |
| Jabłonka | A | 7 | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | G | ⸱ | G | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | G | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | |
| Lola | B | 10 | ⸱ | ⸱ | T | T | ⸱ | ⸱ | ⸱ | ⸱ | G | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | C | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | |
| Łaska | G | 12 | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | G | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | C | ⸱ | G | |
| Romoda | B | 2 | ⸱ | T | T | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | T | T | G | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | A | ⸱ | C | ⸱ | G | |
| Stroma | B | 3 | C | T | ⸱ | ⸱ | T | ⸱ | T | ⸱ | G | T | T | ⸱ | ⸱ | G | C | C | ⸱ | ⸱ | ⸱ | A | C | ⸱ | T | G | |
| Szelda | P | 6 | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | G | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | |
| Warta | M | 2 | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | A | C | ⸱ | T | G | |
| Wołcza | I | 2 | ⸱ | ⸱ | T | ⸱ | ⸱ | ⸱ | ⸱ | G | G | T | T | G | T | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | ⸱ | C | ⸱ | ⸱ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myćka, G.; Klecel, W.; Stefaniuk-Szmukier, M.; Jaworska, J.; Musiał, A.D.; Ropka-Molik, K. Mitochondrial Whole D-Loop Variability in Polish Draft Horses of Sztumski Subtype. Animals 2022, 12, 1870. https://doi.org/10.3390/ani12151870
Myćka G, Klecel W, Stefaniuk-Szmukier M, Jaworska J, Musiał AD, Ropka-Molik K. Mitochondrial Whole D-Loop Variability in Polish Draft Horses of Sztumski Subtype. Animals. 2022; 12(15):1870. https://doi.org/10.3390/ani12151870
Chicago/Turabian StyleMyćka, Grzegorz, Weronika Klecel, Monika Stefaniuk-Szmukier, Joanna Jaworska, Adrianna Dominika Musiał, and Katarzyna Ropka-Molik. 2022. "Mitochondrial Whole D-Loop Variability in Polish Draft Horses of Sztumski Subtype" Animals 12, no. 15: 1870. https://doi.org/10.3390/ani12151870






