Development of an Immunochromatographic Test Based on Rhoptry Protein 14 for Serological Detection of Toxoplasma gondii Infection in Swine
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Ethical Statement
2.2. Parasites and Cell Culture
2.3. Soluble Protein Preparation
2.4. Western Blot
2.5. Preparation of the Recombinant Protein TgROP14 (rTgROP14-His)
2.6. Production of Mouse Monoclonal Antibodies against TgROP14
2.7. Identification of Types and Epitope Specificities of the mAbs
2.8. Preparation of Immunoassay Materials
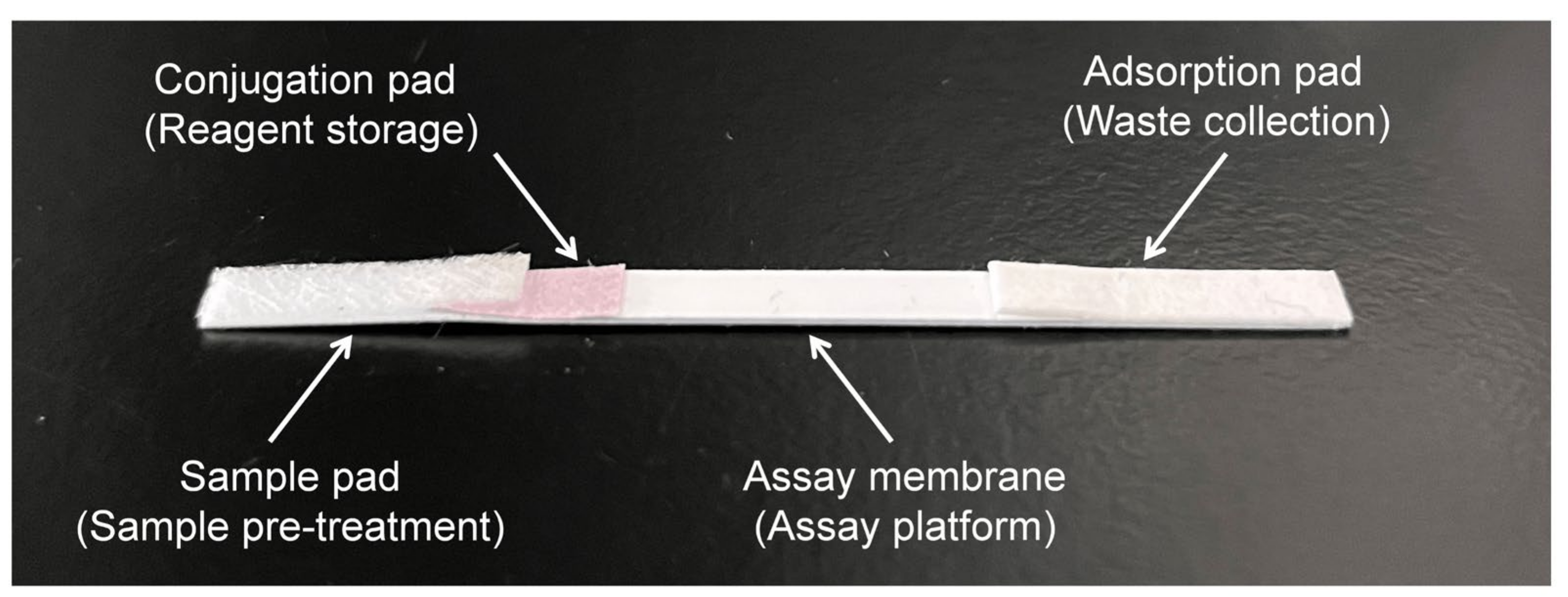
2.9. Preparation of the Immunochromatographic Strip
2.10. Sensitivity, Specificity and Stability of the Immunochromatographic Test
2.11. Detection of T. gondii Infection in Field Samples
3. Results
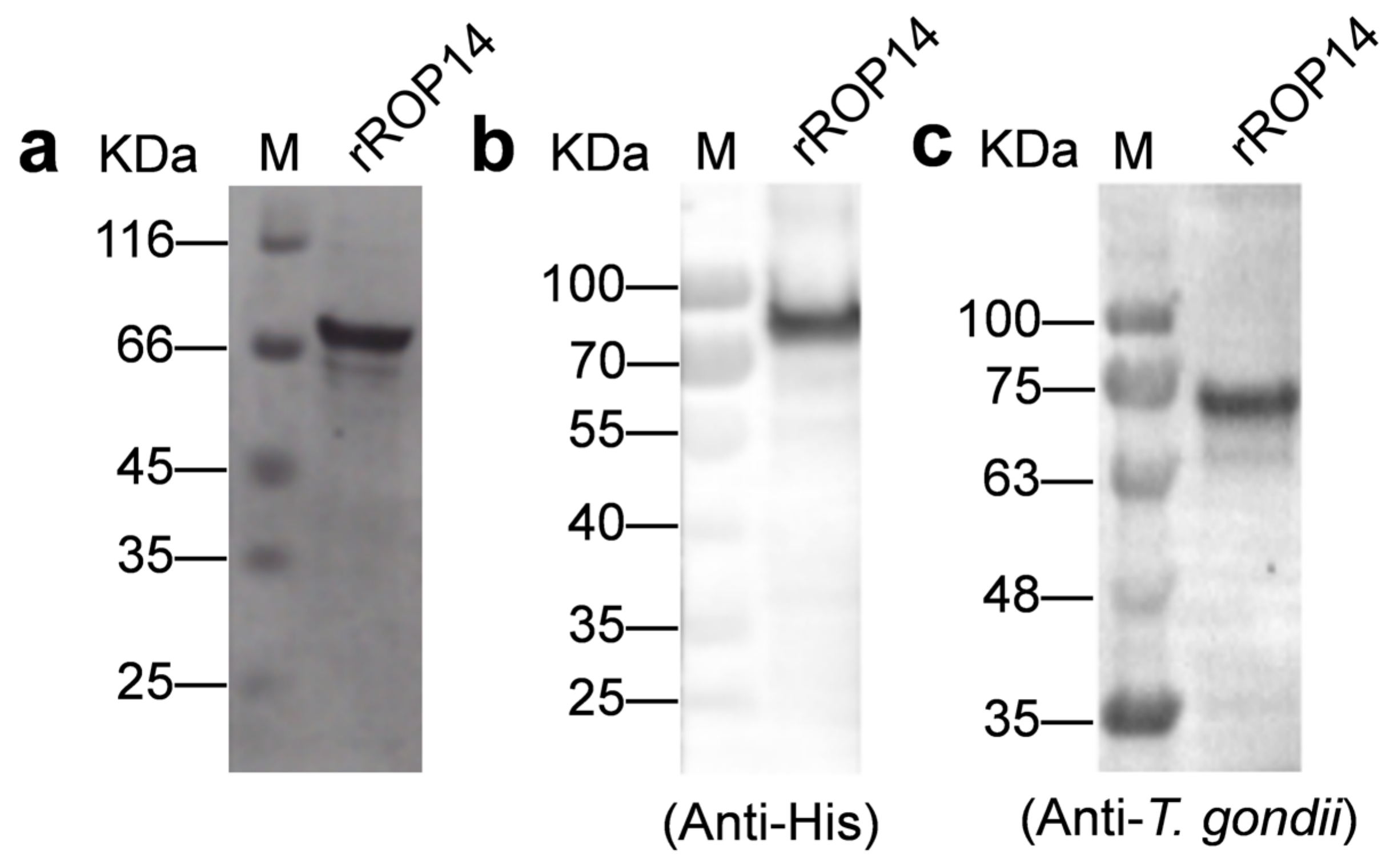
3.1. rTgROP14-His Proteins Are Recognized by Antibodies to T. gondii
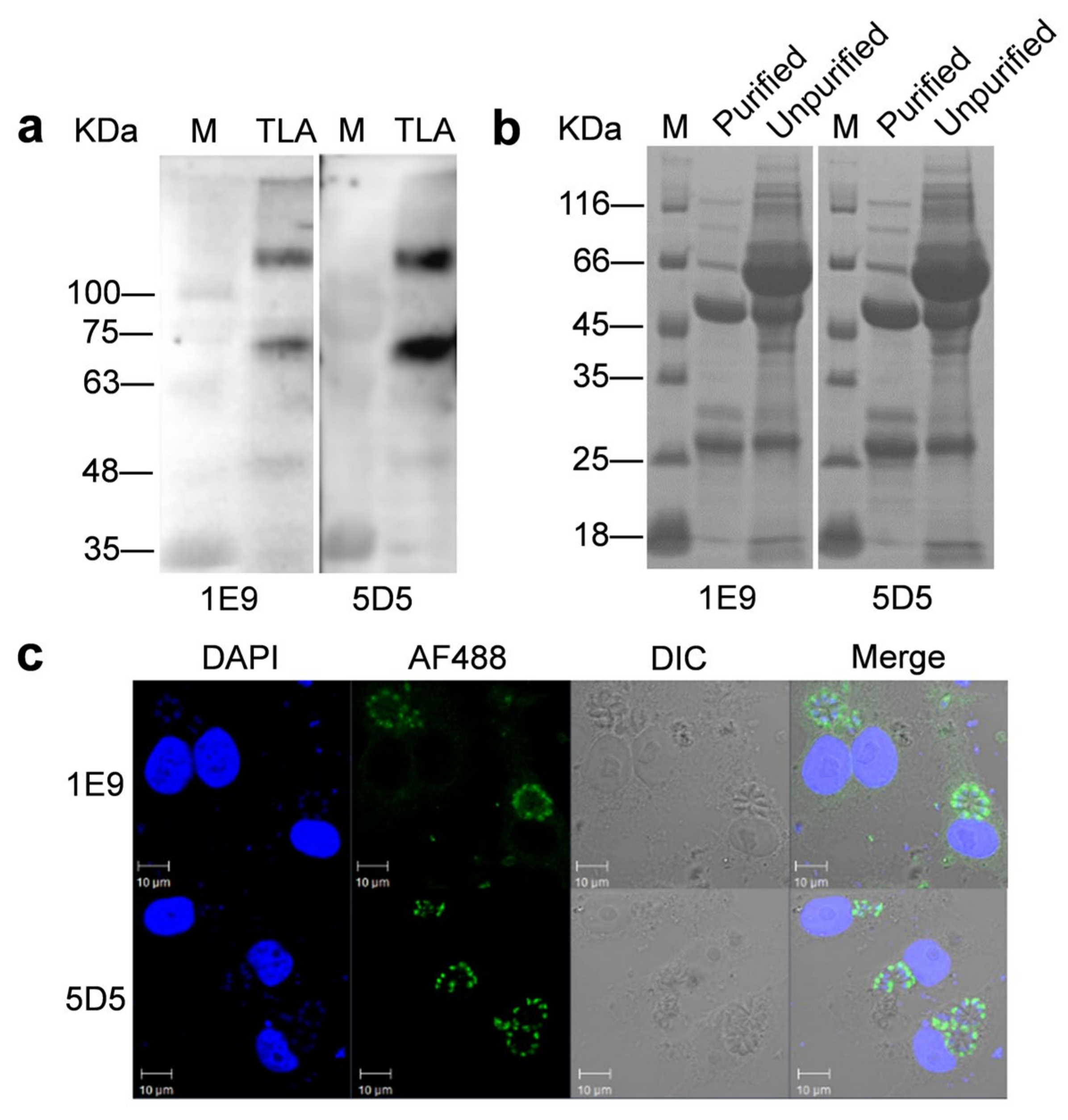
3.2. TgROP14-5D5 Is a Candidate for Colloidal Gold Strip
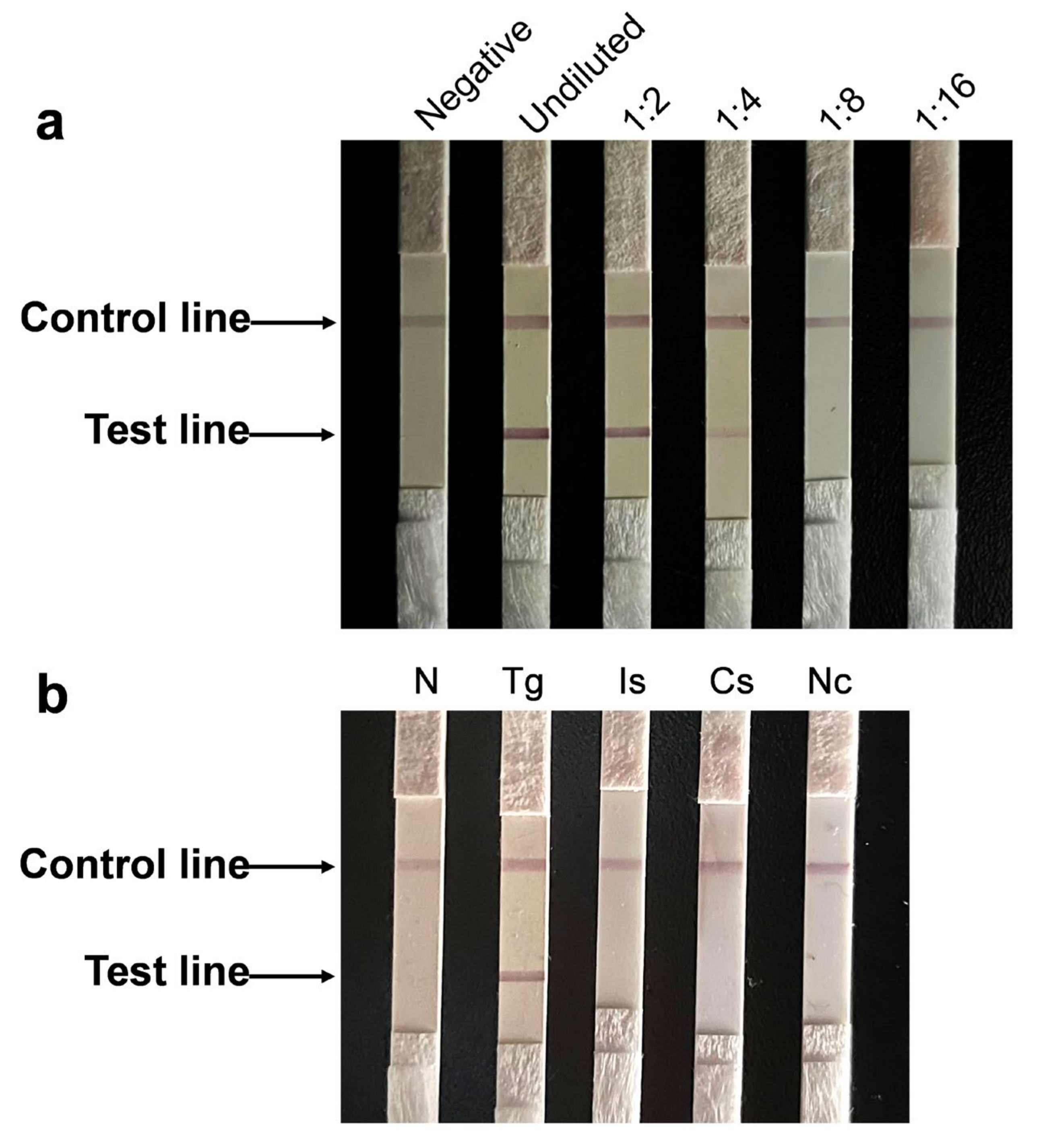
3.3. Immunochromatographic Test Using TgROP14-5D5 Is Sensitive and Specific
3.4. Newly Developed Immunochromatographic Test Is Suitable for Field Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, J.P. History of the discovery of the life cycle of Toxoplasma gondii. Int. J. Parasitol. 2009, 39, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Moncada, P.A.; Montoya, J.G. Toxoplasmosis in the fetus and newborn: An update on prevalence, diagnosis and treatment. Expert Rev. Anti-Infect. Ther. 2012, 10, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Jones, J.L. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 2008, 38, 1257–1278. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P. The History of Toxoplasma gondii-The First 100 Years. J. Eukaryot. Microbiol. 2008, 55, 467–475. [Google Scholar] [CrossRef]
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef] [Green Version]
- Dubey, J.P. Toxoplasmosis in pigs-The last 20 years. Vet. Parasitol. 2009, 164, 89–103. [Google Scholar] [CrossRef]
- Su, R.; Jiang, N.; Lu, Y.; Jian, F.; Wang, H.; Zhang, G.; Zhang, L.; Yang, Y. Low prevalence of viable Toxoplasma gondii in swine from slaughter houses in the central of China. Parasitol. Int. 2020, 76, 102090. [Google Scholar] [CrossRef]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Tao, Q.; Wang, Z.; Feng, H.; Fang, R.; Nie, H.; Hu, M.; Zhou, Y.; Zhao, J. Seroprevalence and risk factors for Toxoplasma gondii infection on pig farms in central China. J. Parasitol. 2011, 97, 262–264. [Google Scholar] [CrossRef]
- Zhou, D.H.; Liang, R.; Yin, C.C.; Zhao, F.R.; Yuan, Z.G.; Lin, R.Q.; Song, H.Q.; Zhu, X.Q. Seroprevalence of Toxoplasma gondii in pigs from southern China. J. Parasitol. 2010, 96, 673–674. [Google Scholar] [CrossRef]
- Rostami, A.; Karanis, P.; Fallahi, S. Advances in serological, imaging techniques and molecular diagnosis of Toxoplasma gondii infection. Infection 2018, 46, 303–315. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.-D.; Huang, S.-Y.; Zhu, X.-Q. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasites Vectors 2015, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Robert-Gangneux, F.; Darde, M.L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Lin, G.; Han, Y.; Li, J. Serological diagnosis of toxoplasmosis and standardization. Clin. Chim. Acta 2016, 461, 83–89. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab A Chip 2013, 13, 2210. [Google Scholar] [CrossRef]
- Huang, X.H.; Xuan, X.N.; Hirata, H.; Yokoyama, N.; Xu, L.S.; Suzuki, N.; Igarashi, I. Rapid immunochromatographic test using recombinant SAG2 for detection of antibodies against Toxoplasma gondii in cats. J. Clin. Microbiol. 2004, 42, 351–353. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.Q.; Sun, H.C.; Zhao, X.F.; Wang, S.H.; Zhuo, X.H.; Yang, Y.; Chen, X.; Yao, C.; Du, A. Development of an immunochromatographic test based on monoclonal antibodies against surface antigen 3 (TgSAG3) for rapid detection of Toxoplasma gondii. Vet. Parasitol. 2018, 252, 52–57. [Google Scholar] [CrossRef]
- Terkawi, M.A.; Kameyama, K.; Rasul, N.H.; Xuan, X.A.; Nishikawa, Y. Development of an Immunochromatographic Assay Based on Dense Granule Protein 7 for Serological Detection of Toxoplasma gondii Infection. Clin. Vaccine Immunol. 2013, 20, 596–601. [Google Scholar] [CrossRef] [Green Version]
- Ybanez, R.H.D.; Kyan, H.; Nishikawa, Y. Detection of antibodies against Toxoplasma gondii in cats using an immunochromatographic test based on GRA7 antigen. J. Vet. Med. Sci. 2020, 82, 441–445. [Google Scholar] [CrossRef] [Green Version]
- Minot, S.; Melo, M.B.; Li, F.; Lu, D.; Niedelman, W.; Levine, S.S.; Saeij, J.P.J. Admixture and recombination among Toxoplasma gondii lineages explain global genome diversity. Proc. Natl. Acad. Sci. USA 2012, 109, 13458–13463. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, C.; Maier, S.; Walker, R.A.; Rehrauer, H.; Joekel, D.E.; Winiger, R.R.; Basso, W.U.; Grigg, M.E.; Hehl, A.B.; Deplazes, P.; et al. An experimental genetically attenuated live vaccine to prevent transmission of Toxoplasma gondii by cats. Sci. Rep. 2019, 9, 1474. [Google Scholar] [CrossRef] [PubMed]
- Aubert, D.; Maine, G.T.; Villena, I.; Hunt, J.C.; Howard, L.; Sheu, M.; Brojanac, S.; Chovan, L.E.; Nowlan, S.F.; Pinon, J.M. Recombinant antigens to detect Toxoplasma gondii-specific immunoglobulin G and immunoglobulin M in human sera by enzyme immunoassay. J. Clin. Microbiol. 2000, 38, 1144–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, J.F.; Jiang, M.; Wang, Y.Y.; Qu, L.L.; Gong, R.J.; Si, J. Evaluation of a Recombinant Multiepitope Peptide for Serodiagnosis of Toxoplasma gondii Infection. Clin. Vaccine Immunol. 2012, 19, 338–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holec-Gasior, L.; Ferra, B.; Hiszczynska-Sawicka, E.; Kur, J. The optimal mixture of Toxoplasma gondii recombinant antigens (GRA1, P22, ROP1) for diagnosis of ovine toxoplasmosis. Vet. Parasitol. 2014, 206, 146–152. [Google Scholar] [CrossRef]
- Li, S.L.; Galvan, G.; Araujo, F.G.; Suzuki, Y.; Remington, J.S.; Parmley, S. Serodiagnosis of recently acquired Toxoplasma gondii infection using an enzyme-linked immunosorbent assay with a combination of recombinant antigens. Clin. Diagn. Lab. Immunol. 2000, 7, 781–787. [Google Scholar] [CrossRef] [Green Version]
- Bradley, P.J.; Ward, C.; Cheng, S.J.; Alexander, D.L.; Coller, S.; Coombs, G.H.; Dunn, J.D.; Ferguson, D.; Sanderson, S.J.; Wastling, J.M.; et al. Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J. Biol. Chem. 2005, 280, 34245–34258. [Google Scholar] [CrossRef] [Green Version]
- Kohler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Friguet, B.; Djavadi-Ohaniance, L.; Pages, J.; Bussard, A.; Goldberg, M. A convenient enzyme-linked immunosorbent assay for testing whether monoclonal antibodies recognize the same antigenic site. Application to hybridomas specific for the beta 2-subunit of Escherichia coli tryptophan synthase. J. Immunol. Methods 1983, 60, 351–358. [Google Scholar] [CrossRef]
- Duan, G.; Tian, Y.M.; Li, B.F.; Yang, J.F.; Liu, Z.L.; Yuan, F.Z.; Zhu, X.Q.; Zou, F.C. Seroprevalence of infection in pet dogs in Kunming, Southwest China. Parasit Vectors 2012, 5, 118. [Google Scholar] [CrossRef] [Green Version]
- Zou, F.C.; Sun, X.T.; Xie, Y.J.; Li, B.; Zhao, G.H.; Duan, G.; Zhu, X.Q. Seroprevalence of Toxoplasma gondii in pigs in southwestern China. Parasitol. Int. 2009, 58, 306–307. [Google Scholar] [CrossRef]
- Holec-Gasior, L. Toxoplasma gondii Recombinant Antigens as Tools for Serodiagnosis of Human Toxoplasmosis: Current Status of Studies. Clin. Vaccine Immunol. 2013, 20, 1343–1351. [Google Scholar] [CrossRef] [Green Version]
- Gamble, H.R.; Andrews, C.D.; Dubey, J.P.; Webert, D.W.; Parmley, S.F. Use of recombinant antigens for detection of Toxoplasma gondii infection in swine. J. Parasitol. 2000, 86, 459–462. [Google Scholar] [CrossRef]
- Jiang, T.; Gong, D.C.; Ma, L.A.; Nie, H.; Zhou, Y.Q.; Yao, B.A.; Zhao, J. Evaluation of a recombinant MIC3 based latex agglutination test for the rapid serodiagnosis of Toxoplasma gondii infection in swines. Vet. Parasitol. 2008, 158, 51–56. [Google Scholar] [CrossRef]
- Duong, H.D.; Taniguchi, Y.; Takashima, Y.; Sekiguchi, S.; Aye, K.M.; Ahmadi, P.; Bui, L.K.; Irie, T.; Nagayasu, E.; Yoshida, A. Diagnostic value of recombinant nanoluciferase fused Toxoplasma gondii antigens in Luciferase-linked Antibody Capture Assay (LACA) for Toxoplasma infection in pigs. J. Vet. Med. Sci. 2022, 84, 905–913. [Google Scholar] [CrossRef]
- Fabian, B.T.; Hedar, F.; Koethe, M.; Bangoura, B.; Maksimov, P.; Conraths, F.J.; Villena, I.; Aubert, D.; Seeber, F.; Schares, G. Fluorescent bead-based serological detection of Toxoplasma gondii infection in chickens. Parasit Vectors 2020, 13, 388. [Google Scholar] [CrossRef]
- Loreck, K.; Mitrenga, S.; Heinze, R.; Ehricht, R.; Engemann, C.; Lueken, C.; Ploetz, M.; Greiner, M.; Meemken, D. Use of meat juice and blood serum with a miniaturised protein microarray assay to develop a multi-parameter IgG screening test with high sample throughput potential for slaughtering pigs. BMC Vet. Res. 2020, 16, 1–14. [Google Scholar] [CrossRef]
- Peng, D.; Hu, S.; Hua, Y.; Xiao, Y.; Li, Z.; Wang, X.; Bi, D. Comparison of a new gold-immunochromatographic assay for the detection of antibodies against avian influenza virus with hemagglutination inhibition and agar gel immunodiffusion assays. Vet. Immunol. Immunopathol. 2007, 117, 17–25. [Google Scholar] [CrossRef]
- Dubey, J.P.; Cerqueira-Cezar, C.K.; Murata, F.H.A.; Kwok, O.C.H.; Hill, D.; Yang, Y.; Su, C. All about Toxoplasma gondii infections in pigs: 2009–2020. Vet. Parasitol. 2020, 288, 109185. [Google Scholar] [CrossRef]
- Su, Y.J.; Ma, Z.D.; Qiao, X.; Wang, P.T.; Kang, Y.T.; Yang, N.A.; Jia, W.; Zhao, Z.J. Geospatial epidemiology of Toxoplasma gondii infection in livestock, pets, and humans in China, 1984–2020. Parasitol. Res. 2022, 121, 743–750. [Google Scholar] [CrossRef]
- Yu, H.J.; Zhang, Z.; Liu, Z.; Qu, D.F.; Zhang, D.F.; Zhang, H.L.; Zhou, Q.J.; Du, A.F. Seroprevalence of Toxoplasma gondii infection in pigs, in Zhejiang Province, China. J. Parasitol. 2011, 97, 748–749. [Google Scholar] [CrossRef]
- Dubey, J.P.; Thulliez, P.; Weigel, R.M.; Andrews, C.; Powell, E. Sensitivity and specificity of various serologic tests for detection of Toxoplasma gondii infection in naturally infected sows. Am. J. Vet. Res. 1995, 56, 1030. [Google Scholar]
| mAb | Isotype | Titer | Additivity Index |
|---|---|---|---|
| 5D5 | IgG2a | 1:3.3 × 107 | 22.80% |
| 1E9 | IgG3 | - |
| ICT | IHA (Reference Standard) | Total | |
|---|---|---|---|
| No. of Positive | No. of Negative | ||
| No. of positive | 90 | 44 | 134 |
| No. of negative | 9 | 293 | 302 |
| Total | 99 | 337 | 436 |
| Test | No. (%) of Positive Samples | No. (%) of Negative Samples |
|---|---|---|
| ICT | 134 (38.7) | 302 (87.3) |
| IHA | 99 (22.7) | 337 (77.3) |
| Test | % (95% Confidence Interval) | |||
|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | |
| IHA as reference | ||||
| ICT | 90.9 (83.6–95.1) | 86.9 (82.9–90.1) | 67.2 (57.4–75.6) | 97.0 (94.6–98.4) |
| Composite reference standard | ||||
| ICT | 93.7 (88.5–96.7) | 100 (98.7–100) | 100 (97.4–100) | 97.0 (94.4–98.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Huang, Y.; Zhao, X.; Lin, M.; Chen, L.; Zhao, M.; Chen, X.; Yang, Y.; Ma, G.; Yao, C.; et al. Development of an Immunochromatographic Test Based on Rhoptry Protein 14 for Serological Detection of Toxoplasma gondii Infection in Swine. Animals 2022, 12, 1929. https://doi.org/10.3390/ani12151929
Yang Y, Huang Y, Zhao X, Lin M, Chen L, Zhao M, Chen X, Yang Y, Ma G, Yao C, et al. Development of an Immunochromatographic Test Based on Rhoptry Protein 14 for Serological Detection of Toxoplasma gondii Infection in Swine. Animals. 2022; 12(15):1929. https://doi.org/10.3390/ani12151929
Chicago/Turabian StyleYang, Yimin, Yechuan Huang, Xianfeng Zhao, Mi Lin, Lulu Chen, Mingxiu Zhao, Xueqiu Chen, Yi Yang, Guangxu Ma, Chaoqun Yao, and et al. 2022. "Development of an Immunochromatographic Test Based on Rhoptry Protein 14 for Serological Detection of Toxoplasma gondii Infection in Swine" Animals 12, no. 15: 1929. https://doi.org/10.3390/ani12151929
APA StyleYang, Y., Huang, Y., Zhao, X., Lin, M., Chen, L., Zhao, M., Chen, X., Yang, Y., Ma, G., Yao, C., Huang, S., & Du, A. (2022). Development of an Immunochromatographic Test Based on Rhoptry Protein 14 for Serological Detection of Toxoplasma gondii Infection in Swine. Animals, 12(15), 1929. https://doi.org/10.3390/ani12151929








