Review of Associated Health Benefits of Algal Supplementation in Cattle with Reference to Bovine Respiratory Disease Complex in Feedlot Systems
Abstract
:Simple Summary
Abstract
1. Introduction
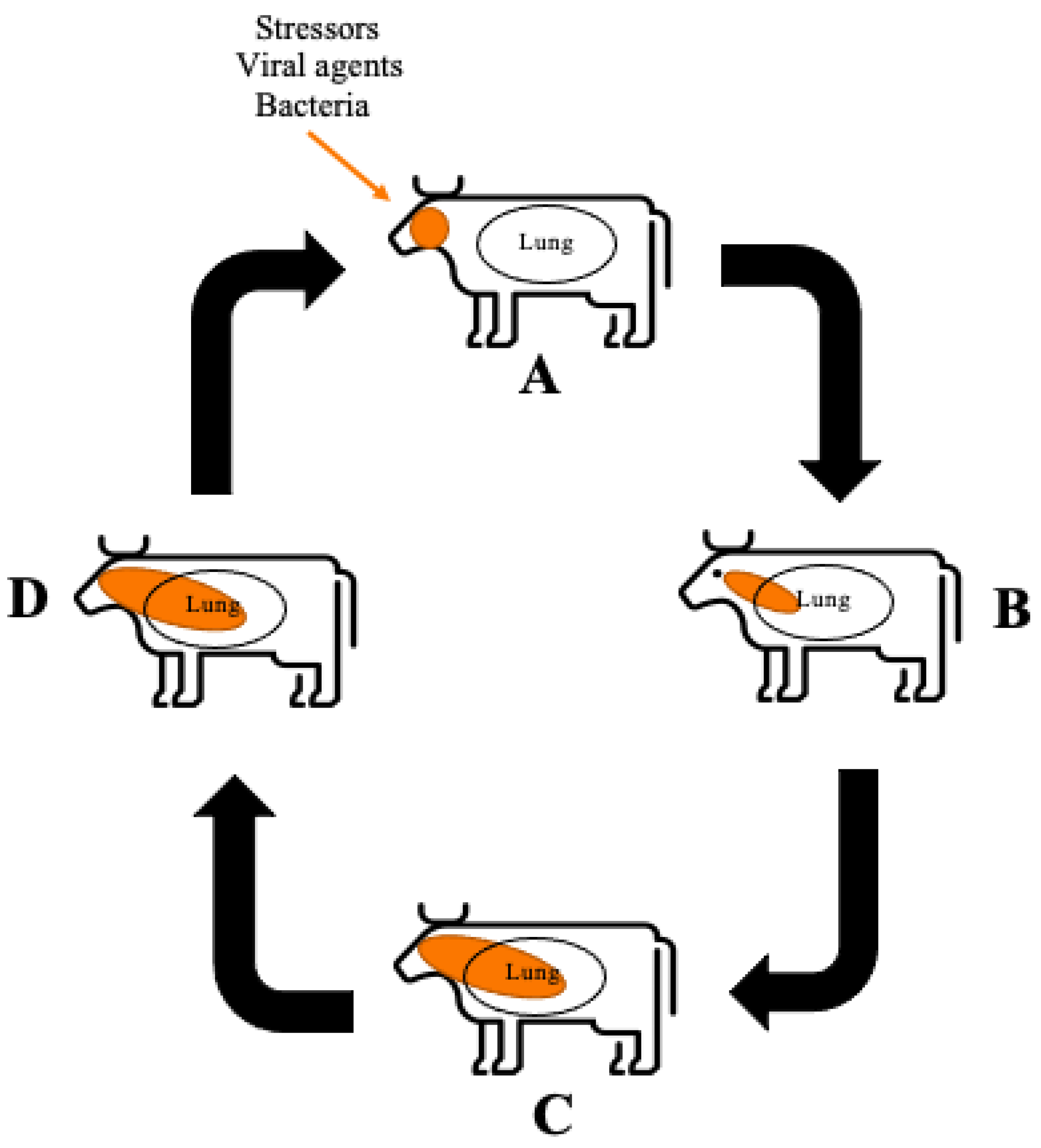
2. Bovine Respiratory Disease
Airway and Lung Epithelia
3. Immune Responses
3.1. Pro-Inflammatory Cytokines
3.2. Neutrophils
3.3. Pattern Recognition Receptors
4. Bioactive Compounds Found in Algae
4.1. Fatty Acids
4.2. Polysaccahrides
4.3. Polyphenols
4.4. Chlorophyll
5. Bioavailability of Algae Fatty Acids in Cattle
6. Health Properties of Algae-Derived Bioactive Compounds
6.1. Increased Reproduction Potential
6.2. Increased Growth Performance
6.3. Antioxidant and Immune Modulating Effects
7. Palatability of Algae Supplementation in Beef Cattle
8. Animal Product Quality
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Greenwood, P.L.; Gardner, G.E.; Ferguson, D.M. Current situation and future prospects for the Australian beef industry—A review. Asian-Australas. J. Anim. Sci. 2018, 31, 992–1006. [Google Scholar] [CrossRef]
- Hay, K.E.; Morton, J.M.; Clements, A.C.; Mahony, T.J.; Barnes, T.S. Associations between feedlot management practices and bovine respiratory disease in Australian feedlot cattle. Prev. Vet. Med. 2016, 128, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Salvin, H.E.; Lees, A.M.; Cafe, L.M.; Colditz, I.G.; Lee, C. Welfare of beef cattle in Australian feedlots: A review of the risks and measures. Anim. Prod. Sci. 2020, 60, 1569–1590. [Google Scholar] [CrossRef]
- Mosier, D. Review of BRD pathogenesis: The old and the new. Anim. Health Res. Rev. 2014, 15, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.A. The immunology of the bovine respiratory disease complex. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 535–550. [Google Scholar] [CrossRef]
- Griffin, D.; Chengappa, M.M.; Kuszak, J.; McVey, D.S. Bacterial pathogens of the bovine respiratory disease complex. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 381–394. [Google Scholar] [CrossRef]
- Chamorro, M.F.; Palomares, R.A. Bovine respiratory disease vaccination against viral pathogens: Modified-live versus inactivated antigen vaccines, intranasal versus parenteral, what is the evidence? Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 461–472. [Google Scholar] [CrossRef]
- Blakebrough-Hall, C.; McMeniman, J.P.; Gonzalez, L.A. An evaluation of the economic effects of bovine respiratory disease on animal performance, carcass traits, and economic outcomes in feedlot cattle defined using four BRD diagnosis methods. J. Anim. Sci. 2020, 98, skaa005. [Google Scholar] [CrossRef]
- Fulton, R.W.; D’Offay, J.M.; Dubovi, E.J.; Eberle, R. Bovine herpesvirus-1: Genetic diversity of field strains from cattle with respiratory disease, genital, fetal disease and systemic neonatal disease and their relationship to vaccine strains. Virus Res. 2016, 223, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Flaga, J.; Korytkowski, L.; Gorka, P.; Kowalski, Z.M. The effect of docosahexaenoic acid-rich algae supplementation in milk replacer on performance and selected immune system functions in calves. J. Dairy Sci. 2019, 102, 8862–8873. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.W. Viruses in bovine respiratory disease in North America: Knowledge advances using genomic testing. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 321–332. [Google Scholar] [CrossRef]
- Coudert, E.; Baéza, E.; Berri, C. Use of algae in poultry production: A review. World’s Poult. Sci. J. 2020, 76, 767–786. [Google Scholar] [CrossRef]
- Nayak, S.; Khozin-Goldberg, I.; Cohen, G.; Zilberg, D. Dietary supplementation with omega6 LC-PUFA-rich algae modulates zebrafish immune function and improves resistance to streptococcal infection. Front. Immunol. 2018, 9, 1960. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Hosseindoust, A.; Goel, A.; Lee, S.; Jha, P.K.; Kwon, I.K.; Chae, B.J. Effects of Ecklonia cava as fucoidan-rich algae on growth performance, nutrient digestibility, intestinal morphology and caecal microflora in weanling pigs. Asian-Australas J. Anim. Sci. 2017, 30, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overland, M.; Mydland, L.T.; Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chojnacka, K.; Wieczorek, P.P.; Schroeder, G.; Michalak, I. Algae Biomass: Characteristics and Applications. Towards Algae-Based Products; Springer: Berlin, Germany, 2018; p. 143. [Google Scholar]
- Kusmayadi, A.; Leong, Y.K.; Yen, H.W.; Huang, C.Y.; Chang, J.S. Microalgae as sustainable food and feed sources for animals and humans—Biotechnological and environmental aspects. Chemosphere 2021, 271, 129800. [Google Scholar] [CrossRef]
- Gutierrez, B.H.; Alvarez, E.; Arrizon, A.A.; Carrasco, R.; Salinas-Chavira, J.; Zinn, R.A. Influence of high-oil algae biomass as a feed intake and growth-performance enhancer in feedlot cattle during period of high ambient temperature. J. Appl. Anim. Res. 2015, 44, 118–120. [Google Scholar] [CrossRef] [Green Version]
- Ghattas, T.A.; Dawoud, E.N.; Mahrous, A.F.; Elgabry, E.A. Effect of Spirulina platensis supplementation on growth, some biochemical and immunological parameters in suckling calves. J. Egypt. Vet. Med. Assoc. 2019, 79, 443–460. [Google Scholar]
- Sharma, K.; Schenk, P.M. Rapid induction of omega-3 fatty acids (EPA) in Nannochloropsis sp. by UV-C radiation. Biotechnolgy Bioeng. 2015, 112, 1243–1249. [Google Scholar] [CrossRef]
- Figueiredo, A.R.P.; da Costa, E.; Silva, J.; Domingues, M.R.; Domingues, P. The effects of different extraction methods of lipids from Nannochloropsis oceanica on the contents of omega-3 fatty acids. Algal. Res. 2019, 41, 1–10. [Google Scholar] [CrossRef]
- Mattos, R.; Staples, C.R.; Arteche, A.; Wiltbank, M.C.; Diaz, F.J. The effects of feeding fish oil on uterine secretion of PGF2α, milk composition, and metabolic status of periparturient holstein cows. J. Dairy Sci. 2004, 87, 921–932. [Google Scholar] [CrossRef] [Green Version]
- Fritsche, K. Fatty acids as modulators of the immune response. Annu Rev. Nutr. 2006, 26, 45–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.A.; Step, D.L.; Woolums, A.R. Bovine respiratory disease: Looking back and looking forward, what do we see? Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 239–251. [Google Scholar] [CrossRef] [PubMed]
- McGill, J.L.; Sacco, R.E. The immunology of bovine respiratory disease: Recent advancements. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.R.; Derscheid, R.; Roth, J.A. Innate immunology of bovine respiratory disease. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 215–228. [Google Scholar] [CrossRef] [Green Version]
- Ferraro, S.; Fecteau, G.; Dubuc, J.; Francoz, D.; Rousseau, M.; Roy, J.P.; Buczinski, S. Scoping review on clinical definition of bovine respiratory disease complex and related clinical signs in dairy cows. J. Dairy Sci. 2021, 7095–7108. [Google Scholar] [CrossRef]
- Timsit, E.; Dendukuri, N.; Schiller, I.; Buczinski, S. Diagnostic accuracy of clinical illness for bovine respiratory disease (BRD) diagnosis in beef cattle placed in feedlots: A systematic literature review and hierarchical Bayesian latent-class meta-analysis. Prev. Vet. Med. 2016, 135, 67–73. [Google Scholar] [CrossRef]
- Bassel, L.L.; Tabatabaei, S.; Caswell, J.L. Host tolerance to infection with the bacteria that cause bovine respiratory disease. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 349–359. [Google Scholar] [CrossRef]
- Angen, O.; Thomsen, J.; Larsen, L.E.; Larsen, J.; Kokotovic, B.; Heegaard, P.M.; Enemark, J.M. Respiratory disease in calves: Microbiological investigations on trans-tracheally aspirated bronchoalveolar fluid and acute phase protein response. Vet. Microbiol. 2009, 137, 165–171. [Google Scholar] [CrossRef]
- Taylor, J.D.; Fulton, R.W.; Lehenbauer, T.W.; Step, D.L.; Confer, A.W. The epidemiology of bovine respiratory disease: What is the eveidence for predisposing factors? Can. Vet. J. 2010, 51, 1095–1102. [Google Scholar] [PubMed]
- Srikumaran, S.; Kelling, C.L.; Ambagala, A. Immune evasion by pathogens of bovine respiratory disease complex. Anim. Health Res. Rev. 2007, 8, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Zecchinon, L.; Fett, T.; Desmecht, D. How Mannheimia haemolytica defeats host defence through a kiss of death mechanism. Vet. Res. 2005, 36, 133–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindholm-Perry, A.K.; Kuehn, L.A.; McDaneld, T.G.; Miles, J.R.; Workman, A.M.; Chitko-McKown, C.G.; Keele, J.W. Complete blood count data and leukocyte expression of cytokine genes and cytokine receptor genes associated with bovine respiratory disease in calves. BMC Res. Notes 2018, 11, 786. [Google Scholar] [CrossRef]
- Gershwin, L.J. Immunology of bovine respiratory syncytial virus infection of cattle. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Czuprynski, C.J.; Charles, J.; Leite, F.; Sylte, M.; Knuckleburg, C.; Schultz, R.; Inzana, T.; Behling-Kelly, E.; Corbeil, L. Complexities of the pathogenesis of Mannheimia haemolytica and Haemophilus somnus infections: Challenges and potential opportunities for prevention? Anim. Health Res. Rev. 2004, 5, 277–282. [Google Scholar] [CrossRef]
- Ozkanlar, Y.; Aktas, M.S.; Kaynar, O.; Ozkanlar, S.; Kirecci, E.; Yildiz, L. Bovine respiratory disease in naturally infected calves: Clinical signs, blood gases and cytokine response. Vet. Med. J. 2012, 163, 123–130. [Google Scholar]
- Burciaga-Robles, L.O.; Step, D.L.; Krehbiel, C.R.; Holland, B.P.; Richards, C.J.; Montelongo, M.A.; Confer, A.W.; Fulton, R.W. Effects of exposure to calves persistently infected with bovine viral diarrhea virus type 1b and subsequent infection with Mannheima haemolytica on clinical signs and immune variables: Model for bovine respiratory disease via viral and bacterial interaction. J. Anim. Sci. 2010, 88, 2179–2188. [Google Scholar] [PubMed]
- Breider, M.A.; Walker, R.D.; Hopkins, F.M.; Schultz, T.W.; Bowersock, T.L. Pulmonary lesions induced by Pasteurella haemolytica in neutrophil sufficient and neutrophil deficient calves. Can. J. Vet. Res. 1988, 52, 205–209. [Google Scholar]
- Bassel, L.L.; Caswell, J.L. Bovine neutrophils in health and disease. Cell Tissue Res. 2018, 371, 617–637. [Google Scholar] [CrossRef] [PubMed]
- Aulik, N.A.; Hellenbrand, K.M.; Klos, H.; Czuprynski, C.J. Mannheimia haemolytica and its leukotoxin cause neutrophil extracellular trap formation by bovine neutrophils. Infect. Immun. 2010, 78, 4454–4466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radi, Z.A.; Caverly, J.M.; Dixon, R.A.; Brogden, K.A.; Ackermann, M.R. Effects of the synthetic selectin inhibitor TBC1269 on tissue damage during acute Mannheimia haemolytica-induced pneumonia in neonatal calves. Am. J. Vet. Res. 2001, 62, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Sukarni; Sudjito; Hamidi, N.; Yanuhar, U.; Wardana, I.N.G. Potential and properties of marine microalgae Nannochloropsis oculata as biomass fuel feedstock. Int. J. Energy Environ. Eng. 2014, 5, 279–290. [Google Scholar] [CrossRef] [Green Version]
- Abdelghany, M.F.; El-Sawy, H.B.; Abd El-Hameed, S.A.A.; Khames, M.K.; Abdel-Latif, H.M.R.; Naiel, M.A.E. Effects of dietary Nannochloropsis oculata on growth performance, serum biochemical parameters, immune responses, and resistance against Aeromonas veronii challenge in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 107, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Y.; Huang, X.; Cheong, K.L. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhuvana, P.; Sangeetha, P.; Anuradha, V.; Ali, M.S. Spectral characterization of bioactive compounds from microalgae: N. oculata and C. Vulgaris. Biocatal. Agric. Biotechnol. 2019, 19, 101094. [Google Scholar] [CrossRef]
- Ma, X.N.; Chen, T.P.; Yang, B.; Liu, J.; Chen, F. Lipid Production from Nannochloropsis. Mar. Drugs 2016, 14, 61. [Google Scholar] [CrossRef] [Green Version]
- Scholz, M.J.; Weiss, T.L.; Jinkerson, R.E.; Jing, J.; Roth, R.; Goodenough, U.; Posewitz, M.C.; Gerken, H.G. Ultrastructure and composition of the Nannochloropsis gaditana cell wall. Eukaryot. Cell 2014, 13, 1450–1464. [Google Scholar] [CrossRef] [Green Version]
- Lubian, L.M.; Montero, O.; Moreno-Garrido, I.; Huertas, I.E.; Sobrino, C.; Gonza’lez-del Valle, M.; Parés, G. Nannochloropsis (Eustigmatophyceae) as source of commercially valuable pigments. J. Appl. Phycol. 2000, 12, 249–255. [Google Scholar] [CrossRef]
- Adarme-Vega, T.C.; Lim, D.K.Y.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Fact. 2012, 11, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Pandeirada, C.O.; Maricato, E.; Ferreira, S.S.; Correia, V.G.; Pinheiro, B.A.; Evtuguin, D.V.; Palma, A.S.; Correia, A.; Vilanova, M.; Coimbra, M.A.; et al. Structural analysis and potential immunostimulatory activity of Nannochloropsis oculata polysaccharides. Carbohydr. Polym. 2019, 222, 114962. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, S.; Sweeney-Jones, A.M.; Mojib, N.; Dale, B.; Gagaring, K.; McNamara, C.W.; Quave, C.L.; Soapi, K.; Kubanek, J. Antibacterial oligomeric polyphenols from the green alga cladophora socialis. J. Org. Chem. 2019, 84, 5035–5045. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimzadeh, M.A.; Khalili, M.; Dehpour, A.A. Antioxidant activity of ethyl acetate and methanolic extracts of two marine algae, Nannochloropsis oculata and Gracilaria gracilis—An in vitro assay. Braz. J. Pharm. Sci. 2018, 54, 1. [Google Scholar] [CrossRef] [Green Version]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Comparison of growth rate and nutrient content of five microalgae species cultivated in greenhouses. Plants 2019, 8, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A comprehensive review of chemistry, sources and bioavailability of Omega-3 fatty acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [Green Version]
- Beauchemin, K.A. Invited review: Current perspectives on eating and rumination activity in dairy cows. J. Dairy Sci. 2018, 101, 4762–4784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, N.R.; Burns, P.D.; Cheatham, R.D.; Romero, R.M.; Nozykowski, J.P.; Bruemmer, J.E.; Engle, T.E. Fish meal supplementation increases bovine plasma and luteal tissue omega-3 fatty acid composition. J. Anim. Sci. 2012, 90, 771–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glasser, F.; Ferlay, A.; Doreau, M.; Schmidely, P.; Sauvant, D.; Chilliard, Y. Long-chain fatty acid metabolism in dairy cows: A meta-analysis of milk fatty acid yield in relation to duodenal flows and de novo synthesis. J. Dairy Sci. 2008, 91, 2771–2785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clayton, E.H. Long-Chain Omega-3 Polyunsaturated Fatty Acids in Ruminant Nutrition: Benefits to Animals and Humans; NSW Department of Primary Industries: Wagga Wagga, NSW, Australia, 2014. [Google Scholar]
- Dewhurst, R.J.; Scollan, N.D.; Lee, M.R.F.; Ougham, H.J.; Humphreys, M.O. Forage breeding and management to increase the beneficial fatty acid content of ruminant products. Proc. Nutr. Soc. 2003, 62, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Mashek, D.G.; Bertics, S.J.; Grummer, R.R. Metabolic fate of long-chain unsaturated fatty acids and their effects on palmitic acid metabolism and gluconeogenesis in bovine hepatocytes. J. Dairy Sci. 2002, 85, 2283–2289. [Google Scholar] [CrossRef] [Green Version]
- Alves, S.P.; Mendonca, S.H.; Silva, J.L.; Bessa, R.J.B. Nannochloropsis oceanica, a novel natural source of rumen-protected eicosapentaenoic acid (EPA) for ruminants. Sci. Rep. 2018, 8, 10269. [Google Scholar] [CrossRef] [PubMed]
- Flakemore, A.R.; Malau-Aduli, B.S.; Nichols, P.D.; Malau-Aduli, A.E.O. Degummed crude canola oil, sire breed and gender effects on intramuscular long-chain omega-3 fatty acid properties of raw and cooked lamb meat. J. Anim. Sci. Technol. 2017, 59, 17. [Google Scholar] [CrossRef] [Green Version]
- Stamey, J.A.; Shepherd, D.M.; de Veth, M.J.; Corl, B.A. Use of algae or algal oil rich in n-3 fatty acids as a feed supplement for dairy cattle. J. Dairy Sci. 2012, 95, 5269–5275. [Google Scholar] [CrossRef] [PubMed]
- Bowen, M.K.; Chudleigh, F.; Buck, S.; Hopkins, K. Productivity and profitability of forage options for beef production in the subtropics of northern Australia. Anim. Prod. Sci. 2018, 58, 332. [Google Scholar] [CrossRef]
- Costa, D.F.A.; Quigley, S.P.; Isherwood, P.; McLennan, S.R.; Poppi, D.P. Supplementaion of cattle fed tropical grasses with microalgae increases microbial protein production and average daily gain. Am. Soc. Anim. Sci. 2016, 94, 2047–2058. [Google Scholar] [CrossRef]
- Rossi, C.A.S.; Compiani, R.; Baldi, G.; Taylor, S.J.; Righi, F.; Simoni, M.; Quarantelli, A. Replacing sodium bicarbonate with half amount of calcareous marine algae in the diet of beef cattle. Rev. Bras. De Zootec. 2019, 48, 1–12. [Google Scholar] [CrossRef]
- Soydan, E.; Şen, U.; Şirin, E. Relationship between dietary fatty acids and reproductive functions in dairy cattle. Turk. J. Agric. Food Sci. Technol. 2017, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Sinedino, L.D.; Honda, P.M.; Souza, L.R.; Lock, A.L.; Boland, M.P.; Staples, C.R.; Thatcher, W.W.; Santos, J.E. Effects of supplementation with docosahexaenoic acid on reproduction of dairy cows. Reproduction 2017, 153, 707–723. [Google Scholar] [CrossRef] [Green Version]
- Faé Neto, W.A.; Borges Mendes, C.R.; Abreu, P.C. Carotenoid production by the marine microalgae Nannochloropsis oculata in different low-cost culture media. Aquac. Res. 2018, 49, 2527–2535. [Google Scholar] [CrossRef]
- Drewery, M.L.; Sawyer, J.E.; Wickersham, T.A. Post-extraction algal residue as a protein supplement for beef steers consuming forage: Palatability and nutrient utilization. Anim. Feed Sci. Technol. 2021, 273, 1–10. [Google Scholar] [CrossRef]
- Abdela, N. Sub-acute ruminal acidosis (SARA) and its consequence in dairy cattle: A review of past and recent research at global prospective. Achiev. Life Sci. 2016, 10, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Gruber, S.L.; Tatum, J.D.; Engle, T.E.; Chapman, P.L.; Belk, K.E.; Smith, G.C. Relationships of behavioral and physiological symptoms of preslaughter stress to beef longissimus muscle tenderness. J. Anim. Sci. 2012, 88, 1148–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Bacteria Species | Viral Species | Reference |
|---|---|---|
| Histophilus somni | Bovine herpesvirus (BHV-1) | [25] |
| Mannheimia haemolytica | Bovine parainfluenza virus 3 (PIV) | [25] |
| Pasteurella multocida | Bovine respiratory syncytial virus (BRSV) | [25] |
| Mycoplasma bovis | Bovine viral diarrhoea virus (BVDV) | [25] |
| Arcanobacterium pyogenes | [30] |
| Algae Species | Effect | Dose Rate Used of Algae | Study Design | Reference |
|---|---|---|---|---|
| Schizochytrium spp. | ↓ feed intake ↓ average daily weight gain ↓ pro-inflammatory cytokine expression | 9, 18, and 27 g DHA-RA/day | 40 female Holstein-Friesian calves, divided into four groups, fed twice daily for 49 days. | [10] |
| Chlorella sp. | ↓ feed intake ↑ N absorption and retention | 1000 g/day | 12 steers fed supplement for 4 days periods with a 3 days washout period. | [73] |
| Spirulina platensis | ↑ leukocyte count ↑ plasma globulin concentration | 6 g/day | 16 Holstein calves, divided into a control and supplement group, fed for 45 days. | [19] |
| Green and blue-green algal biomass | ↑ average daily weight gain ↑ weight gain efficiency | 60 g/day | 60 Holstein steers, divided into four experimental groups, 90 days trial. | [18] |
| Lithothamnium calcareum | ↑ growth rate ↑ feed conversion ↓ acidosis incidence ↑ calmer behaviour traits | 18.2 g/kg of DM and 15.5 g/kg of DM | 180 Charolais bullocks, divided into two groups, 130 days trial. | [69] |
| Algae product containing 10% DHA | ↑ pregnancy on first AI ↓ gestation period ↑ milk yield | 100 g/day | 1800 Holstein cows, primiparous and multiparous, 7-month trial. | [71] |
| Spirulina platensis | ↑ average daily weight gain ↑ dry matter intake ↑ forage quality | 4 g/kg | 42 steers fed supplement with varying basal diets, 10 week trial with an additional 7 day adaptation period. | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willett, M.; Campbell, M.; Schoenfeld, E.; Callcott, E. Review of Associated Health Benefits of Algal Supplementation in Cattle with Reference to Bovine Respiratory Disease Complex in Feedlot Systems. Animals 2022, 12, 1943. https://doi.org/10.3390/ani12151943
Willett M, Campbell M, Schoenfeld E, Callcott E. Review of Associated Health Benefits of Algal Supplementation in Cattle with Reference to Bovine Respiratory Disease Complex in Feedlot Systems. Animals. 2022; 12(15):1943. https://doi.org/10.3390/ani12151943
Chicago/Turabian StyleWillett, Marnie, Michael Campbell, Ebony Schoenfeld, and Esther Callcott. 2022. "Review of Associated Health Benefits of Algal Supplementation in Cattle with Reference to Bovine Respiratory Disease Complex in Feedlot Systems" Animals 12, no. 15: 1943. https://doi.org/10.3390/ani12151943






