Suitable Habitats of Chrysolophus spp. Need Urgent Protection from Habitat Fragmentation in China: Especially Suitable Habitats in Non-Nature Reserve Areas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Occurrence Records
2.2. Predictor Variable Selection
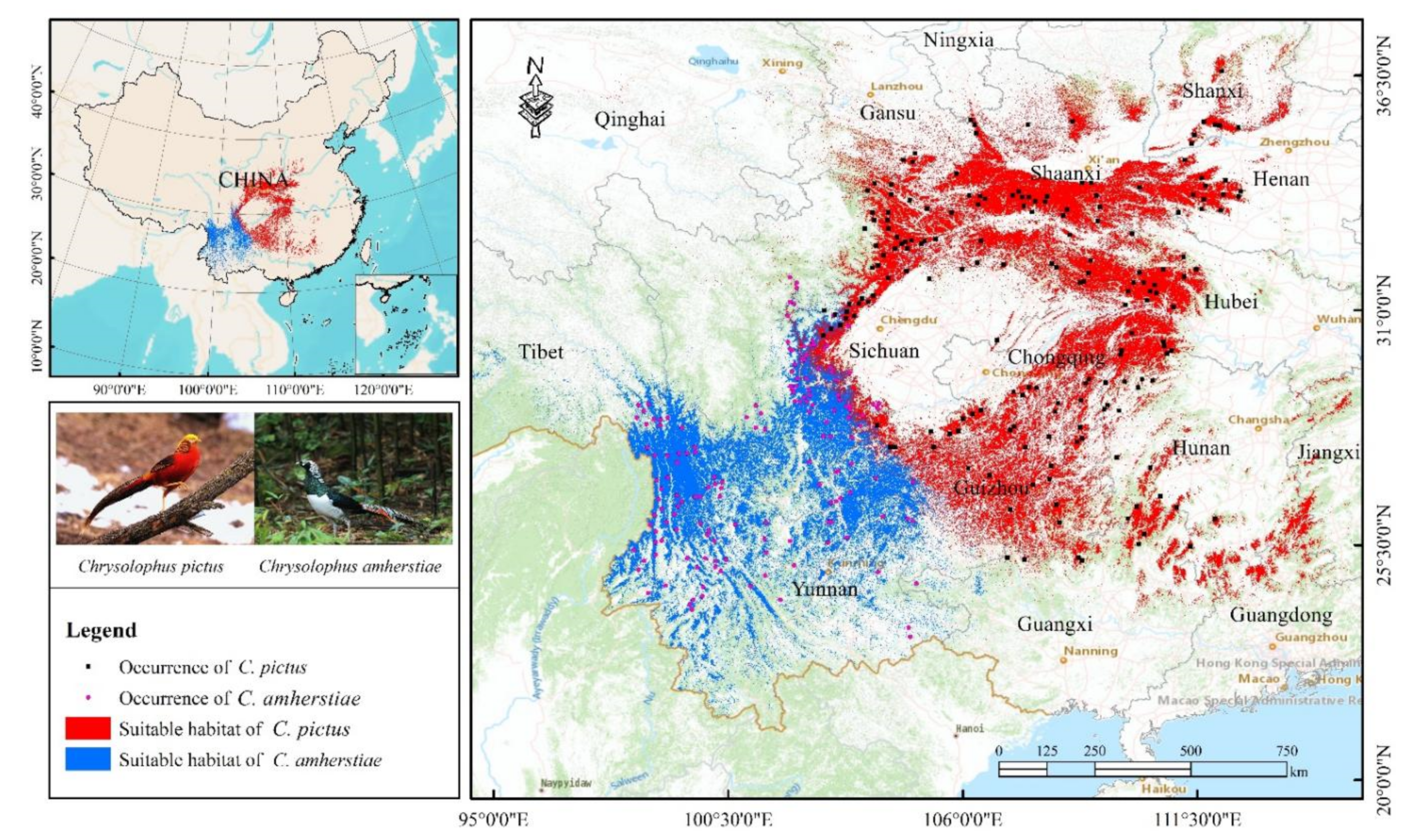
2.3. Suitable Habitat Distribution of Chrysolophus spp. and Niche Differentiation
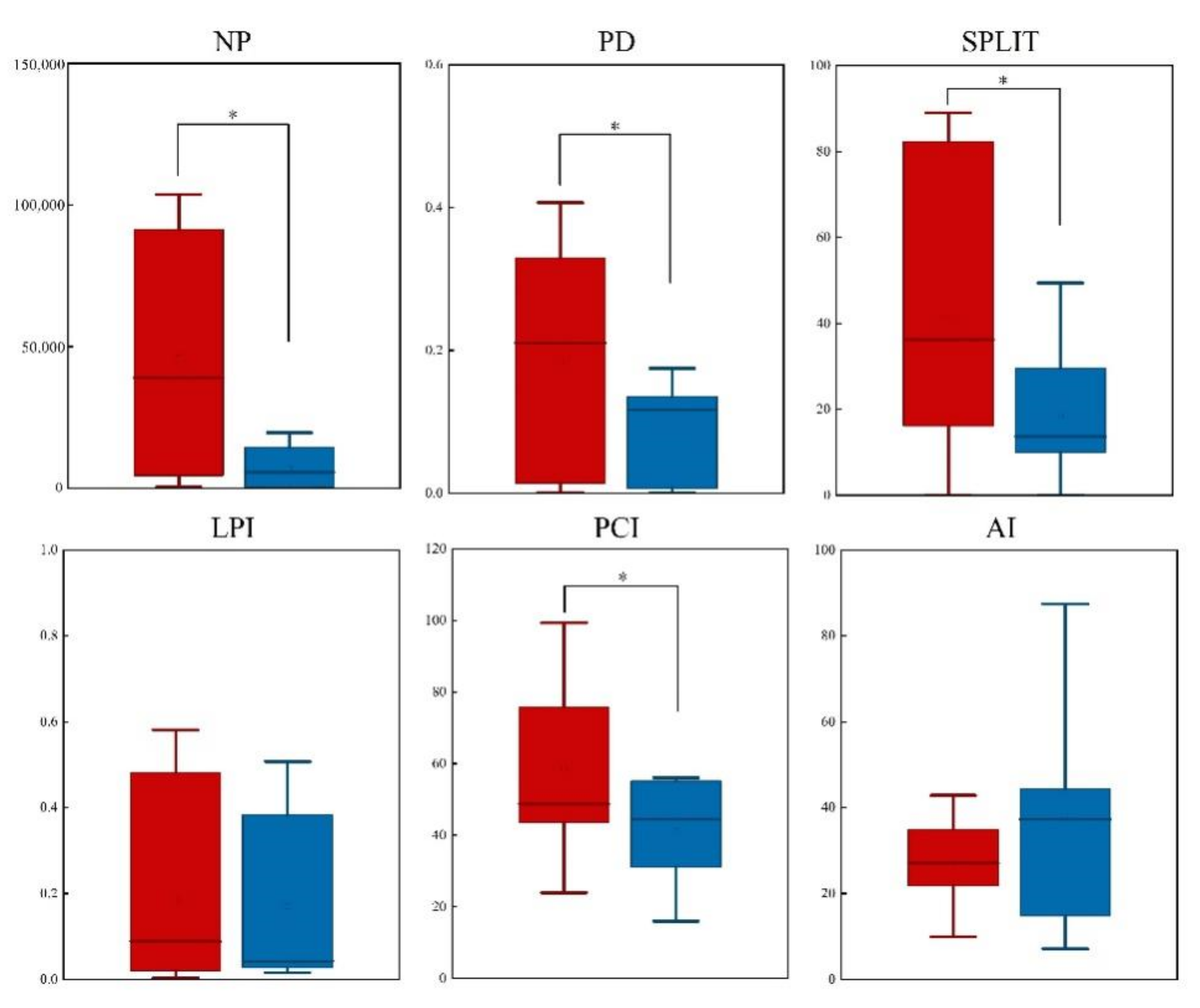
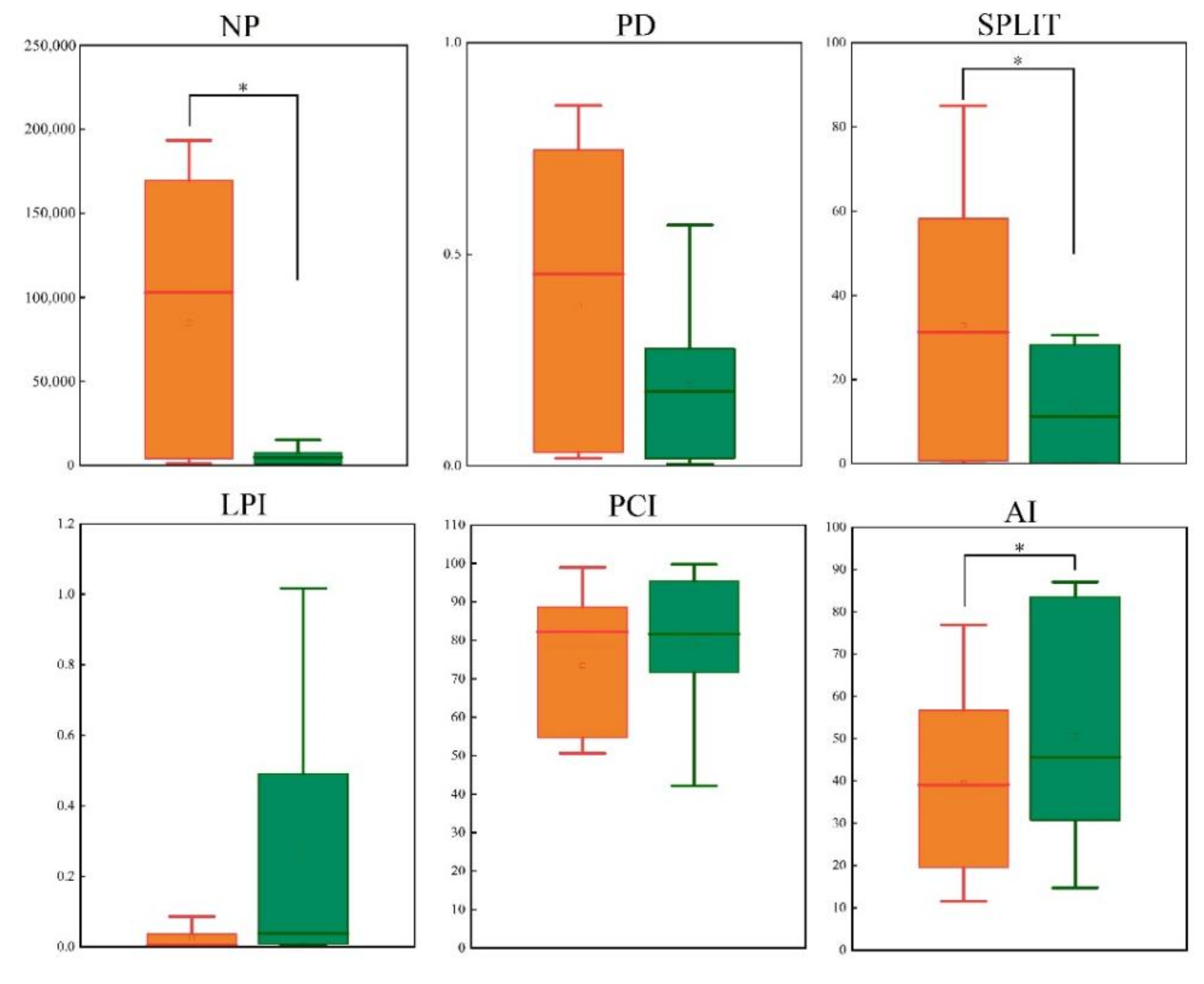
2.4. Landscape Analysis
3. Results
3.1. Model Performance for Chrysolophus spp.
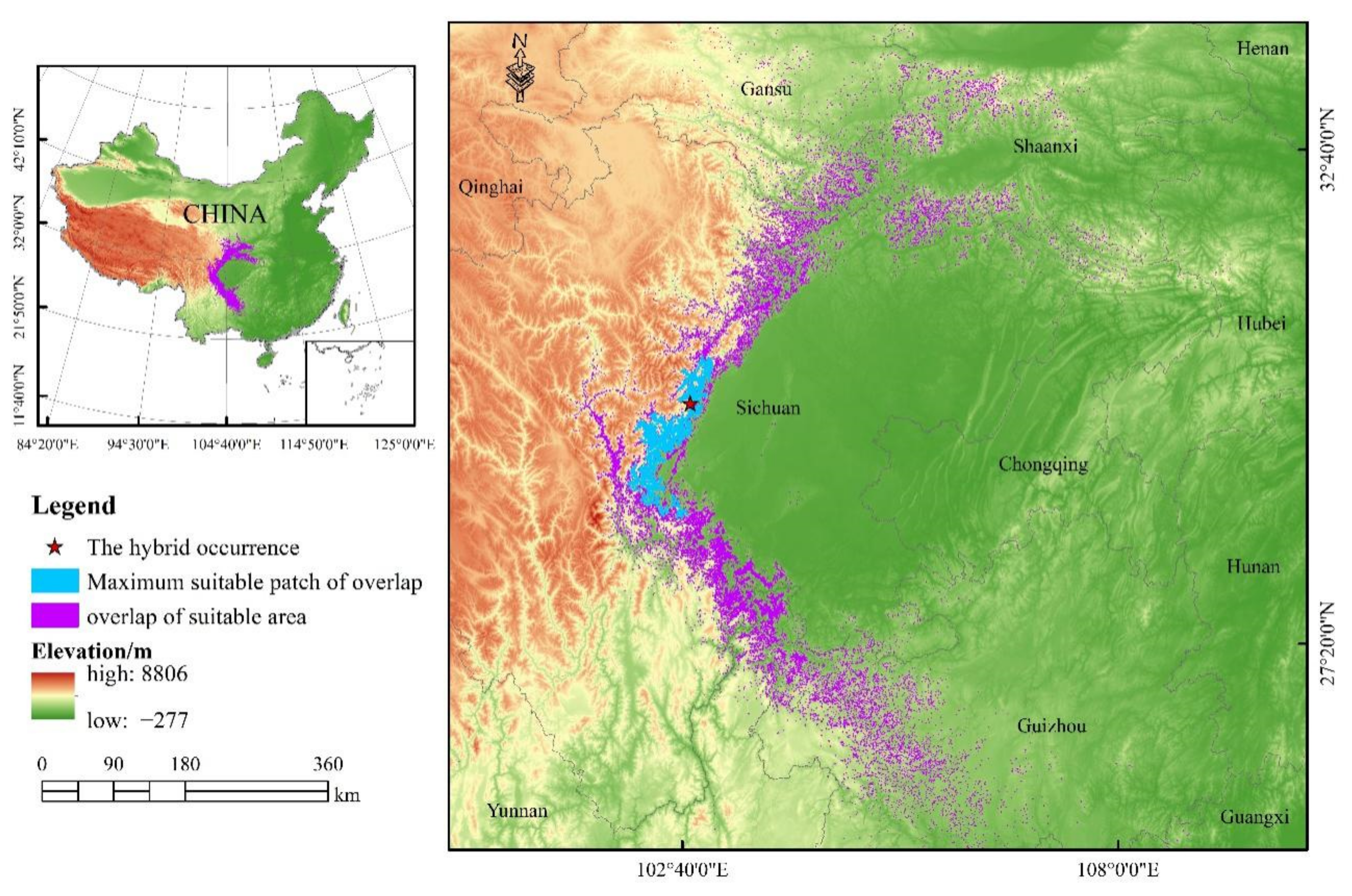
3.2. Potential Suitable Habitat and Niche Differentiation
3.3. Comparison of Potentially Suitable Habitats of Nature Reserves and Non-Nature Reserves
4. Discussion
4.1. MaxEnt Provided a Well-Predicted Potential Distribution of Chrysolophus spp.
4.2. The Existing Suitable Habitat of Chrysolophus spp. Is Shrinking
4.3. It Is Urgent to Protect Suitable Habitats in Non-Nature Reserves
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morrison, M.L.; Marcot, B.G.; Mannan, R.W. Wildlife-habitat relationship: Concepts and applications. J. Range Manag. 2007, 57, 980–981. [Google Scholar]
- Bertrand, R.; Lenoir, J.; Christian Piedallu, C.; Dillon, G.R.; Ruffray, P.D.; Vidal, C.; Pierrat, J.C.; Gégout, J.C. Changes in plant community composition lag behind climate warming in lowland forests. Nature 2011, 479, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ji, C.X.; Li, F. Global status of game hunting. Chin. J. Wildl. 2013, 34, 243–248, (In Chinese with English Abstract). [Google Scholar]
- Gibbs, J.P.; Stanton, E.J. Habitat fragmentation and arthropod community change: Carrion beetles, phoretic mites, and flies. Ecol. Appl. 2001, 11, 79–85. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, Y.; Ran, J.H. predicting suitable habitat of Chinese monal (Lophophorus lhuysii) using ecological niche modeling in the Qionglai Mountains, China. Peer. J. 2017, 5, e3477. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Poor, E.E.; Loucks, C.; Jakes, A.; Urban, D.I. Comparing habitat suitability and connectivity modeling methods for conserving pronghorn migrations. PLoS ONE 2012, 7, e49390. [Google Scholar] [CrossRef]
- Dong, X.; Chu, Y.M.R.; Gu, X.D.; Huang, Q.Y.; Zhang, J.D.; Bai, W.K. 2019. Suitable habitat prediction of Sichuan snub-nosed monkeys (Rhinopithecus roxellana) and its implications for conservation in Baihe nature reserve, Sichuan, China. Environ. Sci. Pollut. Res. 2019, 26, 32374–32384. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, L.; Wu, S.; Xia, W.C.; Yang, L.; Li, M.M.; Chen, M.H.; Luan, X.F. 2020. Use of historical data to improve conservation of the black grouse (Lyrurus tetrix) in Northeast China. Ecosphere 2020, 11, e03090. [Google Scholar] [CrossRef]
- Clements, G.R.; Rayan, D.M.; Aziz, S.A.; Kawanishi, K.; Traeholt, C.; Magintan, D.; Yazi, M.F.A.; Tingley, R. 2012. Predicting the distribution of the Asian tapir in Peninsular Malaysia using maximum entropy modeling. Integr. Zool. 2012, 7, 400–406. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, J.J.; Gao, H.M.; Cai, Z.Y.; Zhou, X.W.; Li, S.Q.; Zhang, T.Z. Musk deer (Moschus spp.) face redistribution to higher elevations and latitudes under climate change in China. Sci. Total Environ. 2019, 704, 135335. [Google Scholar] [CrossRef]
- Harte, J.; Newman, E.A. Maximum information entropy: A foundation for ecological theory. Trends Ecol. Evol. 2014, 29, 384–389. [Google Scholar] [CrossRef]
- Bai, D.F.; Chen, P.J.; Atzeni, L.; Cering, L.; Li, Q.; Shi, K. Assessment of habitat suitability of the snow leopard (Panthera uncia) in Qomolangma national nature reserve based on MaxEnt modeling. Zool. Res. 2018, 39, 373–386. [Google Scholar]
- Tobgay, S.; Mahavik, N. Potential habitat distribution of Himalayan red panda and their connectivity in Sakteng wildlife sanctuary, Bhutan. Ecol. Evol. 2020, 10, 12929–12939. [Google Scholar] [CrossRef]
- Laurance, W.F. Ecosystem Decay of Amazonian Forest Fragments: Implications for Conservation; Springer: Berlin, Germany, 2007; pp. 11–37. [Google Scholar]
- Ewers, R.M.; Didham, R.K. Confounding factors in the detection of species responses to habitat fragmentation. Biol. Rev. 2010, 81, 117–142. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Z.M. Effect of habitat fragmentation on biodiversity: A review. Chin. J. Ecol. 2014, 33, 1946–1952, (In Chinese with English Abstract). [Google Scholar]
- Zuluaga, S.; Speziale, K.L.; Lambertucci, S.A. Flying wildlife may mask the loss of ecological functions due to terrestrial habitat fragmentation. Sci. Total Environ. 2022, 803, 150034. [Google Scholar] [CrossRef]
- Wilson, J.W.; Aarde, R.J.V.; Rensburg, B.J.V. Effects of habitat fragmentation on bird communities of sand forests in southern Mozambique. Ostrich 2007, 78, 37–42. [Google Scholar] [CrossRef]
- Zheng, G.M. Pheasants in China; Higher Education Press: Beijing, China, 2015; pp. 556–616, (In Chinese with English Abstract). [Google Scholar]
- Deng, W.H. Habitat fragmentation and bird life. Acta Ecol. Sin. 2009, 29, 3181–3187, (In Chinese with English Abstract). [Google Scholar]
- Chhetri, N.B.; Dhami, B.; Neupane, B.; Sadadev, B.M.; Thapa, N. Distributional evidence and threats to cheer pheasant (Catreus wallichii) in Annapurna Conservation Area, Nepal. Nepal. J. Zool. 2020, 4, 140–146. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Li, J. The correlation analysis on the landscape pattern index and hydrological processes in the Yanhe watershed, China. J. Hydrol. 2015, 524, 417–426. [Google Scholar] [CrossRef]
- Liu, F.; McShea, W.J.; Li, D.Q. Correlating habitat suitability with landscape connectivity: A case study of Sichuan golden monkey in China. Ecol. Model. 2017, 353, 37–46. [Google Scholar] [CrossRef]
- Zhang, C.; Fan, Y.W.; Chen, M.H.; Xia, W.C.; Wang, J.D.; Zhan, Z.J.; Wang, W.L.; Khan, T.U.; Wu, S.H.; Luan, X.F. Identification of conservation priority areas and a protection network for the siberian musk deer (Moschus moschiferus L.) in Northeast China. Animals 2022, 12, 260. [Google Scholar] [CrossRef]
- XiangYu, J.G.; Yang, L.; Zhang, Y.P. Sequence divergence between Chrysolophus amherstiae and Chrysolophus pictus. HEREDITAS 2000, 22, 225–228, (In Chinese with English Abstract). [Google Scholar]
- Wang, Z.Y.; Zhang, H.J.; Wang, Q. Observation of wintering ecology of the golden pheasant in the middle range of mount Qinling. J. Hanzhong Teach. Coll. (Nat. Sci.) 1997, 15, 47–51, (In Chinese with English Abstract). [Google Scholar]
- Dickinson, C.D. The Howard and Moore Complete Checklist of the Birds of the World, 3rd ed.; Princeton University Press: Princeton, NJ, USA, 2003. [Google Scholar]
- Kang, M.J.; Zheng, G.M. Home range and habitat selection of female lady amherst’s pheasant during breeding period. J. Beijing Norm. Univ. (Nat. Sci.) 2007, 43, 558–562, (In Chinese with English Abstract). [Google Scholar]
- Huang, Z.H.; Long, J.; Zhang, L.X.; Liu, C.B.; Liu, N.F. Taxonomic relationship between Chrysolophus pictus and C. amherstiae based on mitochondrial DNA D-Loop gene. J. JiangXi Norm. Univ. (Nat. Sci.) 2006, 30, 91–94, (In Chinese with English Abstract). [Google Scholar]
- He, J.L.; Li, J.H.; Wei, L.; Zhang, J.F. Notes on some natural hybrids of Chrysolophus amherstiae and Chrysolophus pictus from Dayi county, Sichuan. Zool. Res. 1993, 14, 239–240, (In Chinese with English Abstract). [Google Scholar]
- Zhao, Y.L.; Wang, S.Y.; Duan, C.C.; Jiang, W.X.; Xu, Z.G. Behavioral rhythm of captive Chrysolophus amherstiae in summer and autumn. Chin. J. Ecol. 2018, 37, 2995–3000, (In Chinese with English Abstract). [Google Scholar]
- Liang, W.; Zheng, G.M.; Zhang, Z.W.; Ding, C.Q. Habitat use by golden pheasants (Chrysolophus amherstiae) based on radio-tracking locations. Acta Zool. Sin. 2003, 49, 179–184, (In Chinese with English Abstract). [Google Scholar]
- He, K.; Zhang, P.; Fang, S.G.; Wan, Q.H. Development and characterization of 14 novel microstatellite markers from the golden pheasant (Chrysolophus pictus). Conserv. Genet. 2009, 10, 511–513. [Google Scholar] [CrossRef]
- Huang, Z.H.; Yu, X.P.; Liang, W. Population genetic structure of golden pheasant Chrysolophus pictus in the Qinling Mountains, China. Anim. Biol. 2012, 62, 231–243. [Google Scholar] [CrossRef]
- Zhao, Z.J. A Handbook of the Birds of China (Volume Ⅰ: Non-Passerines); Jilin Science and Technology Press: Changchun, China, 2001; pp. 399–401, (In Chinese with English Abstract). [Google Scholar]
- Schantz, T.V.; Göransson, G.; Andersson, G.; Fröberg, I.; Grahn, M.; Helgée, A.; Wittzell, H. Female choice selects for a viability-based male trait in pheasants. Nature 1989, 337, 166–169. [Google Scholar] [CrossRef]
- Jiang, Z.G.; Jiang, J.P.; Wang, Y.Z.; Zhang, E.; Zhang, Y.Y.; Li, L.L.; Xie, F.; Cai, B.; Cao, L.; Zheng, G.M.; et al. Red List of China’s Vertebrates. Biodivers. Sci. 2016, 24, 500–551, (In Chinese with English Abstract). [Google Scholar]
- Golden Pheasant. Available online: https://www.iucnredlist.org/species/22679355/131874282 (accessed on 19 June 2022).
- Lady Amherst’s Pheasant. Available online: https://www.iucnredlist.org/species/22679358/131905673 (accessed on 19 June 2022).
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Rafael, J.; Alcántara, J.M.; Bastida, J.M.; Rey, P.J. Complex patterns of environmental niche evolution in Iberian columbines (genus Aquilegia, Ranunculaceae). J. Plant Ecol. 2015, 8, 457–467. [Google Scholar]
- Cui, S.P.; Luo, X.; Li, C.W.; Hu, H.J.; Jiang, Z.G. Predicting the potential distribution of white-lipped deer using the MaxEnt model. Biodivers. Sci. 2018, 26, 171–176, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Canran, L.; Matt, W.; Graeme, N.; Richard, P. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar]
- Abdolalizadeh, Z.; Ebrahimi, A.; Mostafazadeh, R. Landscape pattern change in Marakan protected area, Iran. Reg. Environ. Change 2019, 19, 1683–1699. [Google Scholar] [CrossRef]
- Dale, V.H.; Joyce, L.A.; Mcnulty, S.; Neilson, R.P.; Ayres, M.P.; Flannigan, M.D.; Hanson, P.J.; Irland, L.C.; Lugo, A.E.; Peterson, C.J.; et al. Climate change and forest disturbances. BioScience 2001, 51, 723–734. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef]
- Xia, S.S.; Hu, D.M.; Deng, Y.; Zhong, X.; Bai, W.K.; Zhang, J.D.; Wang, B.; Zhou, C.Q. Habitat partitioning between sympatric golden pheasant and temminck’s tragopan at different spatial scales. Acta Ecol. Sin. 2019, 39, 1627–1638, (In Chinese with English Abstract). [Google Scholar]
- Shi, X.J.; Fu, Q.; Wang, L.; Jiang, Z.Y.; Shi, X.G.; Li, S. Notes on the natural hybridization of golden pheasant C. pictus and lady amherst’s pheasant C. amherstiae. Chin. J. Zool. 2018, 53, 660–663, (In Chinese with English Abstract). [Google Scholar]
- Jiang, Z.G.; Fan, E.Y. Exploring the endangered species criteria: Rethinking the IUCN red list criteria. Biodivers. Sci. 2003, 11, 383–392, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Wiens, J.A. Habitat fragmentation: Island versus landscape perspectives on bird conservation. Ibis 1995, 137, 97–104. [Google Scholar] [CrossRef]
- Toledo, M.; Peña-Claros, M.; Bongers, F.; Alarcón, A.; Balcázar, J.; Chuviña, J. Distribution patterns of tropical woody species in response to climatic and edaphic gradients. J. Ecol. 2015, 100, 253–263. [Google Scholar] [CrossRef]
- Lambers, J.H.R. Extinction risks from climate change. Science 2015, 348, 501–502. [Google Scholar] [CrossRef]
- Ye, W.J.; Yang, N.; Yang, B.; Li, Y.; Zhang, J.D.; Chen, D.M.; Zhou, C.Q.; Zhong, X.; Zhang, J. Impacts of climate change on potential geographical distribution of golden pheasant (Chrysolophus pictus), an endemic species in China. Chin. J. Ecol. 2021, 40, 1783–1792, (In Chinese with English Abstract). [Google Scholar]
- Watson, J.E.M.; Dudley, N.; Segan, D.B.; Hockings, M. The performance and potential of protected areas. Nature 2014, 515, 67–73. [Google Scholar] [CrossRef]
- Radeloff, V.C.; Stewart, S.I.; Hawbaker, T.J.; Pidgeon, A.M.; Flather, C.H.; Hammer, R.B.; Helmers, D.P. Housing growth in and near United States protected areas limits their conservation value. Proc. Natl. Acad. Sci. USA 2010, 107, 940–945. [Google Scholar] [CrossRef]
- Leng, X.; Zeng, Y.; Zhou, J.; Yang, F.L.; Ye, J.; Zhang, J.; Wu, R.D. Analysis on habitat protection effectiveness of nature reserve based on landscape fragmentation in southwest China. Chin. J. Ecol. 2022, 41, 569–579, (In Chinese with English Abstract). [Google Scholar]
- Dai, B.; Chen, B.P.; Yue, B.S.; Zeng, T. A study on habitat fragmentation and conservation status of Arborophila rufipectus. Sichuan J. Zool. 2015, 34, 174–180, (In Chinese with English Abstract). [Google Scholar]
- Gao, J.X.; Liu, X.M.; Zhou, D.Q.; Ma, K.P.; Wu, Q.; Li, G.Y. Some opinions on the integration and optimization of natural protected areas in China. Biodivers. Sci. 2021, 29, 290–294, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Xia, W.C.; Zhang, C.; Zhuang, H.F.; Ren, B.P.; Zhou, J.; Shen, J.; Krzton, A.; Luan, X.F.; Li, D.Y. The potential distribution and disappearing of Yunnan snub-nosed monkey: Influences of habitat fragmentation. Glob. Ecol. Conserv. 2020, 21, e00835. [Google Scholar] [CrossRef]


| Variables | Description | Source |
|---|---|---|
| Bio3 | Isothermality | WorldClim database Version 2.0 |
| Bio4 | Temperature seasonality | WorldClim database Version 2.0 |
| Bio10 | Mean temperature of warmest quarter | WorldClim database Version 2.0 |
| Bio17 | Precipitation of driest quarter | WorldClim database Version 2.0 |
| Ele | Elevation | USGS’s Hydro-1K dataset |
| Asp | Aspect | USGS’s Hydro-1K dataset |
| Slo | Slope | USGS’s Hydro-1K dataset |
| LUCC | Classification of land use | National Tibetan Plateau Data Center |
| Pd | Population distribution | Resource and Environment Science and Data Center |
| Vt | Vegetation type | Resource and Environment Science and Data Center |
| Variables | C. pictus | C. amherstiae | ||
|---|---|---|---|---|
| Percent Contribution | Permutation Importance | Percent Contribution | Permutation Importance | |
| Bio3 | 6.5 | 1.7 | - | - |
| Bio4 | 14 | 27.4 | 41.7 | 56.7 |
| Bio10 | - | - | 4.9 | 17.1 |
| Bio17 | 8.2 | 15.1 | 4.2 | 4.1 |
| ELe | 20.1 | 34 | 23.8 | 10.1 |
| Asp | 16.1 | 11.5 | 1.3 | 1.3 |
| Slo | 7.2 | 1.8 | 3.9 | 2.8 |
| LUCC | 5.2 | 2.8 | 3.2 | 1 |
| Pd | 16 | 4.1 | 16.1 | 6.6 |
| Vt | 6.7 | 1.7 | 0.8 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Xia, W.; Zhou, E.; Li, Y.; Hu, J. Suitable Habitats of Chrysolophus spp. Need Urgent Protection from Habitat Fragmentation in China: Especially Suitable Habitats in Non-Nature Reserve Areas. Animals 2022, 12, 2047. https://doi.org/10.3390/ani12162047
Wang P, Xia W, Zhou E, Li Y, Hu J. Suitable Habitats of Chrysolophus spp. Need Urgent Protection from Habitat Fragmentation in China: Especially Suitable Habitats in Non-Nature Reserve Areas. Animals. 2022; 12(16):2047. https://doi.org/10.3390/ani12162047
Chicago/Turabian StyleWang, Peng, Wancai Xia, Enhua Zhou, Yanhong Li, and Jie Hu. 2022. "Suitable Habitats of Chrysolophus spp. Need Urgent Protection from Habitat Fragmentation in China: Especially Suitable Habitats in Non-Nature Reserve Areas" Animals 12, no. 16: 2047. https://doi.org/10.3390/ani12162047






