Long-Term Spatial Restriction Generates Deferred Limited Space Use in a Zoo-Housed Chimpanzee Group
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
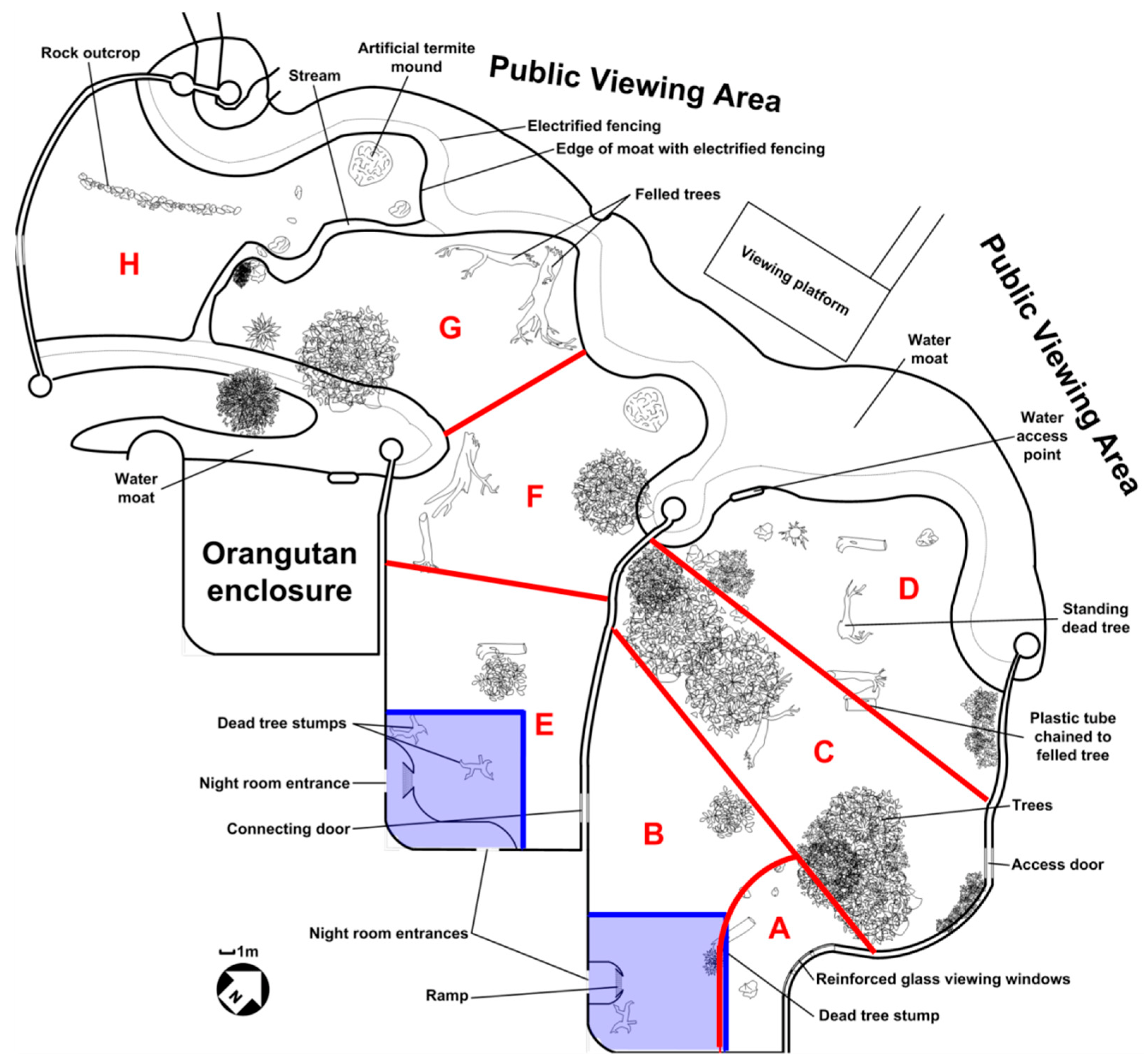
2.1. Study Subjects, Housing and Husbandry
2.2. Sampling Technique
2.2.1. Spatial Sampling
2.2.2. Enclosure Space-Use Patterns
2.2.3. Subgroup Spacing Patterns
2.2.4. Social Influences on Subgroup Spacing
2.3. Data Analysis
2.3.1. Enclosure Space Use
2.3.2. Subgroup Spacing Null Model
2.3.3. Behavioural Effects on Subgroup Spacing
2.3.4. Environmental Effects on Subgroup Spacing
2.3.5. Social Effects on Subgroup Spacing
3. Results
3.1. Space Use
3.2. Subgroup Formation
3.2.1. Behaviour
3.2.2. Environmental and Space Factors
3.2.3. Social Influences on Subgroup Formation and Spacing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hediger, H. Wild Animals in Captivity; Butterworths Scientific Publication: London, UK, 1950. [Google Scholar]
- Shettel-Neuber, J. Second and third-generation zoo exhibits: A comparison of visitor, staff, and animal responses. Environ. Behav. 1988, 20, 452–473. [Google Scholar] [CrossRef]
- Campbell, S. A new zoo? Zoonooz 1984, 55, 4–7. [Google Scholar]
- Ogden, J.J.; Finlay, T.W.; Maple, T.L. Gorilla adaptations to naturalistic environments. Zoo Biol. 1990, 9, 107–121. [Google Scholar] [CrossRef]
- Maple, T.L.; Finlay, T.W. Applied primatology in the modern zoo. Zoo Biol. 1989, 8, 101–116. [Google Scholar] [CrossRef]
- Melfi, V.A.; McCormick, W.; Gibbs, A. A preliminary assessment of how zoo visitors evaluate animal welfare according to enclosure style and the expression of behavior. Anthrozoos 2004, 17, 98–108. [Google Scholar] [CrossRef]
- Young, R.J. Environmental Enrichment for Captive Animals; Blackwell Publishing: Oxford, UK, 2003. [Google Scholar]
- Little, K.A.; Sommer, A. Change of enclosure in langur monkeys: Implications for the evaluation of environmental enrichment. Zoo Biol. 2002, 21, 549–559. [Google Scholar] [CrossRef]
- Stoinski, T.S.; Hoff, M.P.; Maple, T.L. Habitat use and structural preferences of captive Western Lowland gorillas (Gorilla gorilla gorilla): Effects of environmental and social variables. Int. J. Primatol. 2001, 22, 431–447. [Google Scholar] [CrossRef]
- Rose, P.; Evans, C.; Coffin, R.; Miller, R.; Nash, S. Using student-centred research to evidence-base exhibition of reptiles and amphibians: Three species-specific case studies. J. Zoo Aquar. Res. 2014, 2, 25–32. [Google Scholar] [CrossRef]
- Ross, S.R.; Wagner, K.; Schapiro, S.J.; Hau, J.; Lukas, K. Transfer and acclimatization effects on the behavior of two species of African great ape (Pan troglodytes and Gorilla gorilla gorilla) moved to a novel and naturalistic zoo environment. Int. J. Primatol. 2011, 32, 99–117. [Google Scholar] [CrossRef]
- Fàbregas, M.C.; Guillén-Salazar, F.; Garcés-Narro, C. Do naturalistic enclosures provide suitable environments for zoo animals? Zoo Biol. 2012, 31, 362–373. [Google Scholar] [CrossRef]
- Fuller, G.; Sadowski, L.; Cassella, C.; Lukas, K.E. Examining deep litter as environmental enrichment for a family group of wolf’s guenons, Cercopithecus wolfi. Zoo Biol. 2010, 29, 626–632. [Google Scholar] [CrossRef]
- Wehnelt, S.; Bird, S.; Lenihan, A. Chimpanzee Forest exhibit at Chester Zoo. Int. Zoo Yearb. 2006, 40, 313–322. [Google Scholar] [CrossRef]
- Frederick, C.; Fernandes, D. Behavioral changes in pottos (Perodicticus potto): Effects of naturalizing an exhibit. Int. J. Primatol. 1996, 17, 389–399. [Google Scholar] [CrossRef]
- Chamove, A.S.; Rohrhuber, B. Moving callitrichid monkeys from cages to outside areas. Zoo Biol. 1989, 8, 151–163. [Google Scholar] [CrossRef]
- Chang, T.R.; Forthman, D.L.; Maple, T.L. Comparison of confined mandrill (Mandrillus sphinx) behavior in traditional and “ecologically representative” exhibits. Zoo Biol. 1999, 18, 163–176. [Google Scholar] [CrossRef]
- Reamer, L.; Tooze, Z.; Coulson, C.; Semple, S. Correlates of self-directed and stereotypic behaviours in captive red-capped mangabeys (Cercocebus torquatus torquatus). Appl. Anim. Behav. Sci. 2010, 124, 68–74. [Google Scholar] [CrossRef]
- Stricklin, W.R.; Zhou, J.Z.; Gonyou, H.W. Selfish animats and robot ethology: Using artificial animals to investigate social and spatial behavior. Appl. Anim. Behav. Sci. 1995, 44, 187–203. [Google Scholar] [CrossRef]
- Honess, P.E.; Marin, C.M. Enrichment and aggression in primates. Neurosci. Biobehav. Rev. 2006, 30, 413–436. [Google Scholar] [CrossRef]
- Kelling, A.S.; Gaalema, D.E. Postoccupancy evaluations in zoological settings. Zoo Biol. 2011, 30, 597–610. [Google Scholar] [CrossRef]
- Melfi, V.A.; Bowkett, A.; Plowman, A.B.; Pullen, K. Do zoo designers know enough about animals. In Innovation or Replication. Proceedings of the 6th International Symposium on Zoo Design; Whitley Wildlife Conservation Trust: Paignton, UK, 2005; pp. 119–128. [Google Scholar]
- Wilson, M.; Kelling, A.; Poline, L.; Bloomsmith, M.A.; Maple, T. Post-occupancy evaluation of zoo Atlanta’s Giant Panda Conservation Center: Staff and visitor reactions. Zoo Biol. 2003, 22, 365–382. [Google Scholar] [CrossRef]
- Wesley Burgess, J. Social group spacing of rhesus macaque troops (Macaca mulatta) in outdoor enclosures: Environmental effects. Behav. Neural Biol. 1980, 30, 49–55. [Google Scholar] [CrossRef]
- Clarke, A.S.; Juno, C.J.; Maple, T.L. Behavioral effects of a change in the physical environment: A pilot study of captive chimpanzees. Zoo Biol. 1982, 1, 371–380. [Google Scholar] [CrossRef]
- Jeanson, R.; Blanco, S.; Fournier, R.; Deneubourg, J.-L.; Fourcassie, V.; Theraulaz, G. A model of animal movements in a bounded space. J. Theor. Biol. 2003, 225, 443–451. [Google Scholar] [CrossRef]
- Price, E.O. Behavioral development in animals undergoing domestication. Appl. Anim. Behav. Sci. 1999, 65, 245–271. [Google Scholar] [CrossRef]
- Welton, N.J.; McNamara, J.M.; Houston, A.I. Assessing predation risk: Optimal behaviour and rules of thumb. Theor. Popul. Biol. 2003, 64, 417–430. [Google Scholar] [CrossRef]
- Keeling, L.J. Spacing behaviour and an ethological approach to assessing optimum space allocations for groups of laying hens. Appl. Anim. Behav. Sci. 1995, 44, 171–186. [Google Scholar] [CrossRef]
- Keeling, L.J.; Duncan, I.J.H. Social spacing in domestic fowl under seminatural conditions: The effect of behavioural activity and activity transitions. Appl. Anim. Behav. Sci. 1991, 32, 205–217. [Google Scholar] [CrossRef]
- Tamburello, N.; Côté, I.M.; Dulvy, N.K. Energy and the scaling of animal space use. Am. Nat. 2015, 186, 196–211. [Google Scholar] [CrossRef]
- Gordon, D.M. The population consequences of territorial behaviour. Trends Ecol. Evol. 1997, 12, 63–66. [Google Scholar] [CrossRef]
- Estevez, I.; Christman, M.C. Analysis of the movement and use of space of animals in confinement: The effect of sampling effort. Appl. Anim. Behav. Sci. 2006, 97, 221–240. [Google Scholar] [CrossRef]
- Mallapur, A.; Sinha, A.; Waran, N. Influence of visitor presence on the behaviour of captive lion-tailed macaques (Macaca silenus) housed in Indian zoos. Appl. Anim. Behav. Sci. 2005, 94, 341–352. [Google Scholar] [CrossRef]
- Bloomfield, R.C.; Gillespie, G.R.; Kerswell, K.J.; Butler, K.L.; Hemsworth, P.H. Effect of partial covering of the visitor viewing area window on positioning and orientation of zoo orangutans: A preference test. Zoo Biol. 2015, 34, 223–229. [Google Scholar] [CrossRef]
- Brereton, J.E. Directions in animal enclosure use studies. J. Zoo Aquar. Res. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Ross, S.R.; Lukas, K.E. Use of space in a non-naturalistic environment by chimpanzees (Pan troglodytes) and lowland gorillas (Gorilla gorilla gorilla). Appl. Anim. Behav. Sci. 2006, 96, 143–152. [Google Scholar] [CrossRef]
- Ross, S.R.; Schapiro, S.J.; Hau, J.; Lukas, K.E. Space use as an indicator of enclosure appropriateness: A novel measure of captive animal welfare. Appl. Anim. Behav. Sci. 2009, 121, 42–50. [Google Scholar] [CrossRef]
- Ross, S.R.; Calcutt, S.; Schapiro, S.J.; Hau, J. Space use selectivity by chimpanzees and gorillas in an indoor–outdoor enclosure. Am. J. Primatol. 2011, 73, 197–208. [Google Scholar] [CrossRef]
- McBride, G. Theories of animal spacing: The role of flight, fight and social distance. In Behaviour and Environment: The Use of Space by Animals and Men; Esser, A.H., Ed.; Plenum Press: New York, NY, USA, 1971. [Google Scholar]
- Duncan, L.M.; Jones, M.A.; van Lierop, M.; Pillay, N. Chimpanzees use multiple strategies to limit aggression and stress during spatial density changes. Appl. Anim. Behav. Sci. 2013, 147, 159–171. [Google Scholar] [CrossRef]
- De Waal, F.B.M. The myth of a simple relation between space and aggression in captive primates. Zoo Biol. 1989, 8, 141–148. [Google Scholar] [CrossRef]
- Videan, E.N.; Fritz, J. Effects of short- and long-term changes in spatial density on the social behavior of captive chimpanzees (Pan troglodytes). Appl. Anim. Behav. Sci. 2007, 102, 95–105. [Google Scholar] [CrossRef]
- Bettinger, T.; Wallis, J.; Carter, T. Spatial selection in captive adult female chimpanzees. Zoo Biol. 1994, 13, 167–176. [Google Scholar] [CrossRef]
- Hedeen, S.E. Utilization of space by captive groups of Lowland gorillas (Gorilla g. gorilla). Ohio J. Sci. 1982, 82, 27–30. [Google Scholar]
- Nash, L.T.; Chilton, S.-M. Space or novelty?: Effects of altered cage size on Galago behavior. Am. J. Primatol. 1986, 10, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Bateson, P. Measuring Behaviour; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]
- Goodall, J. The Chimpanzees of Gombe: Patterns of Behaviour; Harvard University Press: Cambridge, UK, 1986. [Google Scholar]
- Jones, M.; van Lierop, M.; Pillay, N. All a mother’s fault? Transmission of stereotypy in striped mice Rhabdomys. Appl. Anim. Behav. Sci. 2008, 115, 82–89. [Google Scholar] [CrossRef]
- Plowman, A.B. A note on a modification of the spread of participation index allowing for unequal zones. Appl. Anim. Behav. Sci. 2003, 83, 331–336. [Google Scholar] [CrossRef]
- Rose, P.; Robert, R. Evaluating the activity patterns and enclosure usage of a little-studied zoo species, the sitatunga (Tragelaphus spekii). J. Zoo Aquar. Res. 2013, 1, 14–19. [Google Scholar] [CrossRef]
- Bates, L.; Byrne, R. Sex differences in the movement patterns of free-ranging chimpanzees (Pan troglodytes schweinfurthii): Foraging and border checking. Behav. Ecol. Sociobiol. 2009, 64, 247–255. [Google Scholar] [CrossRef]
- Wrangham, R.W.; Smuts, B.B. Sex differences in the behavioural ecology of chimpanzees in the Gombe National Park, Tanzania. J. Reprod. Fertil. Suppliment 1980, 28, 13–31. [Google Scholar]
- Pederson, A.K.; King, J.E.; Landau, V.I. Chimpanzee (Pan troglodytes) personality predicts behavior. J. Res. Pers. 2005, 39, 534–549. [Google Scholar] [CrossRef]
- Sibbald, A.M.; Shellard, L.J.F.; Smart, T.S. Effects of space allowance on the grazing behaviour and spacing of sheep. Appl. Anim. Behav. Sci. 2000, 70, 49–62. [Google Scholar] [CrossRef]
- Vestal, B.M.; Stoep, A. V Effect of distance between feeders on aggression in captive chamois (Rupicapra rupicapra). Appl. Anim. Ethol. 1978, 4, 253–260. [Google Scholar] [CrossRef]
- Duncan, L.M.; Pillay, N. Shade as a thermoregulatory resource for captive chimpanzees. J. Therm. Biol. 2013, 38, 169–177. [Google Scholar] [CrossRef]
- StatSoft, Inc. STATISTICA (Data Analysis Software System); Tulsa, OK, USA.
- Compix. SimplePCI; Version 5.1.0.0110; Compix: Irvine, CA, USA.
- DeVries, H.; Netto, W.J.; Hanegraaf, P.L.H. Matman: A program for the analysis of sociometric matrices and behavioural transition matrices. Behaviour 1993, 125, 157–175. [Google Scholar]
- Onghena, P.; Edgington, E.S. Randomization tests for restricted alternating treatments designs. Behav. Res. Ther. 1994, 32, 783–786. [Google Scholar] [CrossRef]
- Ryan, E.B.; Proudfoot, K.L.; Fraser, D. The effect of feeding enrichment methods on the behavior of captive Western lowland gorillas. Zoo Biol. 2012, 31, 235–241. [Google Scholar] [CrossRef]
- Blois-Heulin, C.; Martinez-Cruz, B. Influence of food dispersion on feeding activity and social interactions in captive Lophocebus albigena and Cercocebus torquatus torquatus. Primates 2005, 46, 77–90. [Google Scholar] [CrossRef]
- Michels, A.M. Sex differences in food acquisition and aggression in captive common marmosets (Callithrix jacchus). Primates 1998, 39, 549. [Google Scholar] [CrossRef]
- Vignes, S.; Newman, J.D.; Roberts, R.L. Mealworm feeders as environmental enrichment for common marmosets. J. Am. Assoc. Lab. Anim. Sci. 2001, 40, 26–29. [Google Scholar]
- Coe, J.C. Naturalizing habitats for captive primates. Zoo Biol. 1989, 8, 117–125. [Google Scholar] [CrossRef]
- Videan, E.N.; Fritz, J.; Schwandt, M.; Howell, S. Neighbor effect: Evidence of affiliative and agonistic social contagion in captive chimpanzees (Pan troglodytes). Am. J. Primatol. 2005, 66, 131–144. [Google Scholar] [CrossRef]
- Baker, K.C.; Aureli, F. The neighbor effect: Other groups influence intragroup agonistic behavior in captive chimpanzees. Am. J. Primatol. 1996, 40, 283–291. [Google Scholar] [CrossRef]
- Carlstead, K.; Brown, J.L.; Seidensticker, J. Behavioral and adrenocortical responses to environmental changes in leopard cats (Felis bengalensis). Zoo Biol. 1993, 12, 321–331. [Google Scholar] [CrossRef]
- Whitham, J.C.; Miller, L. A Zoo Animal’s Neighbourhood: How Conspecific Neighbours Impact Welfare. Anim. Welfare2 2019, 28, 127–136. [Google Scholar] [CrossRef]
- Anderson, C.; Yngvesson, J.; Boissy, A.; Uvnäs-Moberg, K.; Lidfors, L. Behavioural expression of positive anticipation for food or opportunity to play in lambs. Behav. Processes 2015, 113, 152–158. [Google Scholar] [CrossRef]
- Van der Harst, J.E.; Fermont, P.C.J.; Bilstra, A.E.; Spruijt, B.M. Access to enriched housing is rewarding to rats as reflected by their anticipatory behaviour. Anim. Behav. 2003, 66, 493–504. [Google Scholar] [CrossRef]
- Ratuski, A.S.; Makowska, I.J.; Dvorack, K.R.; Weary, D.M. Using approach latency and anticipatory behaviour to assess whether voluntary playpen access is rewarding to laboratory mice. Sci. Rep. 2021, 11, 18683. [Google Scholar] [CrossRef]
- Sánchez-Vázquez, F.J.; Madrid, J.A. Feeding anticipatory activity. In Food Intake in Fish; Houlihan, D., Boujard, T., Jobling, M., Eds.; Blackwell Science Ltd.: London, UK, 2001; pp. 216–232. [Google Scholar]
- Morgan, K.N.; Tromborg, C.T. Sources of stress in captivity. Appl. Anim. Behav. Sci. 2007, 102, 262–302. [Google Scholar] [CrossRef]
- Wells, D.L. A note on the influence of visitors on the behaviour and welfare of zoo-housed gorillas. Appl. Anim. Behav. Sci. 2005, 93, 13–17. [Google Scholar] [CrossRef]
- Carder, G.; Semple, S. Visitor effects on anxiety in two captive groups of western lowland gorillas. Appl. Anim. Behav. Sci. 2008, 115, 211–220. [Google Scholar] [CrossRef]
- Duncan, L.M.; Pillay, N. Volunteer experience influences the conclusions of behavioural experiments. Appl. Anim. Behav. Sci. 2012, 140, 179–187. [Google Scholar] [CrossRef]
- Hosey, G.R. How does the zoo environment affect the behaviour of captive primates? Appl. Anim. Behav. Sci. 2005, 95, 107–129. [Google Scholar] [CrossRef]
- Fernandez, E.J.; Tamborski, M.A.; Pickens, S.R.; Timberlake, W. Animal-visitor interactions in the modern zoo: Conflicts and interventions. Appl. Anim. Behav. Sci. 2009, 120, 1–8. [Google Scholar] [CrossRef]
- Cook, S.; Hosey, G.R. Interaction sequences between chimpanzees and human visitors at the zoo. Zoo Biol. 1995, 14, 431–440. [Google Scholar] [CrossRef]
- Hyatt, C.W.; Hopkins, W.D. Interspecies object exchange: Bartering in apes? Behav. Processes 1998, 42, 177–187. [Google Scholar] [CrossRef]
- Honess, P.E.; Marin, C.M. Behavioural and physiological aspects of stress and aggression in nonhuman primates. Neurosci. Biobehav. Rev. 2006, 30, 390–412. [Google Scholar] [CrossRef]
- Southwick, C.H. An experimental study of intragroup agonistic behavior in Rhesus monkeys (Macaca mulatta). Behaviour 1967, 28, 182–209. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.M.; Oehlert, G.W.; Carlis, J.V.; Pusey, A.E. Why do male chimpanzees defend a group range? Anim. Behav. 2004, 68, 523–532. [Google Scholar] [CrossRef]
- Wittenberger, J.F. Animal Social Behaviour; Duxbury Press: Boston, MA, USA, 1981. [Google Scholar]
- Doran, D. Influence of seasonality on activity patterns, feeding behavior, ranging, and grouping patterns in Taï chimpanzees. Int. J. Primatol. 1997, 18, 183–206. [Google Scholar] [CrossRef]
- Simon, A.F.; Chou, M.-T.; Salazar, E.D.; Nicholson, T.; Saini, N.; Metchev, S.; Krantz, D.E. A simple assay to study social behavior in Drosophila: Measurement of social space within a group1. Genes Brain Behav. 2012, 11, 243–252. [Google Scholar] [CrossRef]
- Judge, P.G.; de Waal, F.B.M. Rhesus monkey behaviour under diverse population densities: Coping with long-term crowding. Anim. Behav. 1997, 54, 643–662. [Google Scholar] [CrossRef]
- Hedeen, S.E. The use of space by Lowland gorillas (Gorilla g. gorilla) in an outdoor enclosure. Ohio J. Sci. 1983, 83, 183–185. [Google Scholar]
- Ross, S.R.; Shender, M.A. Daily travel distances of zoo-housed chimpanzees and gorillas: Implications for welfare assessments and space requirements. Primates 2016, 57, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Petty, F.; Chae, Y.; Kramer, G.L.; Jordan, S.; Wilson, L. Learned helplessness sensitizes hippocampal norepinephrine to mild restress. Biol. Psychiatry 1994, 35, 903–908. [Google Scholar] [CrossRef]
- Overmier, J.B.; Seligman, M.E. Effects of inescapable shock upon subsequent escape and avoidance responding. J. Comp. Physiol. Psychol. 1967, 63, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Seligman, M.E.; Maier, S.F.; Geer, J.H. Alleviation of learned helplessness in the dog. J. Abnorm. Psychol. 1968, 73, 256–262. [Google Scholar] [CrossRef]
- Maier, S.F.; Seligman, M.E. Learned helplessness: Theory and evidence. J. Exp. Psychol. Gen. 1976, 105, 3–46. [Google Scholar] [CrossRef]
- Maier, S.F. Learned helplessness and animal models of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1984, 8, 435–446. [Google Scholar] [CrossRef]
- Seligman, M.E.P. Learned Helplessness. Annu. Rev. Med. 1972, 23, 407–412. [Google Scholar] [CrossRef]
- Maier, S.F. Learned helplessness and the schedule-shift hypotheses. J. Res. Pers. 1980, 14, 170–186. [Google Scholar] [CrossRef]
- Seligman, M.E.; Beagley, G. Learned helplessness in the rat. J. Comp. Physiol. Psychol. 1975, 88, 534–541. [Google Scholar] [CrossRef]
- Seligman, M.E.; Maier, S.F. Failure to escape traumatic shock. J. Exp. Psychol. 1967, 74, 1–9. [Google Scholar] [CrossRef]
- Musty, R.E.; Jordan, M.P.; Lenox, R.H. Criterion for learned helplessness in the rat: A redefinition. Pharmacol. Biochem. Behav. 1990, 36, 739–744. [Google Scholar] [CrossRef]
- Petty, F.; Kramer, G.L.; Jordan, S. A neuronal model for learned helplessness in the rat. Biol. Psychiatry 1994, 35, 705–706. [Google Scholar] [CrossRef]
- Maier, S.F. Exposure to the stressor environment prevents the temporal dissipation of behavioral depression/learned helplessness. Biol. Psychiatry 2001, 49, 763–773. [Google Scholar] [CrossRef]
- Beasor, T. Great Negotiators: How the Most Successful Negotiators Think and Behave; Gower Publishing Limited: Hampshire, UK, 2006. [Google Scholar]
- Ziglar, Z. See You at the Top, 2nd ed.; Pelican Publishing Company: Gretna, LA, USA, 2005. [Google Scholar]
- D’Egidio, C. Space Use Following Restricted Space Availability in Two Mammals and Two Arthropods. Master’s Dissertation, University of the Witwatersrand, Johannesburg, South Africa, 2012. [Google Scholar]
- Carducci, J.P.; Jakob, E.M. Rearing environment affects behaviour of jumping spiders. Anim. Behav. 2000, 59, 39–46. [Google Scholar] [CrossRef]
- Martin-Ordas, G.; Berntsen, D.; Call, J. Memory for distant past events in chimpanzees and orangutans. Curr. Biol. 2013, 23, 1438–1441. [Google Scholar] [CrossRef]
- Mason, G.; Clubb, R.; Latham, N.; Vickery, S. Why and how should we use environmental enrichment to tackle stereotypic behaviour? Appl. Anim. Behav. Sci. 2007, 102, 163–188. [Google Scholar] [CrossRef] [Green Version]
- Swaisgood, R.R.; Shepherdson, D.J. Scientific approaches to enrichment and stereotypies in zoo animals: What’s been done and where should we go next? Zoo Biol. 2005, 24, 499–518. [Google Scholar] [CrossRef]
- Jones, B.; McGreevy, P. How much space does an elephant need? The impact of confinement on animal welfare. J. Vet. Behav. Clin. Appl. Res. 2007, 2, 185–187. [Google Scholar] [CrossRef]
- Vigilant, L. Chimpanzees. Curr. Biol. 2004, 14, R369–R371. [Google Scholar] [CrossRef]
- Cowlishaw, G.; Dunbar, R. Primate Conservation Biology; University of Chicago Press: London, UK, 2000. [Google Scholar]
- Dunbar, R.; Barrett, L. Cousins: Our Primate Relatives; BBC Worldwide Ltd.: London, UK, 2000. [Google Scholar]
- Janmaat, K.R.L.; Ban, S.D.; Boesch, C. Chimpanzees use long-term spatial memory to monitor large fruit trees and remember feeding experiences across seasons. Anim. Behav. 2013, 86, 1183–1205. [Google Scholar] [CrossRef]
- Rowe, N. The Pictorial Guide to the Living Primates; Pogonias Press: Charlestown, RI, USA, 1996. [Google Scholar]
- Druce, H.C.; Pretorius, K.; Slotow, R. The response of an elephant population to conservation area expansion: Phinda Private Game Reserve, South Africa. Biol. Conserv. 2008, 141, 3127–3138. [Google Scholar] [CrossRef]


| Behaviour | Definition |
|---|---|
| Locomotion | Movement from one location to another, not involving searching for food. Included walking, running and climbing |
| Foraging | Activity related to the searching for, manipulation or consumption of food or drink |
| Socio-negative | Chasing aggressively (characterised by sneering, open and closed grins and compressed lip faces. Usually associated with screams, barks and “wraaa” calls [48]) or overt fighting. Included aggressive gesturing or signalling |
| Socio-positive | Affiliative behaviours such as social grooming and embracing directed at other chimpanzees |
| Play | Social play (wrestling, playful biting and playful chasing characterised by a relaxed face, possibly with a drooping lower lip, or a full play face. Usually silent but may include soft grunts or hoots [48]), object play (play directed at or involving an inanimate object) and locomotor play (solitary active play. Included running, rolling, swinging or somersaulting) |
| Inactivity | Resting, either standing or sitting down, or sleeping |
| Abnormal | Coprophagia/urophagia, self-mutilation, faeces throwing and hair plucking. Other behaviours were scored as abnormal based on the context in which they occurred and whether they occurred repetitively (>3 times in succession [49]). These included nipple pulling, abnormal gait and posturing and chronic masturbation |
| Public Interaction | Attempts by the chimpanzees to engage with the public through the viewing windows or fences |
| Hidden | Chimpanzees were obscured from view or behaviour was not identifiable according to the other categories listed |
| Parameters | Wald χ2 Statistics | Spearman’s rho (ρ) |
|---|---|---|
| Time of day | χ22 = 0.128; p = 0.938 | |
| Section of enclosure | χ21 = 0.01; p = 0.997 | |
| Time of day × Section of enclosure | χ22 = 0.931; p = 0.628 | |
| Weather conditions at start of session | χ21 = 3.240; p = 0.072 | |
| Maximum daily temperature | χ21 = 0.993; p = 0.319 | |
| Percentage available shade in enclosure at start of session | χ21 = 0.604; p = 0.437 | |
| Shade index | χ21 = 0.541; p = 0.462 | |
| Subgroup polygon (with 1 m edge effect) | χ21 = 3.963; p = 0.047 | R = 0.218; p < 0.05 |
| Minimum distance to the public | χ21 = 5.114; p = 0.024 | R = −0.114; p < 0.05 |
| Maximum inter-individual distance | χ21 = 1.137; p = 0.286 | |
| Minimum inter-individual distance | χ21 = 0.040; p = 0.841 | |
| Proportion of enclosure section area used | χ21 = 1.232; p = 0.267 |
| Non-Associations (Pairs That Formed Less Frequently than Expected by Chance) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Daisy | Thabu | Joyce | Charles | Yoda | Amber | Lilly | Zoe | ||
| Associations (Pairs that formed more frequently than expected by chance) | Daisy | - | 14.41 3 | −1.29 | 0.10 | 2.94 2 | −0.48 | −3.51 | −8.61 |
| Thabu | −9.85 | - | 11.07 3 | 9.42 3 | −0.76 | −2.54 | −2.60 | −3.49 | |
| Joyce | 4.15 3 | −9.46 | - | 1.98 | 4.90 3 | 2.55 1 | −0.90 | −3.76 | |
| Charles | 1.55 | −8.57 | −1.10 | - | 6.43 3 | 3.34 2 | 1.35 | −4.24 | |
| Yoda | −4.63 | 0.47 | −6.83 | −8.28 | - | 7.86 3 | 3.15 2 | 5.11 3 | |
| Amber | −1.33 | 1.69 | −5.08 | −5.61 | −8.47 | - | 7.79 3 | 8.55 3 | |
| Lilly | 2.15 2 | 0.18 | −1.24 | −3.90 | −2.79 | −6.02 | - | 10.70 3 | |
| Zoe | 10.75 3 | 1.51 | 3.24 2 | 4.63 3 | −5.23 | −6.77 | −4.86 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duncan, L.M.; D’Egidio Kotze, C.; Pillay, N. Long-Term Spatial Restriction Generates Deferred Limited Space Use in a Zoo-Housed Chimpanzee Group. Animals 2022, 12, 2207. https://doi.org/10.3390/ani12172207
Duncan LM, D’Egidio Kotze C, Pillay N. Long-Term Spatial Restriction Generates Deferred Limited Space Use in a Zoo-Housed Chimpanzee Group. Animals. 2022; 12(17):2207. https://doi.org/10.3390/ani12172207
Chicago/Turabian StyleDuncan, Luke Mangaliso, Chiara D’Egidio Kotze, and Neville Pillay. 2022. "Long-Term Spatial Restriction Generates Deferred Limited Space Use in a Zoo-Housed Chimpanzee Group" Animals 12, no. 17: 2207. https://doi.org/10.3390/ani12172207





