Effect of Chinese Herbs on Serum Biochemical Parameters, Immunity Indices, Antioxidant Capacity and Metabolomics in Early Weaned Yak Calves
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Preparation of Herbal Water Extracts
2.3. Experimental Design
2.4. Sampling Procedure
2.5. Analysis of the Main Active Ingredients of Herbal Root Extract
2.6. Analysis of Serum Biochemical Parameters
2.7. Serum Metabolome Analysis, Bioinformatics, and Statistical Analysis
3. Results
3.1. Main Active Ingredients in the Herbal Root Extracts
3.2. Effect of Herbal Extracts on Serum Biochemical Parameters
3.3. Effect of Herbal Extracts on Serum Immunity Indices
3.4. Effect of Herbal Extracts on Serum Antioxidant Capacity
3.5. Identification and Quantification of LC-MS Compounds in Serum
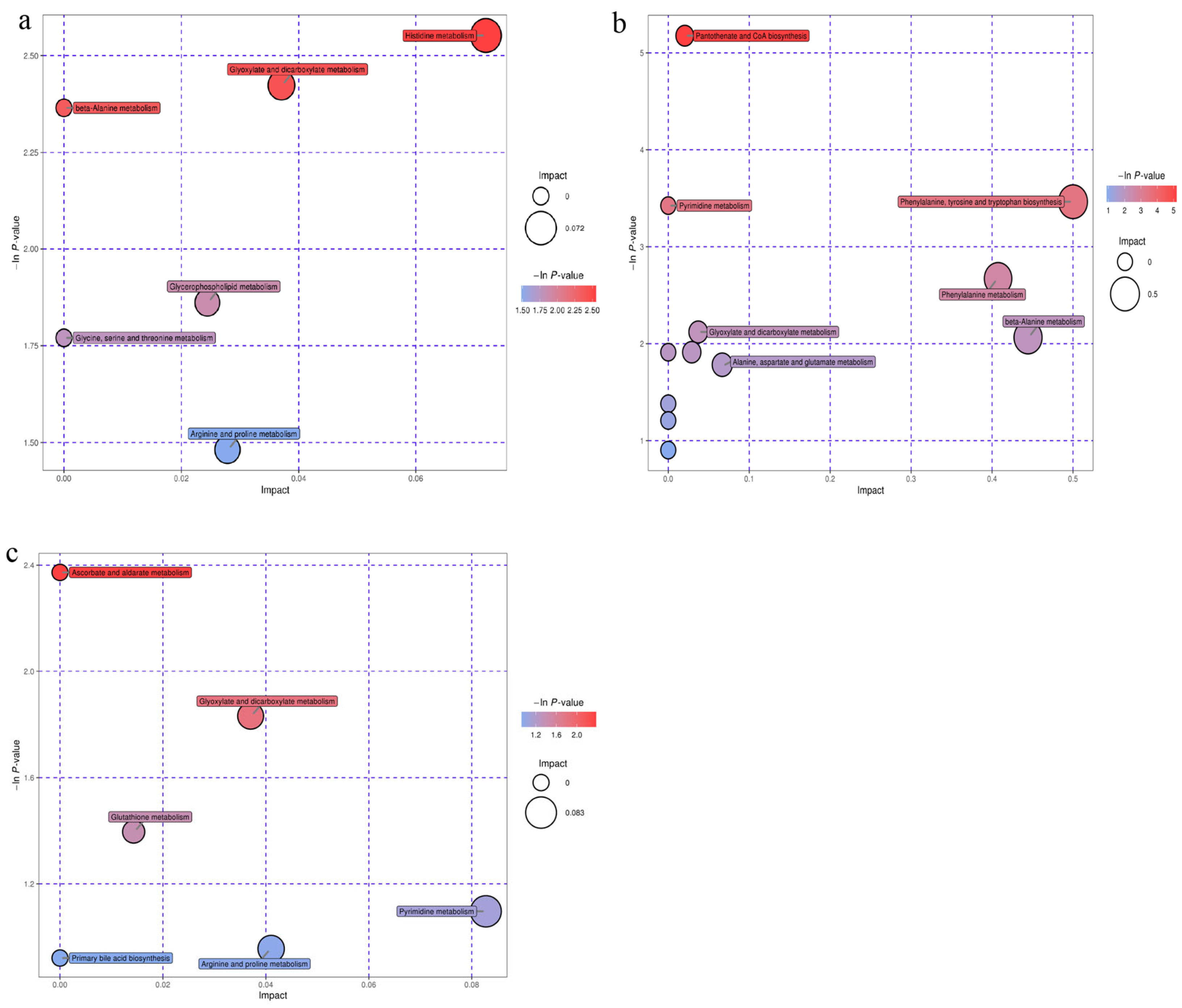
3.6. Metabolomic Profiles in Serum of Early Weaned Yak Calves
4. Discussion
4.1. Effect of Herbs on Serum Biochemical Indices
4.2. Effect of Herbs on Serum Immunity Indices
4.3. Effect of Herbs on Serum Antioxidant Capacity
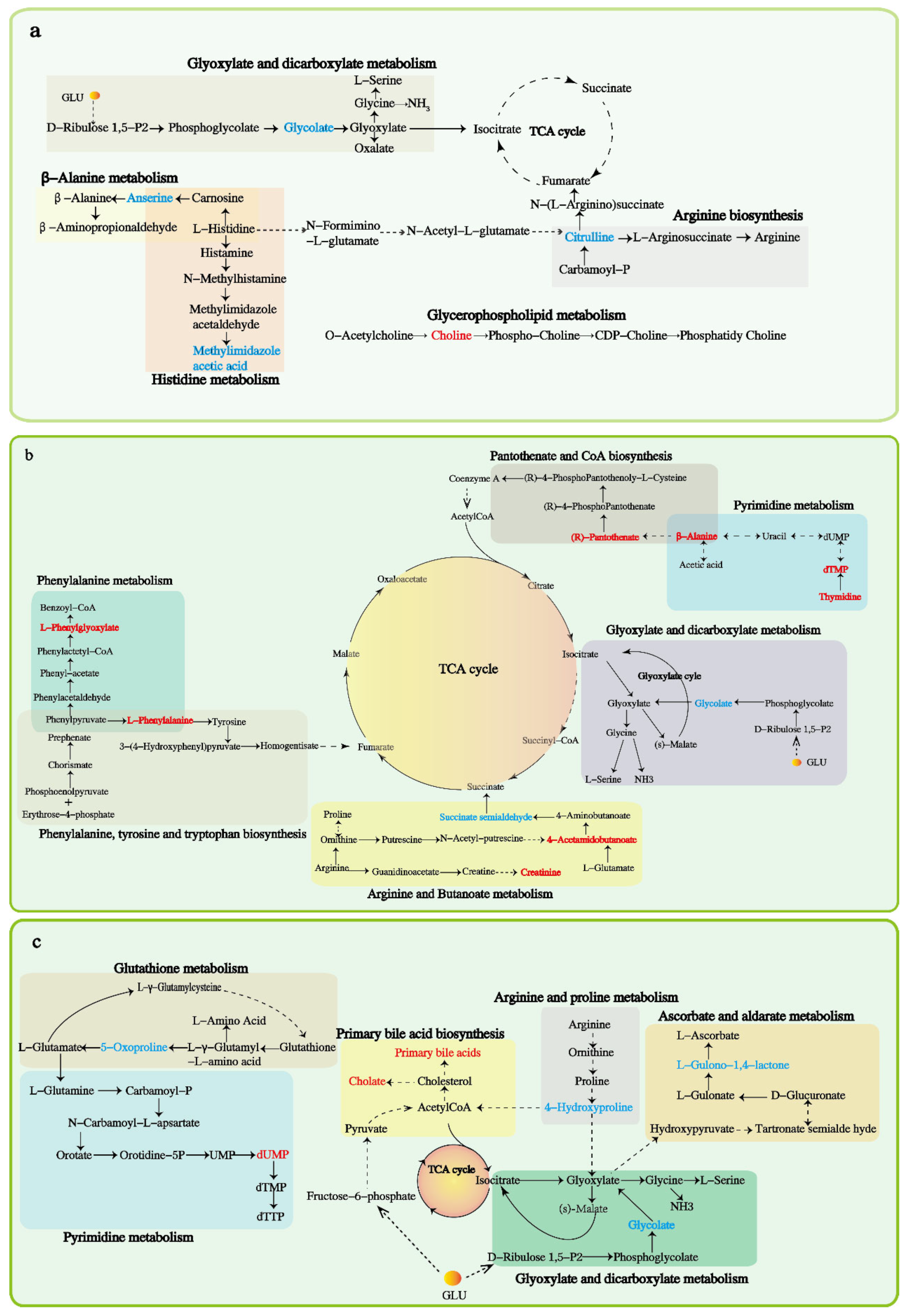
4.4. Serum Metabolome Responses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santi, D.U.; In, H.K. Efficacy of phytogenic feed additive on performance, production and health status of monogastric animals—A Review. Ann. Anim. Sci. 2018, 17, 929–948. [Google Scholar]
- Wei, W.L.; Zeng, R.; Gu, C.M.; Qu, Y.; Huang, L.F. Angelica sinensis in China—A review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. J. Ethnopharmacol. 2016, 190, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.W.; Chiou, W.F.; Chao, S.H.; Lee, M.H.; Chen, C.C.; Tsai, Y.C. Ligustilide prevents LPS-induced iNOS expression in RAW 264.7 macrophages by preventing ROS production and down-regulating the MAPK, NF-kappaB and AP-1 signaling pathways. Int. Immunopharmacol. 2021, 11, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.S.; Yoon, J.J.; Han, B.H.; Jeong, D.H.; Lee, Y.J.; Kang, D.G.; Lee, H.S. Ligustilide attenuates vascular inflammation and activates Nrf2/HO-1 induction and, NO synthesis in HUVECs. Phytomedicine 2018, 38, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Lu, L.; Zhang, R.M.; Luo, L.; Yuan, Z.; Zhang, S.F.; Jiang, J.; Liu, S.M.; Dong, T.T.; Liang, Q.; et al. The extracts of Angelica sinensis inhibit lipid oxidation in fish erythrocytes and improve growth, digestive, absorptive and antioxidant capacity in juvenile Jian carp (Cyprinus carpio var. Jian). Aquac. Nutr. 2019, 25, 119–133. [Google Scholar]
- Li, J.K.; Zhang, X.; Cao, L.Y.; Ji, J.J.; Gao, J.P. Three inulin-type fructans from Codonopsis pilosula (Franch) Nannf roots and their prebiotic activity on Bifidobacterium longum. Molecules 2018, 23, 3123. [Google Scholar] [CrossRef]
- Rahman, S.; Sultana, S. Glycyrrhizin exhibits potential chemopreventive activity on 12-O-tetradecanoyl phorbol-13-acetate-induced cutaneous oxidative stress and tumor promotion in Swiss albino mice. J. Enzym. Inhib. Med. Chem. 2007, 22, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.C.; Wu, C.H.; Yen, G.C. Bioactivity and potential health benefits of licorice. J. Agric. Food Chem. 2014, 62, 542–553. [Google Scholar] [CrossRef]
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef]
- Mehrabadi, M.; Vakili, A.; Mesgaran, M.D.; Valizadeh, R. Evaluation of potential new opportunities for herbal plants as natural products on rumen fermentation patterns in vitro. Iran. J. Appl. Anim. Sci. 2019, 9, 205–216. [Google Scholar]
- Jiang, B.W.; Zhou, Y.X.; Wang, T.; Li, F. Nutritive value and ruminal degradation of seven Chinese herbs as forage for Tibetan sheep. Bioengineered 2020, 11, 1159–1169. [Google Scholar] [CrossRef]
- Liu, P.P.; Dong, Q.M.; Liu, S.J.; Degen, A.; Zhang, J.J.; Qiu, Q.; Jing, X.P.; Shang, Z.H.; Zheng, W.M.; Ding, L.M. Postpartum oestrous cycling resumption of yak cows following different calf weaning strategies under range conditions. Anim. Sci. J. 2018, 89, 1492–1530. [Google Scholar] [CrossRef]
- Liu, P.P.; Liu, S.J.; Degen, A.; Qiu, Q.; Dong, Q.M.; Jing, X.P.; Zhang, J.J.; Yan, Q.; Zheng, W.M.; Ding, L.M. Effect of weaning strategy on performance, behaviour and blood parameters of yak calves (Poephagus grunniens). Rangel. J. 2018, 40, 263–270. [Google Scholar] [CrossRef]
- Liu, P.P.; Zhang, J.J.; Liu, S.J.; Dong, Q.M.; Zheng, W.M.; Zhao, S.N.; Zhou, Y.Q.; Jing, X.P.; Hu, R.; Shao, Y.Q.; et al. Effect of early weaning on grazing yak cows’ and calves’ blood physiological index in Qinghai Lake areas. Chin. J. Anim. Sci. 2016, 52, 79–84. (In Chinese) [Google Scholar]
- Jiang, C.X.; Ding, L.M.; Dong, Q.M.; Wang, X.J.; Wei, H.Y.; Hu, C.S.; Ma, C.F.; Yan, Q.; Zhou, Y.Q.; Degen, A.A. Effects of root extracts of three traditional Chinese herbs as dietary supplements on dry matter intake, average daily gain, rumen fermentation and ruminal microbiota in early weaned yak calves. Anim. Feed Sci. Technol. 2021, 278, 115002. [Google Scholar] [CrossRef]
- Wei, H.Y.; Ding, L.M.; Wang, X.J.; Yan, Q.; Jiang, C.X.; Hu, C.S.; Wang, G.W.; Zhou, Y.Q.; Henkin, Z.; Degen, A.A. Astragalus extract improved average daily gain, immunity, antioxidant status and ruminal microbiota of early weaned yak calves. J. Sci. Food Agric. 2021, 101, 82–90. [Google Scholar] [CrossRef]
- Wang, X.; Hu, C.; Ding, L.; Tang, Y.; Wei, H.; Jiang, C.; Yan, Q.; Dong, Q.; Degen, A.A. Astragalus membranaceus alters rumen bacteria to enhance fiber digestion, improves antioxidant capacity and immunity indices of small intestinal mucosa, and enhances liver metabolites for energy synthesis in Tibetan sheep. Animals 2021, 11, 3236. [Google Scholar] [CrossRef]
- Van Knegsel, A.T.M.; Reilingh, G.D.V.; Meulenberg, S.; Van den Brand, H.; Dijkstra, J.; Kemp, B.; Parmentier, H.K. Natural antibodies related to energy balance in early lactation dairy cows. J. Dairy Sci. 2007, 90, 5490–5498. [Google Scholar] [CrossRef]
- Wang, K.P.; Cao, P.; Shui, W.Z.; Yang, Q.X.; Tang, Z.H.; Zhang, Y. Angelica sinensis polysaccharide regulates glucose and lipid metabolism disorder in prediabetic and streptozotocin-induced diabetic mice through the elevation of glycogen levels and reduction of inflammatory factors. Food Funct. 2015, 6, 902–909. [Google Scholar] [CrossRef]
- Li, X.; Huang, S.M.; Chen, X.; Xu, Q.J.; Ma, Y.X.; You, L.J.; Kulikouskaya, V.; Xiao, J.B.; Piao, J.H. Structural characteristic of a sulfated polysaccharide from Gracilaria lemaneiformis and its lipid metabolism regulation effect. Food Funct. 2020, 11, 10876–10885. [Google Scholar] [CrossRef]
- Mojtahedin, A.; Seifdavati, J.; Seyedsharifi, R. Effects of different levels of dietary citrus limon essential oil on some blood parameters and antioxidant status in Afshari ewes. Cell. Mol. Biol. 2018, 64, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Su, G.Q.; Zhou, X.W.; Wang, Y.; Chen, D.W.; Chen, G.; Li, Y.; He, J. Dietary supplementation of plant essential oil improves growth performance, intestinal morphology and health in weaned pigs. J. Anim. Physiol. Anim. Nutr. 2020, 104, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.C.; Lee, Y.H.; Kim, S.H.; Kim, K.J.; Kim, K.M.; Oh, S.; Jung, Y.S. Hepatoprotective effect of licorice, the root of Glycyrrhiza uralensis Fischer, in alcohol-induced fatty liver disease. BMC Complementary Altern. Med. 2016, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Bera, I.; Tyagi, P.K.; Mir, N.A.; Begum, J.; Dev, K.; Tyagi, P.K.; Biswas, A.; Sharma, D.; Mandal, A.B. Effect of dietary saponin rich soapnut (Sapindus mukorossi) shell powder on growth performance, immunity, serum biochemistry and gut health of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1800–1809. [Google Scholar] [CrossRef]
- Liu, C.; Qu, Y.H.; Guo, P.T.; Xu, C.C.; Ma, Y.; Luo, H.H. Effects of dietary supplementation with alfalfa (Medicago sativa L.) saponins on lamb growth performance, nutrient digestibility, and plasma parameters. Anim. Feed Sci. Technol. 2017, 236, 98–106. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, Y.; Liu, Y.J.; Liu, D.; Liu, Y.; Guo, Y.T.; We, Z.J.; Li, H.Y.; Wang, H. Ursolic acid alleviates lipid accumulation by activating the AMPK signaling pathway in vivo and in vitro. J. Food Sci. 2020, 85, 3998–4008. [Google Scholar] [CrossRef]
- Cozzi, G.; Ravarotto, L.; Gottardo, F.; Stefani, A.L.; Contiero, B.; Moro, L.; Brscic, M.; Dalvit, P. Short communication: Reference values for blood parameters in Holstein dairy cows: Effects of parity, stage of lactation, and season of production. J. Dairy Sci. 2011, 94, 3895–3901. [Google Scholar] [CrossRef]
- Huang, L.X.; Shen, M.Y.; Wu, T.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J.H. Mesona chinensis Benth polysaccharides protect against oxidative stress and immunosuppression in cyclophosphamide-treated mice via MAPKs signal transduction pathways. Int. J. Biol. Macromol. 2020, 152, 766–774. [Google Scholar] [CrossRef]
- Cannas, A.; Pes, A.; Mancuso, R.; Vodret, B.; Nudda, A. Effect of dietary energy and protein concentration on the concentration of milk urea nitrogen in dairy ewes. J. Dairy Sci. 1998, 81, 499–508. [Google Scholar] [CrossRef]
- Van Bibber-Krueger, C.L.; Miller, K.A.; Parsons, G.L.; Thompson, L.K.; Drouillard, J.S. Effects of zilpaterol hydrochloride on growth performance, blood metabolites, and fatty acid profiles of plasma and adipose tissue in finishing steers. J. Anim. Sci. 2015, 93, 2419–2427. [Google Scholar] [CrossRef]
- Zhong, R.Z.; Yu, M.; Liu, H.W.; Sun, H.X.; Cao, Y.; Zhou, D.W. Effects of dietary Astragalus polysaccharide and Astragalus membranaceus root supplementation on growth performance, rumen fermentation, immune responses, and antioxidant status of lambs. Anim. Feed Sci. Technol. 2012, 174, 60–67. [Google Scholar] [CrossRef]
- Hao, X.Y.; Wang, P.J.; Ren, Y.S.; Liu, G.T.; Zhang, J.X.; Leury, B.; Zhang, C.X. Effects of Astragalus membranaceus roots supplementation on growth performance, serum antioxidant and immune response in finishing lambs. Asian-Australas. J. Anim. Sci. 2020, 33, 965–972. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.J.; Liu, F.; Wang, Y.H. Production performance, immunity, and heat stress resistance in Jersey cattle fed a concentrate fermented with probiotics in the presence of a Chinese herbal combination. Anim. Feed Sci. Technol. 2017, 228, 59–65. [Google Scholar] [CrossRef]
- Deng, L.; Xu, H.C.; Liu, P.; Wu, S.Z.; Shi, Y.C.; Lv, Y.W.; Chen, X.Y. Prolonged exposure to high humidity and high temperature environment can aggravate influenza virus infection through intestinal flora and Nod/RIP2/NF-kappa B signaling pathway. Vet. Microbiol. 2020, 251, 108896. [Google Scholar] [CrossRef]
- Song, X.M.; Hu, S.H. Adjuvant activities of saponins from traditional Chinese medicinal herbs. Vaccine 2009, 27, 4883–4890. [Google Scholar] [CrossRef]
- Huang, H.; Luo, S.H.; Huang, D.C.; Cheng, S.J.; Cao, C.J.; Chen, G.T. Immunomodulatory activities of proteins from Astragalus membranaceus waste. J. Sci. Food Agric. 2019, 99, 4174–4181. [Google Scholar] [CrossRef]
- De, X.H.; Cho, S.S.; Lee, S.Y.; Oh, D.S.; Lim, S.K.; Park, D.H. Immune-stimulating effects of Mycoleptodonoides aitchisonii (Agricomycetes) water extract via TNF-alpha and IFN-gamma. Int. J. Med. Mushrooms 2017, 19, 809–815. [Google Scholar]
- Duh, P.D.; Yen, G.C. Antioxidative activity of three herbal water extracts. Food Chem. 1997, 60, 639–645. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef]
- Unyayar, S.; Celik, A.; Cekic, F.O.; Gozel, A. Cadmium-induced genotoxicity, cytotoxicityand lipid peroxidation in Allium sativum and Vicia faba. Mutagenesis 2006, 21, 77–81. [Google Scholar] [CrossRef]
- Esterbauer, H.; Zollner, H.; Schaur, R.J. Hydroxyalkenals: Cytotoxic products of lipid peroxidation. ISI Atlas Sci. Biochem. 1988, 1, 311–317. [Google Scholar]
- Liu, F.; Ooi, V.E.C.; Chang, S.T. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 1997, 60, 763–771. [Google Scholar] [CrossRef]
- Amit, K.G.; Masood, A. Assessment of cytotoxic and genotoxic potential of refinery waste effluent using plant, animal and bacterial systems. J. Hazard. Mater. 2011, 201, 92–99. [Google Scholar]
- Tseng, T.H.; Kao, E.S.; Chu, C.Y.; Chou, F.P.; Wu, H.W.L.; Wang, C.J. Protective effects of dried flower extracts of Hibiscus sabdariffa L. against oxidative stress in rat primary hepatocytes. Food Chem. Toxicol. 1997, 35, 1159–1164. [Google Scholar] [CrossRef]
- Miyake, T.; Shibamoto, T. Antioxidative activities of natural compounds found in plants. J. Agric. Food Chem. 1997, 45, 1819–1822. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Luo, H.L.; Chang, Y.F.; Jiao, L.J.; Liu, K. Effects of liquorice extract on the activity and gene expression level of antioxidant enzymes in Longissimus dorsi muscle of Tan lamb. Small Rumin. Res. 2017, 154, 23–28. [Google Scholar] [CrossRef]
- Bauché, F.; Fouchard, M.H.; Jégou, B. Antioxidant system in rat testicular cells. FEBS Lett. 1994, 349, 392–396. [Google Scholar] [CrossRef]
- Pan, S.K.; Jiang, L.F.; Wu, S.J. Stimulating effects of polysaccharide from Angelica sinensis on the nonspecific immunity of white shrimps (Litopenaeus vannamei). Fish Shellfish Immunol. 2018, 74, 170–174. [Google Scholar] [CrossRef]
- Liu, F.; Geng, C.; Qu, Y.K.; Cheng, B.X.; Zhang, Y.; Wang, A.M.; Zhang, J.H.; Liu, B.; Tian, H.Y.; Yang, W.P.; et al. The feeding of dietary Codonopsis pilosula polysaccharide enhances the immune responses, the expression of immune-related genes and the growth performance of red swamp crayfish (Procambarus clarkii). Fish Shellfish Immunol. 2020, 103, 321–331. [Google Scholar] [CrossRef]
- Wei, W.S.; Tan, J.Q.; Guo, F.; Chen, H.S.; Zhou, Z.Y.; Zhang, Z.H.; Gui, L. Effects of Coriolus versicolor polysaccharides on superoxide dismutase activities in mice. Acta Pharmacol. Sin. 1996, 17, 174–178. [Google Scholar]
- Fan, J.; Feng, H.B.; Yu, Y.; Sun, M.X.; Liu, Y.R.; Li, T.Z.; Sun, X.; Liu, S.J.; Sun, M.D. Antioxidant activities of the polysaccharides of Chuanminshen violaceum. Carbohydr. Polym. 2017, 157, 629–636. [Google Scholar] [CrossRef]
- Lewis, R.M.; Godfrey, K.M.; Jackson, A.A.; Cameron, I.T.; Hanson, M.A. Low serine hydroxymethyltransferase activity in the human placenta has important implications for fetal glycine supply. J. Clin. Endocrinol. Metab. 2005, 90, 1594–1598. [Google Scholar] [CrossRef]
- Wang, W.W.; Wu, Z.L.; Dai, Z.L.; Yang, Y.; Wang, J.J.; Wu, G.Y. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef]
- Danpure, C.J. Molecular aetiology of primary hyperoxaluria type 1. Nephron Exp. Nephrol. 2004, 98, e39–e44. [Google Scholar] [CrossRef]
- Shirfule, A.L.; Sangamwar, A.T.; Khobragade, C.N. Exploring glycolate oxidase (GOX) as an antiurolithic drug target: Molecular modeling and in vitro inhibitor study. Int. J. Biol. Macromol. 2011, 49, 62–70. [Google Scholar] [CrossRef]
- Pi, R.B.; Mao, X.X.; Chao, X.J.; Cheng, Z.Y.; Liu, M.F.; Duan, X.L.; Ye, M.Z.; Chen, X.H.; Mei, Z.G.; Liu, P.Q.; et al. Tacrine-6-ferulic acid, a novel multifunctional dimer, inhibits amyloid-β-mediated Alzheimer’s disease-associated pathogenesis in in vitro and in vivo. PLoS ONE 2012, 7, e31921. [Google Scholar] [CrossRef]
- Sutton, V.R.; Pan, Y.Z.; Davis, E.C.; Craigen, W.J. A mouse model of argininosuccinic aciduria: Biochemical characterization. Mol. Genet. Metab. 2003, 78, 11–16. [Google Scholar] [CrossRef]
- Speranza, L.; Franceschelli, S.; Pesce, M.; Menghini, L.; Patruno, A.; Vinciguerra, I.; Delutiis, M.A.; Felaco, M.; Pelaco, P.; Grilli, A. Anti-inflammatory properties of the plant Verbascum mallophorum. J. Biol. Regul. Homeost. Agents 2009, 23, 189–195. [Google Scholar]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Menon, K.; Marquina, C.; Hoj, P.; Liew, D.; Mousa, A.; de Couten, B. Carnosine and histidine-containing dipeptides improve dyslipidemia: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2020, 78, 939–951. [Google Scholar] [CrossRef]
- Gitomer, W.L.; Tipton, K.F. The role of cytoplasmic aldehyde dehydrogenase in the metabolism of n-tele-methylhistamine. Pharmacol. Biochem. Behav. 1983, 18, 113–116. [Google Scholar] [CrossRef]
- Yao, C.A.; Lin, C.H. Treatment with the herbal formulation Eefooton slows the progression of chronic kidney disease: A case report. Medicine 2019, 98, e17573. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.T.; Zhu, L.B.; Zhang, H.; Yang, J.; Zhao, J.; Du, D.W.; Meng, J.P.; Yang, F.; Zhao, Y.L.; Sun, J.F. Protective effect of a polysaccharide from stem of Codonopsis pilosula against renal ischemia/reperfusion injury in rats. Carbohydr. Polym. 2012, 90, 1739–1743. [Google Scholar] [CrossRef] [PubMed]
- Stentoft, C.; Vestergaard, M.; Lovendahl, P.; Kristensen, N.B.; Moorby, J.M.; Jensen, S.K. Simultaneous quantification of purine and pyrimidine bases, nucleosides and their degradation products in bovine blood plasma by high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2014, 1356, 197–210. [Google Scholar] [CrossRef]
- Tao, S.Y.; Tian, P.; Luo, Y.W.; Tian, J.; Hua, C.F.; Geng, Y.L.; Cong, R.H.; Ni, Y.D.; Zhao, R.Q. Microbiome-metabolome responses to a high-grain diet associated with the hind-gut health of goats. Front. Microbiol. 2017, 8, 1764. [Google Scholar] [CrossRef]
- He, J.W.; Guo, H.D.; Zheng, W.J.; Xue, Y.Q.; Zhao, R.Q.; Yao, W. Heat stress affects fecal microbial and metabolic alterations of primiparous sows during late gestation. J. Anim. Sci. Biotechnol. 2019, 10, 84. [Google Scholar] [CrossRef]
- Parvazi, S.; Sadeghi, S.; Azad, M.; Mohammadi, M.; Arjmand, M.; Vahabi, F.; Sadeghzadeh, S.; Zamani, Z. The effect of aqueous extract of cinnamon on the metabolome of Plasmodium falciparum using HNMR Spectroscopy. J. Trop. Med. 2016, 2016, 3174841. [Google Scholar] [CrossRef][Green Version]
- Ma, L.; Luo, Z.Z.; Chen, J.B.; Du, Z.L.; Zhou, T.; Huang, Y.X.; Yao, X.P.; Shen, L.H.; Yu, S.M.; Shi, X.D.; et al. Effect of Astralus membranaceus root on the serum metabolome of preweaning dairy calves. Agric. Basel 2022, 12, 744. [Google Scholar]
- Manfridi, A.; Brambilla, D.; Mancia, M. Sleep is differently modulated by basal forebrain GABAA and GABAB receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R170–R175. [Google Scholar] [CrossRef]
- Luo, H.; Sun, S.J.; Wang, Y.; Wang, Y.L. Revealing the sedative-hypnotic effect of the extracts of herb pair Semen Ziziphi spinosae and Radix polygalae and related mechanisms through experiments and metabolomics approach. BMC Complement. Med. Ther. 2020, 20, 206. [Google Scholar] [CrossRef]
- Jin, F.H.; Hu, F.D.; Chen, X.; Zhao, L.G.; Feng, S.L. Active comparison of the eluents from different parts of Codonopsis pilosula. J. Chin. Med. Mater. 2009, 32, 112–114. (In Chinese) [Google Scholar]
- Palmer, J.A.; Smith, A.M.; Gryshkova, V.; Donley, E.L.R.; Valentin, J.P.; Burrier, R.E. A targeted metabolomics-based assay using human induced pluripotent stem cell-derived cardiomyocytes identifies structure and functional cardiotoxicity potential. Toxicol. Sci. 2020, 174, 218–240. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Curr. Top. Cell. Regul. 2001, 36, 95–116. [Google Scholar]
- Bando, M.; Hiroshima, Y.; Kataoka, M.; Herzberg, M.C.; Ross, K.F.; Shinohara, Y.; Yamamoto, T.; Nagata, T.; Kido, J. Modulation of calprotectin in human keratinocytes by keratinocyte growth factor and interleukin-1alpha. Immunol. Cell Biol. 2010, 88, 328–333. [Google Scholar] [CrossRef]
- Foyer, C.H. Prospects for enhancement of the soluble antioxidants, ascorbate and glutathione. Biofactors 2001, 15, 75–78. [Google Scholar] [CrossRef]
- Zhou, H.X.; Milne, R.I.; Ma, X.L.; Song, Y.Q.; Fang, J.Y.; Sun, H.; Zha, H.G. Characterization of a L-gulono-1,4-lactone oxidase like protein in the floral nectar of Mucuna sempervirens, Fabaceae. Front. Plant Sci. 2018, 9, 1109. [Google Scholar] [CrossRef]
- Biancalana, A.; Veloso, L.A.; Gomes, L. Obesity affects collagen fibril diameter and mechanical properties of tendons in Zucker rats. Connect. Tissue Res. 2010, 51, 171–178. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Woo, S.; Darrell, K.; Li, P.; Li, X.L.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef]
- Qiao, X.; Ye, M.; Xiang, C.; Bo, T.; Yang, W.Z.; Liu, C.F.; Miao, W.J.; Guo, D.A. Metabolic regulatory effects of licorice: A bile acid metabonomic study by liquid chromatography coupled with tandem mass spectrometry. Steroids 2012, 77, 745–755. [Google Scholar] [CrossRef]

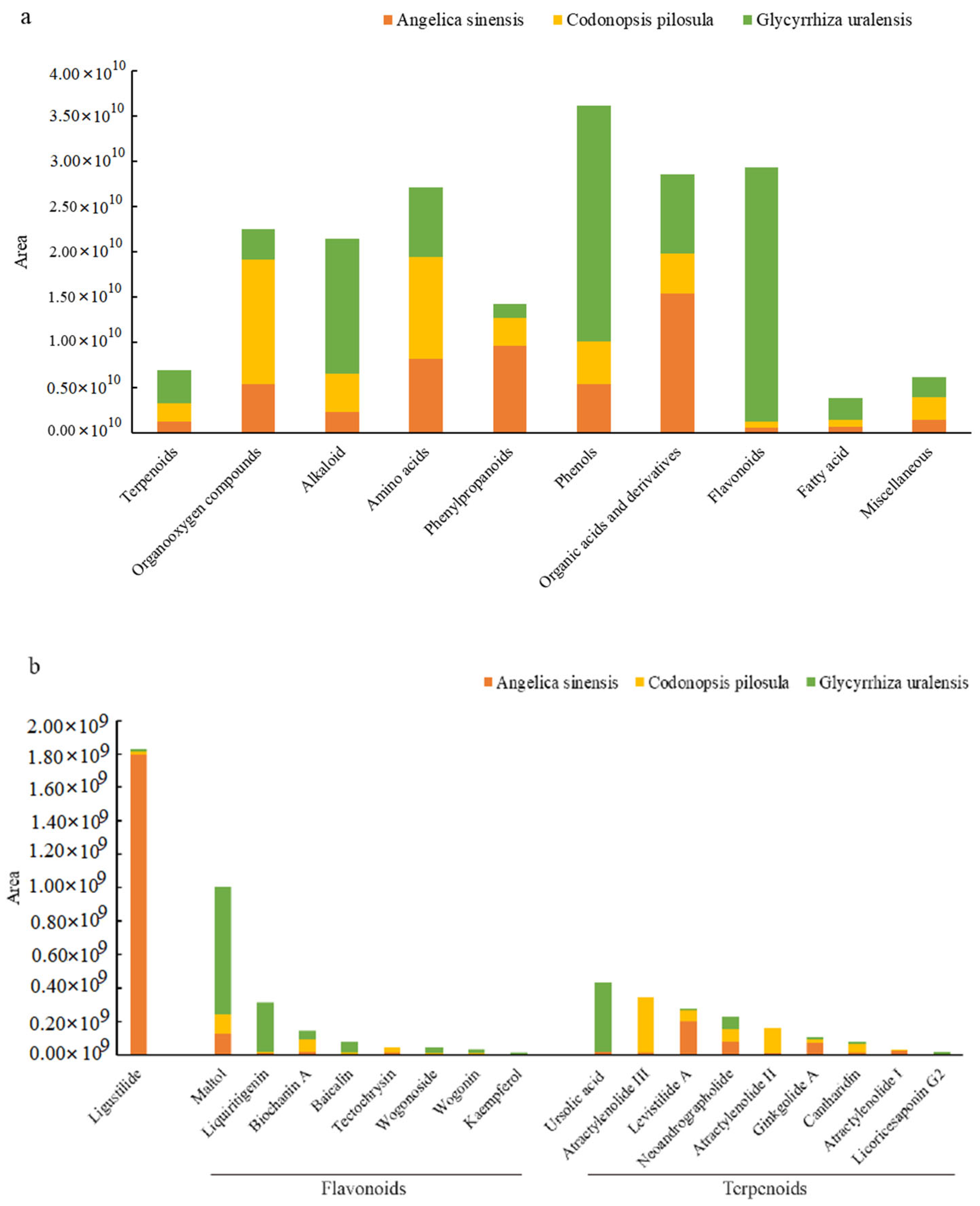
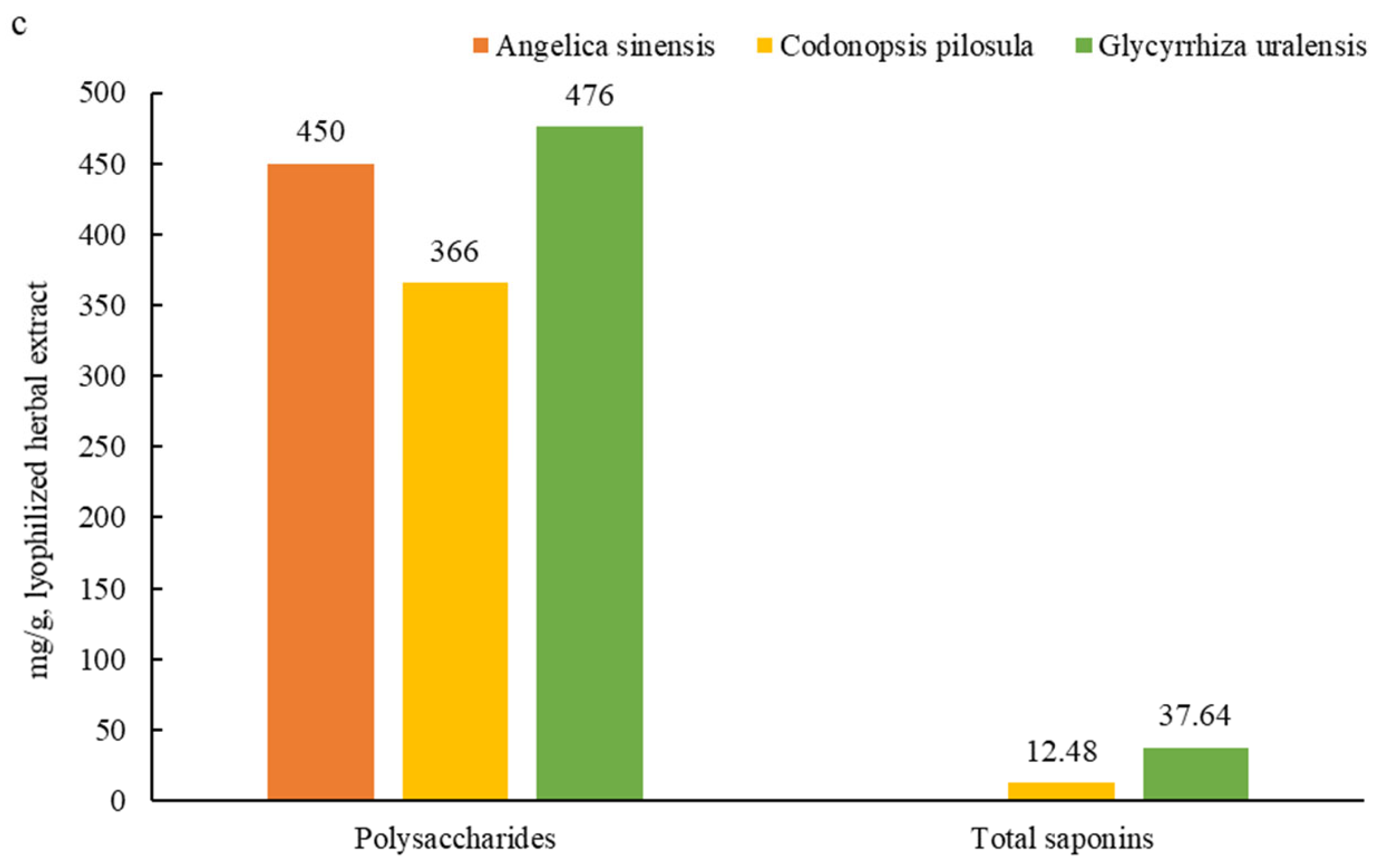
| Items ǂ | Day | Treatment | SEM | p | |||
|---|---|---|---|---|---|---|---|
| Control | Angelica sinensis | Codonopsis pilosula | Glycyrrhiza uralensis | ||||
| TC, mmol/L | 15 | 1.13 | 1.18 | 1.18 | 1.15 | 0.100 | 0.999 |
| 30 | 1.52 a | 0.85 b | 1.10 ab | 1.12 ab | 0.075 | 0.001 | |
| 45 | 1.47 | 1.39 | 1.15 | 1.30 | 0.072 | 0.460 | |
| 60 | 1.31 | 1.23 | 1.15 | 0.98 | 0.091 | 0.616 | |
| p# | L | 0.539 | 0.395 | 0.959 | 0.602 | ||
| Q | 0.287 | 0.697 | 0.969 | 0.599 | |||
| TG, mmol/L | 15 | 0.26 | 0.26 | 0.27 | 0.19 | 0.018 | 0.317 |
| 30 | 0.29 a | 0.28 ab | 0.26 ab | 0.22 b | 0.014 | 0.017 | |
| 45 | 0.35 | 0.30 | 0.29 | 0.28 | 0.023 | 0.566 | |
| 60 | 0.32 a | 0.24 ab | 0.26 ab | 0.17 b | 0.021 | 0.048 | |
| p | L | 0.196 | 0.840 | 0.998 | 0.862 | ||
| Q | 0.266 | 0.103 | 0.974 | 0.052 | |||
| TP, g/L | 15 | 47.1 b | 63.0 a | 58.2 ab | 56.0 ab | 1.987 | 0.017 |
| 30 | 56.8 | 60.9 | 54.0 | 59.9 | 2.058 | 0.708 | |
| 45 | 58.3 | 60.9 | 60.3 | 63.3 | 2.298 | 0.920 | |
| 60 | 49.2 | 60.9 | 54.5 | 53.1 | 1.815 | 0.601 | |
| p | L | 0.581 | 0.314 | 0.756 | 0.756 | ||
| Q | 0.043 | 0.612 | 0.942 | 0.321 | |||
| ALB, g/L | 15 | 27.5 b | 31.7 ab | 32.7 ab | 33.6 a | 0.846 | 0.017 |
| 30 | 24.7 | 27.4 | 27.6 | 29.7 | 0.565 | 0.114 | |
| 45 | 24.6 | 28.5 | 27.4 | 29.9 | 1.092 | 0.115 | |
| 60 | 25.0 b | 31.9 a | 28.4 ab | 30.3 a | 1.104 | 0.041 | |
| p | L | 0.545 | 0.502 | 0.508 | 0.630 | ||
| Q | 0.138 | 0.085 | 0.027 | 0.204 | |||
| GH, ng/mL | 15 | 5.31 | 4.89 | 6.05 | 5.29 | 0.205 | 0.187 |
| 30 | 4.56 | 5.36 | 4.77 | 4.72 | 0.181 | 0.482 | |
| 45 | 5.77 | 6.74 | 7.18 | 7.25 | 0.417 | 0.683 | |
| 60 | 5.03 | 6.06 | 6.50 | 6.86 | 0.298 | 0.130 | |
| p | L | 0.759 | 0.151 | 0.176 | 0.065 | ||
| Q | 0.911 | 0.289 | 0.393 | 0.198 | |||
| FFA, mmol/L | 15 | 0.38 | 0.39 | 0.33 | 0.36 | 0.116 | 0.321 |
| 30 | 0.43 a | 0.36 b | 0.37 b | 0.37 b | 0.011 | 0.021 | |
| 45 | 0.46 a | 0.44 ab | 0.39 b | 0.37 b | 0.015 | 0.018 | |
| 60 | 0.48 a | 0.37 ab | 0.39 ab | 0.34 b | 0.019 | 0.021 | |
| p | L | 0.004 | 0.858 | 0.117 | 0.751 | ||
| Q | 0.014 | 0.728 | 0.047 | 0.074 | |||
| INS, μIU/mL | 15 | 12.06 | 13.21 | 12.77 | 13.60 | 0.291 | 0.282 |
| 30 | 9.36 | 10.14 | 11.89 | 11.20 | 0.499 | 0.182 | |
| 45 | 12.42 | 13.14 | 13.73 | 13.13 | 0.358 | 0.724 | |
| 60 | 9.23 | 10.10 | 9.81 | 9.58 | 0.160 | 0.320 | |
| p | L | 0.243 | 0.255 | 0.257 | 0.024 | ||
| Q | 0.501 | 0.491 | 0.132 | 0.063 | |||
| BUN, mmol/L | 15 | 3.11 | 3.21 | 2.70 | 3.36 | 0.160 | 0.605 |
| 30 | 3.25 | 3.11 | 3.03 | 3.33 | 0.116 | 0.708 | |
| 45 | 3.43 | 3.22 | 3.35 | 3.33 | 0.105 | 0.081 | |
| 60 | 3.71 | 3.76 | 3.25 | 3.52 | 0.143 | 0.643 | |
| p | L | 0.094 | 0.098 | 0.079 | 0.704 | ||
| Q | 0.199 | 0.166 | 0.008 | 0.860 | |||
| Items ǂ | Day | Treatment | SEM | p | |||
|---|---|---|---|---|---|---|---|
| Control | Angelica sinensis | Codonopsis pilosula | Glycyrrhiza uralensis | ||||
| IgA, g/L | 15 | 0.80 | 0.86 | 0.85 | 0.85 | 0.009 | 0.862 |
| 30 | 0.72 | 0.78 | 0.76 | 0.81 | 0.016 | 0.293 | |
| 45 | 0.73 | 0.79 | 0.75 | 0.79 | 0.014 | 0.273 | |
| 60 | 0.75 | 0.76 | 0.84 | 0.81 | 0.016 | 0.195 | |
| p# | L | 0.134 | 0.002 | 0.582 | 0.152 | ||
| Q | 0.059 | 0.004 | 0.001 | 0.121 | |||
| IgG, g/L | 15 | 10.6 | 11.7 | 11.8 | 11.8 | 0.328 | 0.486 |
| 30 | 10.6 | 10.9 | 11.1 | 11.5 | 0.274 | 0.634 | |
| 45 | 11.2 | 11.8 | 12.6 | 11.7 | 0.204 | 0.107 | |
| 60 | 11.4 | 12.1 | 10.7 | 10.3 | 0.369 | 0.084 | |
| p | L | 0.278 | 0.450 | 0.670 | 0.097 | ||
| Q | 0.560 | 0.539 | 0.595 | 0.159 | |||
| IgM, g/L | 15 | 2.59 | 3.46 | 2.94 | 3.37 | 0.163 | 0.253 |
| 30 | 2.72 | 2.76 | 3.09 | 3.02 | 0.056 | 0.292 | |
| 45 | 2.87 | 2.96 | 3.16 | 2.81 | 0.088 | 0.611 | |
| 60 | 2.75 | 2.85 | 2.76 | 2.90 | 0.065 | 0.763 | |
| p | L | 0.502 | 0.13 | 0.491 | 0.107 | ||
| Q | 0.679 | 0.11 | 0.252 | 0.148 | |||
| IL-2, pg/mL | 15 | 332 | 334 | 329 | 324 | 2.72 | 0.757 |
| 30 | 253 | 232 | 246 | 240 | 4.73 | 0.403 | |
| 45 | 271 | 279 | 267 | 285 | 4.85 | 0.173 | |
| 60 | 188 | 185 | 200 | 190 | 2.00 | 0.657 | |
| p | L | <0.001 | 0.001 | <0.001 | 0.001 | ||
| Q | <0.001 | 0.007 | 0.003 | 0.003 | |||
| IL-6, pg/mL | 15 | 137.0 | 141.4 | 143.2 | 136.6 | 1.60 | 0.483 |
| 30 | 118.3 | 110.5 | 119.6 | 118.2 | 2.83 | 0.746 | |
| 45 | 128.6 | 129.6 | 127.5 | 126.6 | 1.16 | 0.447 | |
| 60 | 100.2 | 95.8 | 91.6 | 93.19 | 2.368 | 0.401 | |
| p | L | <0.001 | 0.007 | 0.009 | 0.004 | ||
| Q | <0.001 | 0.031 | 0.039 | 0.016 | |||
| TNF-α, pg/mL | 15 | 64.4 | 64.6 | 66.1 | 62.1 | 0.913 | 0.214 |
| 30 | 52.8 | 51.6 | 56.8 | 54.9 | 0.964 | 0.251 | |
| 45 | 56.8 b | 54.6 b | 64.2 a | 64.0 a | 1.556 | 0.016 | |
| 60 | 53.5 | 58.0 | 56.8 | 58.6 | 2.278 | 0.120 | |
| p | L | 0.889 | 0.825 | 0.131 | 0.523 | ||
| Q | 0.001 | 0.004 | 0.319 | 0.259 | |||
| Items ǂ | Day | Treatment | SEM | p | |||
|---|---|---|---|---|---|---|---|
| Control | Angelica sinensis | Codonopsis pilosula | Glycyrrhiza uralensis | ||||
| MDA, nmol/mL | 15 | 2.90 a | 1.94 b | 1.98 b | 2.25 b | 0.129 | 0.003 |
| 30 | 3.08 a | 2.75 ab | 2.10 c | 2.38 bc | 0.122 | 0.002 | |
| 45 | 2.78 | 2.23 | 2.35 | 2.38 | 0.108 | 0.336 | |
| 60 | 1.91 a | 1.55 ab | 1.68 ab | 1.35 b | 0.069 | 0.004 | |
| p# | L | 0.216 | 0.002 | 0.216 | 0.022 | ||
| Q | 0.003 | 0.001 | 0.003 | 0.001 | |||
| GSH-Px, U/mL | 15 | 397 | 445 | 436 | 428 | 9.2 | 0.299 |
| 30 | 310 c | 406 b | 448 ab | 466 a | 18.9 | <0.001 | |
| 45 | 342 c | 428 b | 411 b | 470 a | 14.4 | <0.001 | |
| 60 | 324 c | 398 b | 383 b | 553 a | 15.6 | <0.001 | |
| p | L | 0.051 | 0.112 | 0.007 | <0.001 | ||
| Q | 0.026 | 0.292 | 0.009 | <0.001 | |||
| SOD, U/mL | 15 | 85.2 | 85.1 | 88.2 | 87.3 | 0.78 | 0.422 |
| 30 | 91.2 | 92.2 | 92.6 | 93.6 | 0.85 | 0.841 | |
| 45 | 91.9 | 93.1 | 94.7 | 94.7 | 0.69 | 0.443 | |
| 60 | 92.4 | 92.8 | 94.5 | 92.8 | 0.59 | 0.687 | |
| p | L | 0.018 | 0.022 | 0.005 | 0.024 | ||
| Q | 0.023 | 0.007 | 0.008 | 0.002 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Dong, Q.; Xin, X.; Degen, A.A.; Ding, L. Effect of Chinese Herbs on Serum Biochemical Parameters, Immunity Indices, Antioxidant Capacity and Metabolomics in Early Weaned Yak Calves. Animals 2022, 12, 2228. https://doi.org/10.3390/ani12172228
Jiang C, Dong Q, Xin X, Degen AA, Ding L. Effect of Chinese Herbs on Serum Biochemical Parameters, Immunity Indices, Antioxidant Capacity and Metabolomics in Early Weaned Yak Calves. Animals. 2022; 12(17):2228. https://doi.org/10.3390/ani12172228
Chicago/Turabian StyleJiang, Cuixia, Quanmin Dong, Xiaoping Xin, Abraham Allan Degen, and Luming Ding. 2022. "Effect of Chinese Herbs on Serum Biochemical Parameters, Immunity Indices, Antioxidant Capacity and Metabolomics in Early Weaned Yak Calves" Animals 12, no. 17: 2228. https://doi.org/10.3390/ani12172228
APA StyleJiang, C., Dong, Q., Xin, X., Degen, A. A., & Ding, L. (2022). Effect of Chinese Herbs on Serum Biochemical Parameters, Immunity Indices, Antioxidant Capacity and Metabolomics in Early Weaned Yak Calves. Animals, 12(17), 2228. https://doi.org/10.3390/ani12172228







