Simple Summary
Can urea nitrogen metabolite contribute to implementing the ideal protein concept in monogastric animals? This work aims to critically analyse how this metabolite can contribute to accurately implementing the ideal protein concept in monogastric animals, particularly in pig, poultry, and rabbit nutrition. This information will contribute to evaluating its potential and limitations as biomarker, as well as to standardizing the use of this metabolite in precise amino acidic monogastric nutrition.
Abstract
The ideal protein concept refers to dietary protein with an amino acid profile that exactly meets an animal’s requirement. Low-quality protein levels in the diet have negative implications for productive and reproductive traits, and a protein oversupply is energetically costly and leads to an excessive N excretion, with potentially negative environmental impact. Urea Nitrogen (UN), which corresponds to the amount of nitrogen in the form of urea circulating in the bloodstream, is a metabolite that has been widely used to detect amino acid imbalances and deficiencies and protein requirements. This review aims to critically analyse how UN can contribute to accurately implementing the ideal protein concept in monogastric animals, particularly in pig, poultry, and rabbit nutrition (14,000 animals from 76 published trials). About 59, 37, and 4% of trials have been conducted in pigs, poultry, and rabbits, respectively. UN level was negatively correlated to main performance traits (Pearson Correlation Coefficient [PCC] of −0.98 and −0.76, for average daily gain and feed conversion ratio, respectively), and lower UN level was related to higher milk yield and concentration. High level of UN was positively correlated to N excretion (PCC = 0.99) and negatively correlated to protein retention (PCC = −0.99). Therefore, UN in blood seems to be a proper indicator of amino acid imbalance in monogastric animals. Great variability in the use of UN was observed in the literature, including uses as determination medium (blood, plasma, or serum), units, and feeding system used (ad libitum or restricted), among others. A standardization of the methods in each of the species, with the aim to harmonize comparison among works, is suggested. After review, UN measurement in plasma and, whenever possible, the utilization of the same nutritional methodology (ad libitum conditions or restriction with blood sampling after refeeding at standardised time) are recommended. More studies are necessary to know the potential of UN and other bioindicators for amino acid deficiencies evaluation to get closer to the ideal protein concept.
1. Introduction
The ideal protein concept refers to dietary protein with an amino acid profile that exactly meets an animal’s requirement [1]. When applying this concept, all essential and non-essential dietary amino acids can be equally co-limiting. Formulating according to the ideal protein concept can improve animal performance, increasing protein retention [2], and can contribute to reducing N excretion [3,4,5]. Nevertheless, to use the ideal protein concept, it is necessary to combine precise knowledge on feed evaluation and an animal’s nutritional requirements. An animal’s amino acids requirements are dynamic, since they can vary according to different factors including genetics, age, and physiological state [6]. Amino acid requirements have also shown to vary individually [7] within the same animal type in growing pigs. Currently, it is well accepted that amino acid requirements and feed evaluation should be determined at ileal true digestible level, because this information provides a more accurate estimation compared to total amino acids content at dietary or faecal level.
In addition, there is an increasing interest in the use of low-protein diets as a tool to improve the sustainability of animal production [8]. Previously, protein requirements were determined by dose–response experiments [9,10,11,12,13,14,15,16,17,18]. Commonly, dose–response studies only deal with a specific amino acid, without considering amino acid interactions [19]. These limitations could be overcome by acquiring information on the metabolic profile of animals that are fed with different levels of essential amino acids. This approach could reveal metabolic phenotypes related to the presence of specific limiting amino acids. At present, metabolomics can be used as a tool to investigate the relationship between nutritional status and metabolic phenotyping [20]. Among these metabolites, urea nitrogen (UN) level in blood has been widely used to detect amino acid imbalances and deficiencies.
This review aims to critically analyse how UN determination in blood can contribute to accurately implementing the ideal protein concept in monogastric animals, particularly in pig, poultry, and rabbit nutrition. This information will contribute to evaluating its potential and limitations, as well as to standardizing the use of this metabolite in precise amino acidic monogastric nutrition.
To achieve this goal, a critical review was carried out using all the articles published on monogastric species where UN was analysed in any of its forms. The different aspects studied in these works were studied and analysed jointly through correlation coefficients.
2. Monogastrics’ Protein Metabolism
Protein are macro-biomolecules, and monogastrics carry out protein synthesis through translation, creating their own protein using the amino acids available after cellular digestion. Amino acids become available as end-products after protein digestion in an animal’s digestive tract. Protein and amino acids oversee many functions in animals (structural, regulatory, transporter, defensive, enzymatic, or even contractile). There are two types of amino acids: non-essential and essential [21,22] Essential amino acids depend on the animal species [10,11,23,24]. Starting from the diet, animals can synthesize their own protein. However, protein efficiency, usually measured by dividing protein retention by protein intake and multiplying by 100, is low, and only a part of ingested protein can be digested, metabolised, and effectively used by the animal [2,25,26,27].
Figure 1 shows how only a portion of total ingested crude protein (CP) is digested and converted into amino acids that can be absorbed in the small intestine. The amino acids that are absorbed until ileum correspond to the ileal digestible CP. The rest (ileal indigestible CP) undergo processes into the large intestine where they can be used or modified by microbial action and finally eliminated in faeces (faecal indigestible CP). It is therefore reasonable to believe that ileal amino acid digestibility (and especially true ileal digestibility, which takes into account the endogenous losses) allows us to know the quantity of amino acids that are available for the animal [28], giving valuable information on both the animal’s requirements and nutritional balance of the diet.
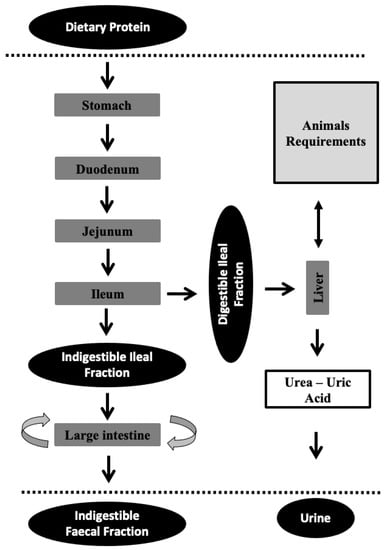
Figure 1.
The protein partitioning and utilization in monogastric animals. The dotted lines symbolize the interior of the animal. The different arrows in the large intestine refer to the conversion of some amino acids into others produced by the microbiota.
Animals synthetize specific proteins depending on their requirements [29]. Protein synthesis mainly depends on animal requirements, determined by DNA transcription, and amino acids provided by the diet [30]. When there is no amino acid limitation, protein synthesis can happen adequately. However, if there is any amino acid limitation, the specific protein will not be synthesized.
3. When Protein Is Synthesized from Balanced Diets: Protein Requirements
If the amino acids of the protein to be synthesized by the animals match with the amino acids available at the end of digestion, protein synthesis will occur. Total protein requirements are calculated factorially by summation of the requirements for maintenance and production as well as the immune status and the typical characteristics of the species.
Protein requirements for maintenance are the amino acids used by the animal when its body composition remains constant. The determination of protein requirements for maintenance in monogastrics is controversial, and the results vary depending on the consulted bibliographical source [16,31,32,33,34,35,36,37,38,39,40].
The growth pattern of an animal also determines its protein requirements. The growth of all body components can be investigated by slaughtering and analysing animals at successive stages of growth, determining retained nitrogen [41]. This retained nitrogen is related to body weight and nitrogen intake by the animals [32,33,34,42,43,44,45].
An animal’s requirements are also affected by reproduction. In spite that, the quantities of protein required to produce spermatozoa by mammals are small and of little significance [30], and all female mammals increase protein requirements in the reproductive period. Females must mobilize protein for ovulation, implantation process, foetal development, and milk production. [46,47,48,49].
The health status (immunity responses) of the animals also influences their protein requirements and vice versa [25,50,51,52]. Research has shown differences in immunity responses to be functions of dietary protein level in all monogastric species [53].
There is another peculiarity in a monogastric that must be considered when calculating protein requirements, in the case of lagomorphs, which practice caecotrophy. The caecotrophy contribution to the total CP intake is around 17% [25]. Furthermore, protein of soft faeces is rich in essential amino acids [54,55].
4. When Protein Is Not Synthesized: Urea Nitrogen Formation
No dietary amino acids are absorbed in a total form for utilization by the animal [56]. In addition, not all of the absorbed fraction is used by the animal (e.g., due to the presence of some limiting amino acid). These amino acids (absorbed but not used) together with the ones coming from cell renewal are catabolized, in the urea cycle (Figure 1), in the mitochondria and cell cytosol of liver cells. Urea is the end-product, and it passes into the bloodstream and from there to the kidneys, which excrete it in urine. UN can be measured in blood (blood urea nitrogen; BUN), concentrated plasma (plasma urea nitrogen; PUN), or serum (serum urea nitrogen; SUN), depending on whether clotting factors are included or not [57]; in this review, UN measurement will encompass all three. The urea cycle requires the utilization of energy in urea production. Therefore, diets that induce high urea levels will result in a reduction in animal performance (related to growth traits [58], health status [25], and reproductive traits, among other factors), while an oversupply will be energetically costly and will lead to pollution [1,3,59]. That is why works that enable knowing the amino acid needs of animals are very important.
As it has been said previously, amino acid requirements are usually determined by dose–response trials [9,10,11,12,13,14,15,16,17,18]. In addition to dose–response trials, animals have showed a certain ability to modulate their amino acid profile intake when they have the opportunity to choose between diets with different levels of amino acids in a choice feeding trial [60,61,62]. This ability of animals (to choose the diet that fits their nutritional requirements) should also be considered in future works that study the ideal protein profile of the diets. Nevertheless, amino acids requirements determination is complex, because there are many interactions among them and between amino acids and other nutrients. In general terms, when comparing the animal amino acid requirements with the amino acid composition of their targeted diets, it is shown that the ‘first-limiting’ amino acid for pigs is Lysine, whereas for poultry and rabbits, it is commonly Methionine, although Lysine and Arginine may also be limitans.
As it has been explained previously, on one hand, UN is related with the parts of a protein that were absorbed but non-utilized, and on the other hand, implementing the ideal protein concept in monogastric animals’ nutrition could improve animal performance and reduce pollution. In this moment, a question arises: Could UN be used as a measurement of the ideal protein concept in monogastrics?
5. Use of Urea Nitrogen to Detect Amino Acid Imbalances
Animals fed with a balanced diet show low UN levels in blood, indicating a decrease in protein catabolism, more efficient total N utilization, and thus a decrease in urea synthesis. Although the first research using UN as an indicator of CP requirements was carried out in the 1970s [63,64], the use of this metabolite has become more common in recent years, because it is a rapid and cheap response criterion to determine amino acids requirements [65,66].
Table 1 summarizes studies where UN has been used as an indicator for improving productive or reproductive traits (to these works must be added those that relate UN with N excretion). In these studies, around 14,000 animals were used in 76 different trials. On average, the number of treatments used in each trial was around five, with approximately 48 animals per treatment. About 59, 37, and 4% of the trials were conducted in pigs, poultry, and rabbits, respectively (excluding experiments carried out on rats). In both pig and poultry studies, a range from reproductive animals to noticeably young individuals can be found. Nevertheless, in rabbit studies, UN has only been determined in growing rabbits. According to the dietary treatment, only 5% of the studies compared protein source, 17% studied CP, and 78% studied amino acids level.

Table 1.
Parameters studied in the published works where urea nitrogen (UN) is used to determine nutritional requirements in monogastrics and related with performance and reproductive traits.
5.1. Causes of Variability in UN Measurements
Despite the extensive use of this metabolite, there are discrepancies in its use that must be considered when comparing studies. Like any blood metabolite, UN presents daily circadian variations. Therefore, evaluation of UN evolution in time in each animal species is needed, considering particularly feeding behaviour. Of the total published works, only 5% have evaluated this issue [56,63,74,87]. In fact, no research was found in broilers or hens addressing this matter.
As it has been explained previously, UN level depends on protein intake, and it is for this reason that feed behaviour can alter UN measurements. For example, 20% more PUN (p < 0.05) has been observed in samples obtained 4 h after a refeeding in animals that were submitted to a fasting trial than those animals (for the same animal type and with the same experimental diet) that were fed ad libitum. About 30 and 70% of the studies were performed in animals fed ad libitum and restricted diets, respectively [87]. In those animals fed ad libitum, the circadian evolution of UN followed a pattern very similar to that of feed intake, when animals were fed with balanced diets. However, in the case of animals subjected to fasting, the UN pattern after refeeding increases for the first 3–4 h after feeding and thereafter reaches a plateau. UN concentrations as a function of time after refeeding must be considered to compare results between works (the sample collection presents a large variability, from 1 h to 16 h after refeeding, in the consulted literature).
Regarding the standardization of feeding management, we recommend feeding in ad libitum conditions or drawing blood samples 4 h after refeeding (since it has been shown that the highest UN levels are reached after ingestion). It would be advisable to specify the type of feed management.
In addition to the above, these studies showed different experimental designs such as randomized blocks, incomplete blocks, Latin square, repeated Latin square, and even repeated measures [88]. Another observed discrepancy may be due to the type of measurement. As previously mentioned, UN can be determined in blood, plasma, or serum. Independently of the species and physiological state, 66, 28, and 14% of the works determined UN in plasma, serum, or blood, respectively. This is a factor of variability that causes deviations in UN results, which must be considered when comparing trials. Furthermore, there is also great diversity in the units used to express UN. Most studies express it in mg/dL, although there are some others that express it with other units such as mmol/L [75]. Is possible to convert from one unit to another, with 1 mmol/L = 6.006 mg/dL.
Despite the mentioned methodological differences, of the total of these studies, 86% showed that UN could be used for the detection of amino acid imbalances (see Table 1). Next, we proceed to analyse the different relationships of this metabolite with performance and environmental nutrient load.
5.2. Urea Nitrogen and Performance/Reproductive Traits
Most of the studies where UN was used were carried out in growing animals. The main traits controlled to evaluate growing performance were feed intake, average daily gain (ADG), and feed conversion ratio (FCR), as well as other less frequent traits such as carcass quality. In general terms, UN values are negatively correlated with protein utilization and growth performance traits. Figure 2 (n = 348 animals) shows the linear relationship between UN and ADG (Figure 2A) and FCR (Figure 2B) obtained with different experimental diets varying in three essential amino acids (Lysine, Sulphur amino acids, and Threonine) in growing rabbits [2,60,87,89]. Data on this figure reflects how UN levels are negatively correlated to ADG (Pearson Correlation Coefficient [PCC] = −0.98; p < 0.05) and positively correlated to FCR (PCC = 0.81; p < 0.05). Along this same line, Figure 3 (n = 2154 animals) shows the linear relationship between UN and FCR obtained in different trials in growing pigs [65,66,73,75,76,78]. As it has been shown in rabbits, UN levels in pigs are positively correlated to FCR (PCC = 0.70; p < 0.05). It should be considered that in making comparisons between animals of different ages and genetic types, the relationships between UN and ADG or FCR may be compromised. That is why the relationship with FCR is stronger (FCR is corrected for the marked differences in ADG and feed intake in animals of different ages). As can be extracted from Figure 2 and Figure 3, for every 2.5 and 1.55 mg/dL more units of UN in plasma, the FCR increases by 0.1 in rabbits and pigs, respectively (p < 0.05). This could be because low UN levels imply more efficient use of protein, lower imbalance in the amino acid profile, and less energy addressed to catabolizing non-used amino acids and UN metabolization. Therefore, nutrients are more available for growth and fitted to the requirements. Other trials have been conducted to investigate the relationship between UN and reproductive performance traits in sows [44] or laying hens. No trials have been performed on reproductive rabbits until recently. In these studies, parameters such as milk yield, composition yield, and egg production have been analysed. These studies have shown that lower levels of UN are usually related to higher milk yield and concentration (even increasing the quantity of immunoglobulins in colostrum [90]).
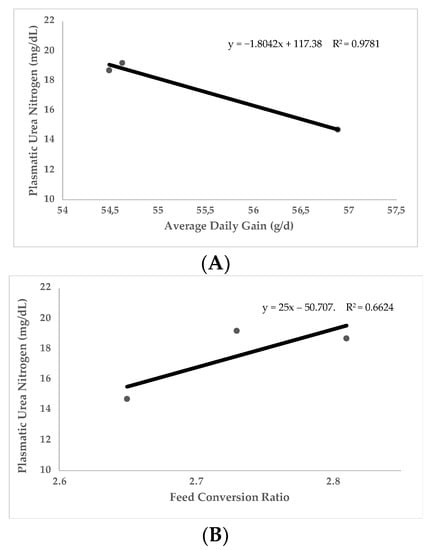
Figure 2.
Relationship of plasmatic urea nitrogen (PUN) with average daily gain (A) and with feed conversion ratio (B). Data obtained from four published studies in growing rabbits [2,60,87,89].
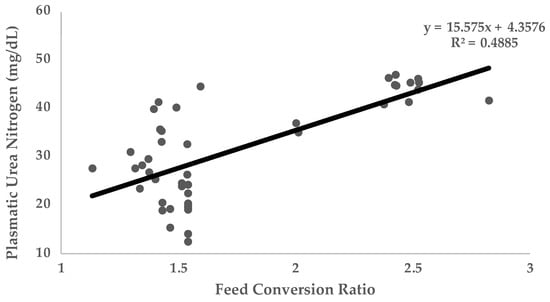
Figure 3.
Relationship of plasmatic urea nitrogen (PUN) with feed conversion ratio. Data obtained from four published studies in growing pigs [65,66,73,75,76,78].
5.3. Urea Nitrogen and Environmental Nutrient Load
Nitrogen pollution from livestock should be studied, as it is a serious problem for the environment [91]. Contamination of groundwater is a serious environmental issue of global concern because of its direct adverse effect on human health and biodiversity, among other effects [92]. A total of nine studies have been conducted to evaluate the relationship between UN level and N excretion. All the works observed a relationship between UN and nitrogen contamination [66,71,74,88,90,93,94,95,96]. As it can be seen in Figure 4 (starting from two experimental trials conducted in pigs [71,86]), UN level in blood seems to be positively correlated to N excretion (PCC = 0.99; p < 0.05) and negatively correlated to protein retention (PCC = −0.99; p < 0.05). For all the above, diets that induce higher UN levels have negative implications for productive and reproductive traits and lead to an excessive N excretion, with potentially negative environmental impact.
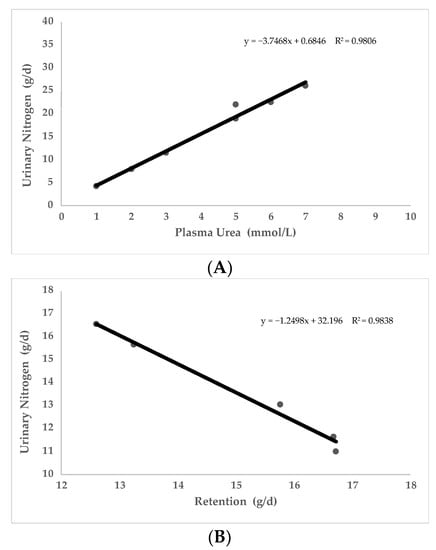
Figure 4.
Relationship of plasma urea nitrogen (PUN) with urinary nitrogen excreted (A) and relationship of urinary nitrogen excreted with Nitrogen retained (B). Data obtained from two published studies in sows and growing pigs [71,86].
6. Future Perspectives
As a summary, UN level in blood has been shown to be a promising indicator of protein and amino acid use, sensible to amino acid imbalance in pigs, poultry, and rabbits. It has been validated as a useful response criterion related with performance and nitrogen excretion data. Its use would have the potential to obtain indications of possible deficiencies or imbalances of dietary amino acids, and some recommendations for extending the use of this metabolite as a rapid and economical response indicator for amino acid imbalances have been proposed.
The ideal protein concept could be used not only in animal production and environment, as it can also be applied in other areas such as animal conservation. Some wild animal populations seem to be limited by protein supply of their ecosystems. In this case, meeting nutritional requirements would improve adaptive success of wild animals, and specific vegetation management programs could be carried out for the conservation of these animals [97,98].
On the other hand, there are a multitude of metabolites that could complement UN use to improve the concept of the ideal protein. Metabolomics arises as a valuable tool to identify and quantify biomarkers related to the presence of limiting amino acid. Some studies have proposed as candidate metabolites plasma glychocolic acid, taurocholic acid, and ascorbic acid, among others [99]. However, more studies are needed to complement UN with other metabolites to implement the ideal protein concept in monogastric animals.
An important factor is the interaction between protein nutrition and heat stress caused by climate change. The activity of feeding (amino acid included) and the metabolism caused by digestion and assimilation of food increase an animal’s heat production [100]. It is known that the metabolism of amino acids in excess produces the most heat among all nutrients, negatively influencing animal performance. The ideal protein concept could reduce the heat of metabolism, allowing animals to withstand thermal stress better, and UN could be used along this line [101].
7. Conclusions
The use of urea nitrogen in blood is proven as an indicator of amino acid imbalance in monogastric animals. However, there are some discrepancies in the methodology used in the different studies, which make it difficult to compare them. The standardization of the methods in each of the species is recommended. From the analysis of the literature, this study recommends the measurement of UN in plasma (PUN) and whenever possible trying to always use the same feeding management (ad libitum conditions or drawing blood samples 4 h after refeeding). More studies are necessary to know the potential of UN and other bioindicators for amino acid deficiencies evaluation to get closer to the ideal protein concept.
Author Contributions
Conceptualization, P.J.M.-G., E.B., M.C.-L. and J.J.P.; methodology, M.C.L.-L.; software, P.J.M.-G.; validation, P.J.M.-G.; formal analysis, P.J.M.-G.; investigation, P.J.M.-G., E.B., M.C.-L., J.J.P., L.L. and M.C.L.-L.; resources, P.J.M.-G.; data curation, P.J.M.-G.; writing—original draft preparation, P.J.M.-G.; writing—review and editing, P.J.M.-G.; visualization, P.J.M.-G., E.B., M.C.-L. and J.J.P.; supervision, P.J.M.-G.; project administration, P.J.M.-G.; funding acquisition, P.J.M.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Conselleria de Innovación, Universidades, Ciencia y Sociedad Digital from the Generalitat Valenciana grant number GV/2021/115. And The APC was invited to Pablo Jesús Marín García.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study was supported by the Conselleria de Innovación, Universidades, Ciencia y Sociedad Digital from the Generalitat Valenciana (GV/2021/115).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| BUN | Blood Urea Nitrogen |
| CP | Crude Protein |
| PCC | Pearson Correlation Coefficient |
| PUN | Plasma Urea Nitrogen |
| SUN | Serum Urea Nitrogen |
| UN | Urea Nitrogen |
References
- Van Milgen, J.; Dourmad, J.-Y. Concept and application of ideal protein for pigs. J. Anim. Sci. Biotechnol. 2015, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Marín-García, P.; Ródenas, L.; Martínez-Paredes, E.; Cambra-López, M.; Blas, E.; Pascual, J. A moderate protein diet does not cover the requirements of growing rabbits with high growth rate. Anim. Feed Sci. Technol. 2020, 264, 114495. [Google Scholar] [CrossRef]
- Langner, J.; Bergström, R.; Foltescu, V. Impact of climate change on surface ozone and deposition of sulphur and nitrogen in Europe. Atmos. Environ. 2005, 39, 1129–1141. [Google Scholar] [CrossRef]
- Hou, Y.; Bai, Z.; Lesschen, J.P.; Staritsky, I.G.; Sikirica, N.; Ma, L.; Velthof, G.; Oenema, O. Feed use and nitrogen excretion of livestock in EU-27. Agric. Ecosyst. Environ. 2016, 218, 232–244. [Google Scholar] [CrossRef]
- Pomar, C.; Andretta, I.; Remus, A. Feeding Strategies to Reduce Nutrient Losses and Improve the Sustainability of Growing Pigs. Front. Veter-Sci. 2021, 8, 742220. [Google Scholar] [CrossRef]
- Pesti, G.M.; Miller, B.R. Modelling for Precision Nutrition. J. Appl. Poult. Res. 1997, 6, 483–494. [Google Scholar] [CrossRef]
- Remus, A.; Hauschild, L.; Létourneau-Montminy, M.-P.; Pomar, C. Estimating Amino Acid Requirements in Real-Time for Precision-Fed Pigs: The Challenge of Variability among Individuals. Animals 2021, 11, 3354. [Google Scholar] [CrossRef]
- Attia, Y.A.; Bovera, F.; Wang, J.; Al-Harthi, M.A.; Kim, W.K. Multiple Amino Acid Supplementations to Low-Protein Diets: Effect on Performance, Carcass Yield, Meat Quality and Nitrogen Excretion of Finishing Broilers under Hot Climate Conditions. Animals 2020, 10, 973. [Google Scholar] [CrossRef]
- De Blas, C.; Mateos, G.G. Feed Formulation. In Nutrition of the Rabbit; De Blas, C., Wiseman, J., Eds.; CABI: Wallingford, UK, 2010; pp. 222–232. ISBN 978-1-84593-669-3. [Google Scholar]
- Taboada, E.; Mendez, J.; Mateos, G.; de Blas, C. The response of highly productive rabbits to dietary lysine content. Livest. Prod. Sci. 1994, 40, 329–337. [Google Scholar] [CrossRef]
- Taboada, E.; Mendez, J.; De Blas, J.; De Blas, C. The response of highly productive rabbits to dietary sulphur amino acid content for reproduction and growth. Reprod. Nutr. Dev. 1996, 36, 191–203. [Google Scholar] [CrossRef]
- de Blas, C.; Taboada, E.; Nicodemus, N.; Campos, R.; Piquer, J.; Méndez, J. Performance response of lactating and growing rabbits to dietary threonine content. Anim. Feed Sci. Technol. 1998, 70, 151–160. [Google Scholar] [CrossRef]
- Adamson, I.; Fisher, H. Amino Acid Requirement of the Growing Rabbit: An Estimate of Quantitative Needs. J. Nutr. 1973, 103, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Berchiche, M.; Lebas, F. Supplémentation en Méthionine dun Aliment a Base de Féverole: Effets sur la Croissance, le Rendement a L’abattage et la Composition de la Carcasse Chez le Lapin. World Rabbit Sci. 2010, 2, 135–140. [Google Scholar] [CrossRef]
- Cloutier, L.; Pomar, C.; Montminy, M.L.; Bernier, J.; Pomar, J. Evaluation of a method estimating real-time individual lysine requirements in two lines of growing–finishing pigs. Animal 2015, 9, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.K.; Baker, D.H. Efficiency of Dietary Methionine Utilization by Young Pigs. J. Nutr. 1992, 122, 1862–1869. [Google Scholar] [CrossRef]
- Morris, T.R.; Al-Azzawi, K.; Gous, R.M.; Simpson, G.L. Effects of protein concentration on responses to dietary lysine by chicks. Br. Poult. Sci. 1987, 28, 185–195. [Google Scholar] [CrossRef]
- Bunchasak, C. Role of Dietary Methionine in Poultry Production. J. Poult. Sci. 2009, 46, 169–179. [Google Scholar] [CrossRef]
- García, P.J.M. Lysine, Amino Acids and Threonine Requirements of Growing Rabbits from a Line Selected by Growth Rate. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, 2019. [Google Scholar]
- The Human Serum Metabolome (HUSERMET) Consortium; Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- McDonald, P.; Greenhalg, J.F.D.; Morgan, C.A.; Edvards, R.; Sinclair, L.A.; Wilkinson, R.G. Animal Nutrition; Pearson: London, UK, 2011; ISBN 978-1-4082-0423-8. [Google Scholar]
- Marín-García, P.; Llobat, L. How Does Protein Nutrition Affect the Epigenetic Changes in Pig? A Review. Animals 2021, 11, 544. [Google Scholar] [CrossRef]
- Rezaei, R.; Knabe, D.A.; Tekwe, C.D.; Dahanayaka, S.; Ficken, M.D.; Fielder, S.E.; Eide, S.J.; Lovering, S.L.; Wu, G. Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids 2013, 44, 911–923. [Google Scholar] [CrossRef]
- Fisher, H.; Johnson, D. The Amino Acid Requirement of the Laying Hen. J. Nutr. 1956, 60, 275–282. [Google Scholar] [CrossRef]
- Carabaño Luengo, R.M.; Villamide Díaz, M.J.; García, J.; Nicodemus Martin, N.; Llorente, A.; Chamorro, S.; Menoyo Luque, D.; Garcia Rebollar, P.; García Ruiz, A.I.; Blas Beorlegui, J.C.D. New concepts and objectives for protein-amino acid nutrition in rabbits: A review. World Rabbit Sci. 2010, 17, 01–14. [Google Scholar] [CrossRef]
- Noblet, J.; Henry, Y.; Dubois, S. Effect of Protein and Lysine Levels in the Diet on Body Gain Composition and Energy Utilization in Growing Pigs. J. Anim. Sci. 1987, 65, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.K.; Baker, D.H. Quantitative Evaluation of Nonspecific Nitrogen Sources for the Growing Chick. Poult. Sci. 1974, 53, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Lemme, A.; Ravindran, V.; Bryden, W. Ileal digestibility of amino acids in feed ingredients for broilers. World’s Poult. Sci. J. 2004, 60, 423–438. [Google Scholar] [CrossRef]
- Eagle, H. Amino Acid Metabolism in Mammalian Cell Cultures. Science 1959, 130, 432–437. [Google Scholar] [CrossRef]
- Labadan, M.C.; Hsu, K.-N.; Austic, R.E. Lysine and Arginine Requirements of Broiler Chickens at Two- to Three-Week Intervals to Eight Weeks of Age. Poult. Sci. 2001, 80, 599–606. [Google Scholar] [CrossRef]
- Fraga, M.J.; Lorente, M.; Carabaño, R.M.; de Blas, J.C. Effect of diet and of remating interval on milk production and milk composition of the doe rabbit. Anim. Sci. 1989, 48, 459–466. [Google Scholar] [CrossRef]
- Partridge, G.G.; Garthwaite, P.H.; Findlay, M. Protein and energy retention by growing rabbits offered diets with increasing proportions of fibre. J. Agric. Sci. 1989, 112, 171–178. [Google Scholar] [CrossRef]
- Fernández, C.; Fraga, M.J. Effect of fat inclusion in diets for rabbits on the efficiency of digestible energy and protein utilization. World Rabbit Sci. 2010, 4, 19–23. [Google Scholar] [CrossRef][Green Version]
- Ferreira, W.M.; Fraga, M.J.; Carabañco, R. Inclusion of grape pomace, in substitution for alfalfa hay, in diets for growing rabbits. Anim. Sci. 1996, 63, 167–174. [Google Scholar] [CrossRef]
- Whittemore, C.; Green, D.; Knap, P. Technical review of the energy and protein requirements of growing pigs: Protein. Anim. Sci. 2001, 73, 363–373. [Google Scholar] [CrossRef]
- Iii, H.M.E.; Baker, D.H.; Fernandez, S.R.; Parsons, C.M. Maintenance threonine requirement and efficiency of its use for accretion of whole-body threonine and protein in young chicks. Br. J. Nutr. 1997, 78, 111–119. [Google Scholar] [CrossRef][Green Version]
- Edwards, H.; Fernandez, S.R.; Baker, D. Maintenance lysine requirement and efficiency of using lysine for accretion of whole-body lysine and protein in young chicks. Poult. Sci. 1999, 78, 1412–1417. [Google Scholar] [CrossRef]
- Heger, J.; Van Phung, T.; Křížová, L. Efficiency of amino acid utilization in the growing pig at suboptimal levels of intake: Lysine, threonine, sulphur amino acids and tryptophan. J. Anim. Physiol. Anim. Nutr. 2002, 86, 153–165. [Google Scholar] [CrossRef]
- Heger, J.; Van Phung, T.; Krizova, L.; Sustala, M.; Simecek, K. Efficiency of amino acid utilization in the growing pig at suboptimal levels of intake: Branched-chain amino acids, histidine and phenylalanine + tyrosine. J. Anim. Physiol. Anim. Nutr. 2003, 87, 52–65. [Google Scholar] [CrossRef]
- Velu, J.G.; Baker, D.H.; Scott, H.M. Protein and Energy Utilization by Chicks Fed Graded Levels of a Balanced Mixture of Crystalline Amino Acids. J. Nutr. 1971, 101, 1249–1256. [Google Scholar] [CrossRef]
- Birolo, M.; Trocino, A.; Tazzoli, M.; Xiccato, G. Effect of feed restriction and feeding plans on performance, slaughter traits and body composition of growing rabbits. World Rabbit Sci. 2017, 25, 113–122. [Google Scholar] [CrossRef]
- Carr, J.R.; Boorman, K.N.; Cole, D.J.A. Nitrogen retention in the pig. Br. J. Nutr. 1977, 37, 143–155. [Google Scholar] [CrossRef]
- Ames, D.R.; Brink, D.R. Effect of Temperature on Lamb Performance and Protein Efficiency Ratio. J. Anim. Sci. 1977, 44, 136–144. [Google Scholar] [CrossRef]
- Badillo, D.; Herzka, S.Z.; Viana, M.T. Protein Retention Assessment of Four Levels of Poultry By-Product Substitution of Fishmeal in Rainbow Trout (Oncorhynchus mykiss) Diets Using Stable Isotopes of Nitrogen (δ15N) as Natural Tracers. PLoS ONE 2014, 9, e107523. [Google Scholar] [CrossRef] [PubMed]
- Meluzzi, A.; Sirri, F.; Tallarico, N.; Franchini, A. Nitrogen retention and performance of brown laying hens on diets with different protein content and constant concentration of amino acids and energy. Br. Poult. Sci. 2001, 42, 213–217. [Google Scholar] [CrossRef]
- Whittemore, C.; Morgan, C. Model components for the determination of energy and protein requirements for breeding sows: A review. Livest. Prod. Sci. 1990, 26, 1–37. [Google Scholar] [CrossRef]
- Pettigrew, J.E.; Yang, H. Protein nutrition of gestating sows. J. Anim. Sci. 1997, 75, 2723–2730. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.A.; Herron, K.M. Effects of energy and protein allowances during lay on the reproductive performance of broiler breeder hens. Br. Poult. Sci. 1981, 22, 227–239. [Google Scholar] [CrossRef]
- Begin, J.J.; Insko, W.M. The Effects of Dietary Protein Level on the Reproductive Performance of Coturnix Breeder Hens. Poult. Sci. 1972, 51, 1662–1669. [Google Scholar] [CrossRef]
- Kidd, M. A treatise on chicken dam nutrition that impacts on progeny. World’s Poult. Sci. J. 2003, 59, 475–494. [Google Scholar] [CrossRef]
- Lochmiller, R.L.; Vestey, M.R.; Boren, J.C. Relationship between Protein Nutritional Status and Immunocompetence in Northern Bobwhite Chicks. Ornithology 1993, 110, 503–510. [Google Scholar] [CrossRef]
- Le Floc’H, N.; Wessels, A.; Corrent, E.; Wu, G.; Bosi, P. The relevance of functional amino acids to support the health of growing pigs. Anim. Feed Sci. Technol. 2018, 245, 104–116. [Google Scholar] [CrossRef]
- Hermes, R.G.; Molist, F.; Ywazaki, M.; Nofrarías, M.; De Segura, A.G.; Gasa, J.; Pérez, J.F. Effect of dietary level of protein and fiber on the productive performance and health status of piglets1. J. Anim. Sci. 2009, 87, 3569–3577. [Google Scholar] [CrossRef]
- Spreadbury, D. A study of the protein and amino acid requirements of the growing New Zealand White rabbit with emphasis on lysine and the sulphur-containing amino acids. Br. J. Nutr. 1978, 39, 601–613. [Google Scholar] [CrossRef] [PubMed]
- García, A.I.; de Bias, J.C.; Carabaño, R. Effect of type of diet (casein-based or protein-free) and caecotrophy on ileal endogenous nitrogen and amino acid flow in rabbits. Anim. Sci. 2004, 79, 231–240. [Google Scholar] [CrossRef]
- Coma:, J.; Zimmerman, D.R.; Carrion, D. Lysine requirement of the lactating sow determined by using plasma urea nitrogen as a rapid response criterion. J. Anim. Sci. 1996, 74, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Barelli, S.; Crettaz, D.; Thadikkaran, L.; Rubin, O.; Tissot, J.-D. Plasma/serum proteomics: Pre-analytical issues. Expert Rev. Proteom. 2007, 4, 363–370. [Google Scholar] [CrossRef]
- Hussein, M.A.A.; El-Kloub, K.; El Moustafa, M.; Gad El-hak, M.K.; Abbas, A.M. Optimal metabolizable energy and crude protein levels for Sinai laying hens. Egypt Poult. Sci. 2010, 30, 1073–1095. [Google Scholar]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, and Identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Marín-García, P.; López-Luján, M.; Ródenas, L.; Martínez-Paredes, E.; Cambra-López, M.; Blas, E.; Pascual, J. Do Growing Rabbits with a High Growth Rate Require Diets with High Levels of Essential Amino Acids? A Choice-Feeding Trial. Animals 2021, 11, 824. [Google Scholar] [CrossRef]
- Kyriazakis, I.; Emmans, G.C.; Whittemore, C.T. Diet selection in pigs: Choices made by growing pigs given foods of different protein concentrations. Anim. Sci. 1990, 51, 189–199. [Google Scholar] [CrossRef]
- Siegel, P.B.; Picard, M.; Nir, I.; Dunnington, E.; Willemsen, M.H.; Williams, P. Responses of meat-type chickens to choice feeding of diets differing in protein and energy from hatch to market weight. Poult. Sci. 1997, 76, 1183–1192. [Google Scholar] [CrossRef]
- Eggum, B.O. Blood urea measurement as a technique for assessing protein quality. Br. J. Nutr. 1970, 24, 983–988. [Google Scholar] [CrossRef]
- Lewis, A.J.; Speer, V.C. Lysine Requirement of the Lactating Sow. J. Anim. Sci. 1973, 37, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Waguespack, A.M.; Bidner, T.D.; Payne, R.L.; Southern, L.L. Valine and isoleucine requirement of 20- to 45-kilogram pigs. J. Anim. Sci. 2012, 90, 2276–2284. [Google Scholar] [CrossRef] [PubMed]
- Toledo, J.; Furlan, A.; Pozza, P.; Piano, L.; Carvalho, P.; Peñuela-Sierra, L.; Huepa, L. Effect of the reduction of the crude protein content of diets supplemented with essential amino acids on the performance of piglets weighing 6–15kg. Livest. Sci. 2014, 168, 94–101. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, J.; Yoon, S.; Jin, Z.; Choi, J.; Piao, X.; Kim, B.; Ohh, S.; Wang, M.; Chae, B. Early energy and protein reduction: Effects on growth, blood profiles and expression of genes related to protein and fat metabolism in broilers. Br. Poult. Sci. 2009, 50, 218–227. [Google Scholar] [CrossRef]
- Yang, H.-M.; Wang, W.; Wang, Z.-Y.; Yang, Z.; Wan, Y.; Hou, B.-H.; Huang, K.-H.; Lu, H. Effects of early energy and protein restriction on growth performance, clinical blood parameters, carcass yield, and tibia parameters of broilers. J. Integr. Agric. 2016, 15, 1825–1832. [Google Scholar] [CrossRef]
- Yuan, J.; Karimi, A.; Zornes, S.; Goodgame, S.; Mussini, F.; Lu, C.; Waldroup, P.W. Evaluation of the role of glycine in low-protein amino acid-supplemented diets. J. Appl. Poult. Res. 2012, 21, 726–737. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, X.; Zhu, Q.; Li, J.; Yin, H.; Gilbert, E.R.; Zhang, Y.; Liu, Y.; Wang, Y.; Li, D.; et al. Effects of Dietary Lysine Levels on Carcass Performance and Biochemical Characteristics of Chinese Local Broilers. Ital. J. Anim. Sci. 2015, 14, 3840. [Google Scholar] [CrossRef]
- Zervas, S.; Zijlstra, R.T. Effects of dietary protein and fermentable fiber on nitrogen excretion patterns and plasma urea in grower pigs. J. Anim. Sci. 2002, 80, 3247. [Google Scholar] [CrossRef]
- Yin, Y.; Baidoo, S.K.; Boychuk, J.L.L.; Simmins, H.H. Performance and carcass characteristics of growing pigs and broilers fed diets containing micronised barley, ground barley, wheat and maize. J. Sci. Food Agric. 2001, 81, 1487–1497. [Google Scholar] [CrossRef]
- Lenehan, M.A. The Optimal True Ileal Digestible Lysine and Threonine Requirement for Nursery Pigs between 25 and 55 Lb; Kansas State University: Manhattan, KS, USA, 2003; pp. 61–66. [Google Scholar]
- Brown, J.A.; Cline, T.R. Urea Excretion in the Pig: An Indicator of Protein Quality and Amino Acid Requirements. J. Nutr. 1974, 104, 542–545. [Google Scholar] [CrossRef]
- Guzik, A.C.; Southern, L.L.; Bidner, T.D.; Kerr, B.J. The tryptophan requirement of nursery pigs. J. Anim. Sci. 2002, 80, 2646–2655. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.; Southern, L.; Kerr, B.; Bidner, T. The Lysine and Total Sulfur Amino Acid Requirements of Six- to Twelve-Kilogram Pigs1. Prof. Anim. Sci. 2007, 23, 527–535. [Google Scholar] [CrossRef]
- Kerr, B.J.; Yen, J.T.; Nienaber, J.A.; Easter, R.A. Influences of dietary protein level, amino acid supplementation and environmental temperature on performance, body composition, organ weights and total heat production of growing pigs. J. Anim. Sci. 2003, 81, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Nieto, R.; Barea, R.; Lara, L.; Palma-Granados, P.; Aguilera, J. Lysine requirement relative to total dietary protein for optimum performance and carcass protein deposition of Iberian piglets. Anim. Feed Sci. Technol. 2015, 206, 48–56. [Google Scholar] [CrossRef]
- Azzam, M.M.M.; Dong, X.; Dai, L.; Zou, X.T. Effect of excess dietary L-valine on laying hen performance, egg quality, serum free amino acids, immune function and antioxidant enzyme activity. Br. Poult. Sci. 2015, 56, 72–78. [Google Scholar] [CrossRef]
- Li, F.; Ning, H.; Duan, X.; Chen, Z.; Xu, L. Effect of dietary l-arginine of broiler breeder hens on embryonic development, apparent metabolism, and immunity of offspring. Domest. Anim. Endocrinol. 2021, 74, 106537. [Google Scholar] [CrossRef]
- Roth-Maier, D.A.; Ott, H.; Roth, F.X.; Paulicks, B.R. Effects of the level of dietary valine supply on amino acids and urea concentration in milk and blood plasma of lactating sows: Valine for Lactating Sows. J. Anim. Physiol. Anim. Nutr. 2004, 88, 39–45. [Google Scholar] [CrossRef]
- Schneider, J.D.; Nelssen Jim, L. Determining the Total Sulfur Amino Acid to Lysine Requirement of the Lactating Sow; Agricultural Experiment Station and Cooperative Extension Service; Kansas State University: Manhattan, KS, UK, 2006; pp. 47–51. [Google Scholar]
- Sohail, M.A.; Cole, D.J.A.; Lewis, D. Amino acid requirements of the breeding sow: 2. The Dietary Lysine Requirement of the Lactating Sow. Br. J. Nutr. 1978, 40, 369–376. [Google Scholar] [CrossRef]
- Soltwedel, K.T.; Easter, R.A.; Pettigrew, J.E. Evaluation of the order of limitation of lysine, threonine, and valine, as determined by plasma urea nitrogen, in corn-soybean meal diets of lactating sows with high body weight loss. J. Anim. Sci. 2006, 84, 1734–1741. [Google Scholar] [CrossRef]
- Figueroa, J.L.; Lewis, A.J.; Miller, P.S.; Fischer, R.L.; Gómez, R.S.; Diedrichsen, R.M. Nitrogen metabolism and growth performance of gilts fed standard corn-soybean meal diets or low-crude protein, amino acid-supplemented diets1. J. Anim. Sci. 2002, 80, 2911–2919. [Google Scholar] [CrossRef]
- Cuaron, J.A.; Chapple, R.P.; Easter, R.A. Effect of Lysine and Threonine Supplementation of Sorghum Gestation Diets on Nitrogen Balance and Plasma Constituents in First-Litter Gilts. J. Anim. Sci. 1984, 58, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Marín-García, P.J.; López-Luján, M.D.C.; Ródenas, L.; Martínez-Paredes, E.; Blas, E.; Pascual, J.J. Plasma urea nitrogen as an indicator of amino acid imbalance in rabbit diets. World Rabbit Sci. 2020, 28, 63–72. [Google Scholar] [CrossRef]
- Kohn, R.A.; Dinneen, M.M.; Russek-Cohen, E. Using blood urea nitrogen to predict nitrogen excretion and efficiency of nitrogen utilization in cattle, sheep, goats, horses, pigs, and rats1. J. Anim. Sci. 2005, 83, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Marín-García, P.J.; López-Luján, M.C.; Ródenas, L.; Martínez-Paredes, E.; Blas, E.; Pascual, J.J. Plasmatic Urea Nitrogen in Growing Rabbits with Different Combinations of Dietary Levels of Lysine, Sulphur Amino Acids and Threonine. Animals 2020, 10, 946. [Google Scholar] [CrossRef]
- Atinmo, T.; Pond, W.G.; Barnes, R.H. Effect of Dietary Energy vs. Protein Restriction on Blood Constituents and Reproductive Performance in Swine. J. Nutr. 1974, 104, 1033–1040. [Google Scholar] [CrossRef]
- Lassaletta, L.; Billen, G.; Garnier, J.; Bouwman, L.; Velazquez, E.; Mueller, N.D.; Gerber, J. Nitrogen use in the global food system: Past trends and future trajectories of agronomic performance, pollution, trade, and dietary demand. Environ. Res. Lett. 2016, 11, 095007. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Kim, K.; Powell, M.A. Managing Groundwater Nitrate Contamination from Livestock Farms: Implication for Nitrate Management Guidelines. Curr. Pollut. Rep. 2016, 2, 178–187. [Google Scholar] [CrossRef]
- Mosenthin, R.; Sauer, W.C.; Henkel, H.; Ahrens, F.; De Lange, C. Tracer studies of urea kinetics in growing pigs: II. The effect of starch infusion at the distal ileum on urea recycling and bacterial nitrogen excretion1. J. Anim. Sci. 1992, 70, 3467–3472. [Google Scholar] [CrossRef]
- Mosenthin, R.; Sauer, W.C.; De Lange, C. Tracer studies of urea kinetics in growing pigs: I. The effect of intravenous infusion of urea on urea recycling and the site of urea secretion into the gastrointestinal tract. J. Anim. Sci. 1992, 70, 3458–3466. [Google Scholar] [CrossRef]
- Van Barneveld, R.J.; Batterham, E.S.; Skingle, D.C.; Norton, B.W. The effect of heat on amino acids for growing pigs: 4. Nitrogen Balance and Urine, Serum and Plasma Composition of Growing Pigs Fed on Raw or Heat-Treated Field Peas (Pisum Sativum). Br. J. Nutr. 1995, 73, 259–273. [Google Scholar] [CrossRef]
- Zervas, S.; Zijlstra, R.T. Effects of dietary protein and oathull fiber on nitrogen excretion patterns and postprandial plasma urea profiles in grower pigs. J. Anim. Sci. 2002, 80, 3238–3246. [Google Scholar] [CrossRef]
- Marín-García, P.J.; Llobat, L. What Are the Keys to the Adaptive Success of European Wild Rabbit (Oryctolagus cuniculus) in the Iberian Peninsula? Animals 2021, 11, 2453. [Google Scholar] [CrossRef] [PubMed]
- Llobat, L.; Marín-García, P.J. Application of protein nutrition in natural ecosystem management for European rabbit (Oryctolagus cuniculus) conservation. Biodivers. Conserv. 2022, 31, 1435–1444. [Google Scholar] [CrossRef]
- Soumeh, E.A.; Hedemann, M.S.; Poulsen, H.D.; Corrent, E.; van Milgen, J.; Nørgaard, J.V. Nontargeted LC–MS Metabolomics Approach for Metabolic Profiling of Plasma and Urine from Pigs Fed Branched Chain Amino Acids for Maximum Growth Performance. J. Proteome Res. 2016, 15, 4195–4207. [Google Scholar] [CrossRef] [PubMed]
- Musharaf, N.; Latshaw, J. Heat increment as affected by protein and amino acid nutrition. World’s Poult. Sci. J. 1999, 55, 233–240. [Google Scholar] [CrossRef]
- Qaid, M.; Al-Garadi, M. Protein and Amino Acid Metabolism in Poultry during and after Heat Stress: A Review. Animals 2021, 11, 1167. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).