1. Introduction
Tilapia is a group of fish with great economic importance. The global production of Nile tilapia, one of the most cultivated tilapiine species, exceeded 4.4 million tons in 2020, accounting for 9% of the total production of finfish in inland aquaculture [
1]. Nile tilapia originate from tropical, subtropical Africa and the Middle East, and are widely distributed in Nile and Niger River basins and in lakes Tanganyika, Albert, Edward and George [
2]. The water temperature of the native habitats for tilapia species ranges from 28 °C to 34 °C in warm seasons and from 22 °C to 26 °C in cold seasons; the lowest temperature that tilapia may encounter in the natural habitat is 17 °C to 24 °C in high-altitude lakes [
2,
3]. Due to the warm environment during evolution, tilapia fishes become cold-sensitive, which stop growth and feeding below 22 °C and 20 °C, respectively [
4], and generally die within a few days when exposed to low temperatures of 10 °C to 12 °C [
5]. The lowest winter temperature in the main culturing zone for tilapia, such as in China, is readily below the thermal minimum of tilapia.
The susceptibility of tilapia to low temperatures represents a major economic concern, which limits growing seasons and leads to mass over-winter mortality [
6]. Except for estimating the cold resistance of different selected species or strains [
6,
7,
8], the mechanisms determining cold resistance (or susceptibility) of tilapia have attracted intensive research interest. Histological investigations revealed that exposure of Nile tilapia to acute cold stress (13 °C) resulted in pathological tissue structure changes, such as vacuolation of neuropil and curling of secondary gill lamellae [
9]. Cold-induced changes to blood biochemical parameters of tilapia were also characterized, which reflect the status of body health. Exposure to cold stress markedly decreased activities of antioxidant enzymes such as superoxide dismutase and catalase [
10,
11,
12], indicating impairment of antioxidant functions. Nile tilapia exposed to cold temperature demonstrated significantly decreased concentrations of glucose and triglyceride in the serum [
11,
13], suggesting depletion of energy deposition. Furthermore, exposure of tilapia to cold stress increased activities of serum aspartate aminotransferase, alanine aminotransferase and lactate dehydrogenase [
11] and concentrations of urea and uric acid [
14], indicating the occurrence of tissue damage.
Genetic factors were reported to play dominant functions in determining cold resistance of tilapia based on variations in the ability of different species and their hybrids to survive lethal cold stress [
10,
15]. Comparative transcriptomic studies for species and strains with distinct cold resistance were conducted to explore the genetic pathways underlying the cold resistance of tilapia. Biological pathways including FoxO signaling and metabolic regulation were suggested to account for the differential low-temperature limit between Nile tilapia and zebrafish (
Danio rerio) [
16]. The cold-tolerant strain of blue tilapia (
Oreochromis aureus) demonstrated downregulation of biological processes including glycolysis/gluconeogenesis (in gills) and amino-acid biosynthesis (in liver) upon cold stress in comparison with the cold-sensitive strain [
17].
Cold-responsive genes (CRGs) in tissues such as the liver and kidney of Nile tilapia were identified through RNA sequencing and subjected to functional enrichment analysis to shed light on the biological functions influenced by cold stress. The CRGs of Nile tilapia liver were highly enriched in biological processes such as nucleic acid synthesis and metabolism, apoptosis and immunity [
18], while those of the kidney were mainly associated with metabolism and immunity [
14]. Cold-induced alternative splicing in tissues including testis, brain and kidney of Nile tilapia were characterized and many differentially spliced genes were found to be involved in the circadian clock pathway [
19]. Small RNA sequencing was conducted to identify cold-regulated miRNAs in the head kidney of Nile tilapia and the predicted target genes of the cold-responsive miRNAs are mainly associated with lipid metabolic processes and response to temperature stimulus [
12].
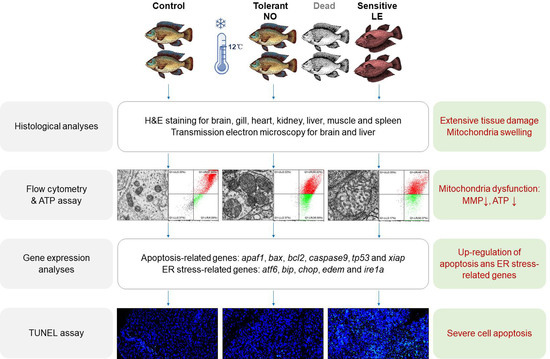
The previous studies have expanded our understanding of the cold-elicited adverse effects and the genetic programs associated with cold resistance of tilapia; however, the cellular and molecular mechanisms underlying cold susceptibility of tilapia remain to be further investigated. The cellular events and molecular pathways leading to tissue dysfunction upon severe cold stress are largely unknown. In this study, we characterized the cold-resistance ability of juvenile Nile tilapia and classified the individuals as cold-tolerant and cold-sensitive based on their capability to withstand lethal cold exposure. Histological analyses were performed for multiple tissues from the individuals with different cold resilience. We found that mitochondrial swelling was the most prominent subcellular change caused by exposure to lethal cold stress. Subsequent experiments proved that hardiness of mitochondrial function, expression of apoptosis and ER stress-associated genes are intimately related to cold resilience of Nile tilapia. Our data suggest that mitochondria dysfunction and mitochondria-mediated cell apoptosis limit resistance of Nile tilapia to lethal cold stress.
2. Materials and Methods
2.1. Fish Maintenance
Larvae of Nile tilapia were obtained from the Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences (Wuxi, China). The fish were raised in aquariums supplied with recycling water, as previously described [
20]. Water temperature was maintained at 28 ± 0.5 °C and the fish room was illuminated with 12 h/12 h light cycles (from 8 AM to 8 PM). Water quality parameters including pH, dissolved oxygen and ammonia were measured as 7.0–7.5, >5 mg/L and <0.01 mg/L, respectively. The fish at larval stage were fed with nauplii of brine shrimp and the fingerlings were fed with a commercial floating fish feed (crude protein ≥ 36%, crude fats ≥ 5.0%, crude fiber ≤ 4.0% and ash ≤ 14%). The fish were fed twice daily to satiation. Three- to six-month-old fish were used for the experiments.
2.2. Cold Treatment of Nile Tilapia
The facilities used for cold exposure are shown in
Figure 1A, consisting of a PC200 A40 ARCTIC Refrigerated Circulator from Thermo Scientific (Waltham, MA, USA) and a customized plastic box. Two mini pumps were used for water circulation between chambers of the refrigerated circulator and the plastic box. A siphon pipe was used for water volume balance. The water was aerated continuously using an air stone connected to an air pump. The experimental fish were held in the plastic box (filled with 100 L water).
The fish were fasted for 24 h before being transferred into the exposure box. They were acclimated to the experimental condition at 28 °C for 24 h. After that, the temperature was decreased from 28 °C to 20 °C (2 °C/h) and maintained for 20 h. The temperature was further decreased to 12 °C (2 °C/h) and then maintained for the remaining part of the experiment (
Figure 1B). The fish were not fed and checked every 12 h during cold exposure. Death was judged by a lack of response to agitation with a thin stick. Death was further ensured by placing the dead fish into water of normal temperature where none of them could revive. The dead fish were removed and subjected to standard length and body weight measurements. Survival time of the fish at 12 °C was used as the index for cold resistance. The experiment was repeated 3 times and 20 fish were included in each treatment.
During the experiments, we found that the cold-injured fish usually lost equilibrium (LE,
Figure 1A, shown in red) at 12 to 24 h before death. At a given time point, the individuals with LE symptoms couldn’t survive when returned to normal temperature (28 °C), while those that swam normally (NO) could revive. This indicated that the LE fish were more susceptible to cold stress and had higher extent of cellular and tissue damages than the NO ones. To explore the cellular and molecular mechanisms underlying cold susceptibility of Nile tilapia, the fish were treated at 12 °C for 72 h and the survivors were classified as NO and LE, respectively. Samples collected from the untreated (Ctrl), NO and LE fish were subjected to subsequent histological, biochemical and gene expression analyses.
2.3. Histological Analysis
Histological analysis was conducted to investigate the effects of cold exposure on tissue structure. The fish were exposed to cold stress as described above. After cold exposure, the fish were euthanized by immersion into ice-slurry for 3 min as previously described [
21]. The fish were dissected and tissues including brain (cerebellum), gill, heart, kidney, liver, muscle and spleen were collected. The samples were fixed in 4% PFA (Beyotime Biotechnology, Shanghai, China) at 4 °C overnight. After fixation, the samples were dehydrated with alcohol gradients, embedded with paraffin and sectioned into 4 μm slices. The slices were stuck to glass slides, soaked in xylene for dewaxing and rehydrated in ethanol gradients. Finally, the slides were subjected to H&E staining. Photographs of the tissue sections were taken using an Aperio VERSA Brightfield, Fluorescence & FISH Digital Pathology Scanner from Leica (Wetzlar, Germany). Subjective analyses were performed for the photographs to identify changes of tissue structure among the samples from different experimental groups. The photographs were examined independently by different investigators to avoid personal preconceptions when making their judgments.
2.4. Transmission Electron Microscopy
Transmission electron microscopy was performed as previously reported [
22] to investigate effects of cold exposure on the subcellular structure of the brain (cerebellum) and liver. After ultrathin sectioning and staining, photographs of the sections were taken using a HT7700 transmission electron microscope (Hitachi High-Tech, Tokyo, Japan). The photographs were analyzed using ImageJ2 [
23] to measure mitochondria size (the largest distance between the ends of mitochondria).
2.5. Mitochondrial Membrane Potential Measurement
Brain and liver were collected as described above. To measure mitochondrial membrane potential (MMP), mitochondria were isolated from the samples using the Tissue Mitochondria Isolation Kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions. The isolated mitochondria were suspended with 180 μL PBS (pH 7.4) in 1.5 mL centrifuge tubes. After that, 20 μL JC-1 working solution (obtained from Beyotime, Shanghai, China) was added into the mitochondria suspension. The samples were mixed thoroughly by gently flicking the tubes. Flow cytometry was performed using a CytoFLEX S Flow Cytometry (Beckman Coulter, Brea, CA, USA).
2.6. ATP Concentration Measurement
ATP content of the brain and liver tissues was analyzed using the Enhanced ATP Assay Kit (Beyotime, Shanghai, China) following the manufacturer’s instructions. Briefly, the tissue homogenate was centrifuged at 4 °C, 12,000 rpm for 5 min. After centrifugation, the supernatant was collected and mixed with the ATP detection reagent. Fluorescence was measured using a GloMax® 20/20 Luminometer (Promega Biotech, San Luis Obispo, CA, USA). A standard curve was generated using the ATP solution included in the kit. Protein concentration of the samples was measured using the Enhanced BCA Protein Assay Kit (Beyotime, Shanghai, China). Six biological replicates for each group were included in the assay. ATP concentration was normalized to the amount of total protein (nM/mg protein).
2.7. Total RNA Extraction and Quantitative Real-Time PCR
Quantitative real-time PCR (qPCR) was performed as previously reported to analyze gene expression [
22]. Brain and liver were collected and subjected to total RNA extraction using TRIzol Reagent (Invitrogen, Waltham, MA, USA). The samples were homogenized in TRIzol using a LyserPro Procedural Cryogenic Tissue Grinder from Monad (Shanghai, China). Concentration of total RNA was measured using a Q5000 UV–Vis Spectrophotometer (Quawell, Fremont, CA, USA) and quality of RNA was examined by agarose gel electrophoresis. One microgram of total RNA was used for synthesis of the first-strand cDNA using the TransScript® All-in-One All-in-one First Strand cDNA synthesis SuperMix from TransGen (Beijing, China). The primers used for qPCR assays were designed using the online software Primer3Plus (
www.primer3plus.com, assessed on 12 September 2022). The sequence, amplicon size and efficiency of the qPCR primers are listed in
Table 1. A CFX Duet Real-Time PCR System (Bio-Rad, Hercules, CA, USA) was used for qPCR assays. The reagents, program and method of data analysis were the same as the previous study [
22]. Expression of two commonly used internal references,
ef1α and
gapdh, were analyzed and the geometric average of their relative expression was used to normalize expression of the target genes.
2.8. TUNEL Assay
TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labeling) assay was conducted for brain and liver to assess the effects of cold exposure on cell apoptosis. The samples were collected, fixed, embedded and sectioned using the same protocol as histological analysis. The sections were deparaffinized in xylene and rehydrated in gradient ethanol solutions. After rehydration, the sections were treated with a proteinase K solution (obtained from Servicebio Technology Co., Ltd., Wuhan, China) for 25 min at 37 °C. Then, the slides were thoroughly washed using PBS (pH 7.4) and the reaction solution contained in the One Step TUNEL Apoptosis Assay Kit (Beyotime, Shanghai, China) was added onto the sections. The sections were placed into a flat wet box and incubated at 37 °C for 2 h. After being washed 3 times with PBS (pH 7.4), the sections were stained using DAPI solution at room temperature for 10 min. Subsequently, the slides were mounted and observed using a Leica SP8 confocal microscope (Wetzlar, Germany). Photos for the sections were taken and analyzed with ImageJ2 [
23] for quantification of TUNEL signal (fluorescence intensity).
2.9. Statistical Analysis
Statistical analysis was performed using IBM SPSS statistics version 25 (Armonk, NY, USA). The correlation between survival time and variables including standard length, body weight and conditional factor was analyzed by bivariate Pearson correlation. Median survival time (LT50) of Nile tilapia upon 12 °C cold stress was estimated by analyzing the data of survival time and average death rate through Probit regression. One-way analysis of variance (ANOVA) followed by multiple comparisons was performed to test significance of difference among the experimental groups. Significant difference (p < 0.05) between means of the treatment groups was marked using different letters.
4. Discussion
Cold resistance is an important economic trait of farmed fish. A large number of investigations have been conducted to explore the mechanisms underlying cold susceptibility of tilapia; however, the cellular basis and molecular pathways leading to tissue damage upon cold exposure remain largely unknown. Here, we report the characterization of cold resistance of Nile tilapia, expressed as the survival time of individuals under lethal low-temperature stress (12 °C). Although the roles of genetic factors in determining cold resistance of tilapia have been clearly established [
10,
15], the effects of body size are inconsistent. No correlation between cold tolerance and fish size (within the range of 23–105 mm standard length) was identified for
Oreochromis mossambicus,
O. aureus and their F1 and F2 hybrids [
15]. In another study, body size (136 vs. 220 mm) significantly affected mortality of Nile tilapia under cold stress; the smaller fish were less tolerant to low temperature than the larger ones [
26]. In our study, no significant correlation was identified between survival time and factors including standard length, body weight and conditional factor. The discrepancy between these results may be ascribed to the distinct physiological status of the experimental fish used for different studies.
During characterization of cold resistance, we found that loss of equilibrium (LE) was the most prominent symptom for the fish injured by cold exposure. Upon suffering the same degree of cold stress (exposure time), the individuals with LE symptom could not revive upon returning to normal temperature, while those that swam normally (NO) could readily resume. These observations indicate that the NO and LE individuals had differential ability to withstand lethal cold stress. Furthermore, this difference cannot be attributed to physiological factors including body size and conditional factor. The fish were exposed to 12 °C low temperature for 72 h and the survivors were classified into the NO and LE groups. The individuals with distinct cold resilience were subjected to histological, biochemical and gene expression assays to explore the cellular and molecular changes underlying cold endurance. The results of histological analysis indicate that exposure to lethal cold stress caused extensive damage to the tissues of Nile tilapia. Cold exposure caused the generation of vacuoles in the brain and liver, atrophy of cardiac and skeletal muscle fibers, collapse and disintegration of gill lamella, shrinkage of renal tubule epithelial cells and reduction of red blood cell numbers in the spleen. These observations shed new light on the cellular changes underlying cold-induced impairments to body functions including motility, metabolism, respiration, osmotic regulation and hematopoiesis.
As the brain is the organ controlling body equilibrium and the liver was found to be more susceptible to cold stress than other organs, they were chosen for the subsequent analyses. Mitochondrial swelling was the most marked subcellular change upon cold exposure for both the brain and liver cells, followed by ER structural disturbance. Measurements of mitochondria size indicate that the LE samples had more severe mitochondria structure disruption than the NO fish. Mitochondrial swelling can be induced by toxicants, calcium overloading and oxidative stress [
27,
28,
29], which reflects the opening of the mitochondrial permeability transition pore and mitochondrial dysfunction [
27]. Mitochondrial swelling is reversible and the extent of mitochondrial swelling determines whether the cell can recover from an adverse event [
30]. Inability of the LE fish to revive under normal temperature may be ascribed to severe mitochondrial damage that exceeded the reversible point.
Consistently, results of MMP and tissue ATP concentration assays demonstrate that cold exposure significantly decreased cellular MMP and tissue ATP concentration. The LE fish demonstrated significantly lower tissue ATP concentration than the NO ones. These results indicate that exposure to acute cold stress impairs the structure and function of mitochondria and that hardiness of mitochondria is intimately related to an individuals’ cold resistance. Cold-induced damage to mitochondria was also reported for Japanese eel
Anguilla japonica, where electron microscopy revealed mitochondrial degeneration in hepatocytes [
31]. Mitochondrial dysfunction is a major inducer of mitochondria-mediated apoptosis and necrosis, which releases proapoptotic factors such as cytochrome c [
32]. Expressions of several genes involved in mitochondria-dependent apoptosis were analyzed by qPCR to connect cold-induced mitochondria dysfunction with cell apoptosis. The results demonstrate that genes such as
apaf1,
bax and
tp53 were significantly upregulated in both the brain and liver of the LE fish, while the NO samples demonstrated less upregulated apoptosis-related genes than the LE fish. Our study links mitochondrial dysfunction with the ability of fish to resist cold stress.
ER and mitochondria are physically connected cellular organelles, and induction of ER stress can affect both the morphology and function of mitochondria [
25]. A previous study indicated that exposure to cold stress elicited ER stress and activated the intrinsic apoptotic pathway (mitochondria-mediated apoptotic pathway) in zebrafish through disrupting calcium homeostasis [
33]. Disturbance of ER structure was observed for liver samples of both the LE and NO fish, suggesting that ER stress may be elicited in Nile tilapia tissues upon cold stress. Upregulation of the ER stress-activated genes such as
atf6,
bip and
chop in the tissues of cold-treated fish confirmed this postulation. Interestingly, upregulation of different ER stress-related genes was found for the brain and liver of the cold-injured Nile tilapia, suggesting that a different branch of ER stress response was activated by cold stress in these two tissues. The molecular mechanisms underlying this phenomenon remain to be investigated.
Finally, as expected from the results of histological, biochemical and gene expression analyses, severe cell apoptosis was identified in tissues of the cold-treated Nile tilapia. Furthermore, cell apoptosis indices for both the brain and liver of the LE fish were significantly higher than those of the NO samples. The degree of cell apoptosis reflects the extent of mitochondria dysfunction and determines whether the fish can survive the cold stress. Taken together, our data indicate that exposure of Nile tilapia to acute cold stress causes ER stress, mitochondria dysfunction, cell apoptosis and systemic tissue damage; the overall adverse effects ultimately result in organism death. We propose that mitochondria dysfunction and cell apoptosis limit the resistance of Nile tilapia to lethal cold stress.