Aquaponics as a Promising Strategy to Mitigate Impacts of Climate Change on Rainbow Trout Culture
Abstract
:Simple Summary
Abstract
1. Introduction
2. Rainbow Trout Farming, Biological Aspects and Effects of Climate Change
2.1. Rainbow Trout Farming Systems and Production Trends
2.2. Biological Aspects of Rainbow Trout
2.3. Climate Change as a Huge Challenge for Rainbow Trout Aquaculture
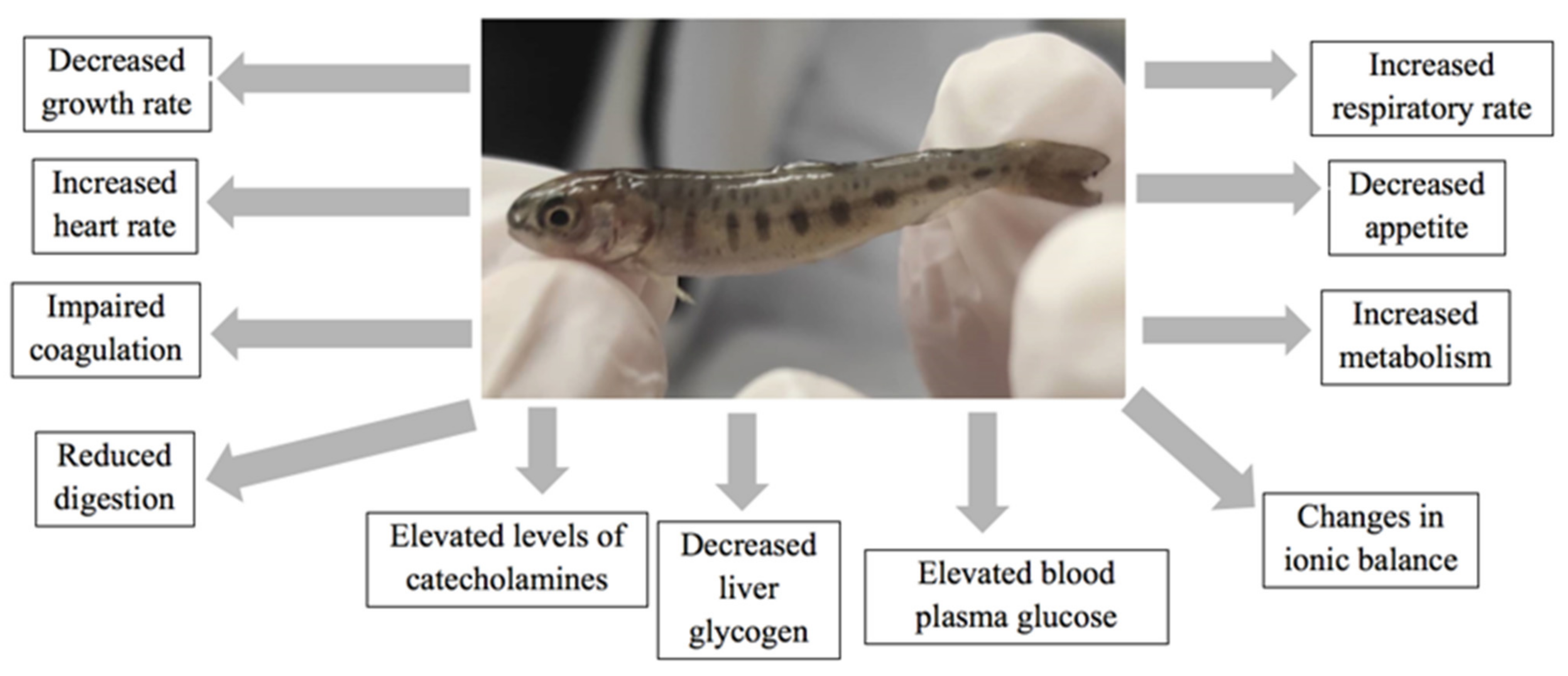
Climate Change and Physiological Homeostasis
3. The Potential of Rainbow Trout Farming in Aquaponics
3.1. Aquaponics as a Novel Technology
3.2. Description and Operation of an Aquaponic System
3.3. Perspectives and Requirements of Rainbow Trout Farming in an Aquaponic System
| Requirements | Values |
|---|---|
| Dissolved oxygen (DO) | >5.0–5.5 ppm |
| Water quality | Good flow, clean freshwater |
| pH | 6.7–8.2 |
| Growth rate | Rapid |
| Marketable size | 350 gr up to one kilo in less than a year |
| Protein content | High (50%) |
| Circular tank containing | 300 gallons of water |
| Density | 7.26–20 kg/m3 |
| Temperature | 9–21 °C |
| Nitrates | <75 mg/L |
3.4. Comparative Yields of Rainbow Trout with Other Fish Reared in Aquaponics Systems
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Georgoulis, I.; Feidantsis, K.; Kouvas, D.; Lattos, A.; Delis, G.A.; Theodoridis, A.; Michaelidis, B.; Giantsis, I.A. The effect of seawater physical parameters in bivalve farming: Could systematic monitoring and early warning prevent negative impacts: A review focused on Vistonikos Gulf, North Aegean Sea. Int. J. Agric. Resour. Gov. Ecol. 2022, 18, 22–37. [Google Scholar] [CrossRef]
- Kalaitzidou, M.P.; Alvanou, M.V.; Papageorgiou, K.V.; Lattos, A.; Sofia, M.; Kritas, S.K.; Petridou, E.; Giantsis, I.A. Pollution Indicators and HAB-Associated Halophilic Bacteria Alongside Harmful Cyanobacteria in the Largest Mussel Cultivation Area in Greece. Int. J. Environ. Res. Public Health 2022, 19, 5285. [Google Scholar] [CrossRef] [PubMed]
- Feidantsis, K.; Giantsis, I.A.; Vratsistas, A.; Makri, S.; Pappa, A.Z.; Drosopoulou, E.; Anestis, A.; Mavridou, E.; Exadactylos, A.; Vafidis, D.; et al. Correlation between intermediary metabolism, Hsp gene expression, and oxidative stress-related proteins in long-term thermal-stressed Mytilus galloprovincialis. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2020, 319, 264–281. [Google Scholar] [CrossRef]
- Georgoulis, I.; Feidantsis, K.; Giantsis, I.A.; Kakale, A.; Bock, C.; Pörtner, H.O.; Sokolova, I.M.; Michaelidis, B. Heat hardening enhances mitochondrial potential for respiration and oxidative defence capacity in the mantle of thermally stressed Mytilus galloprovincialis. Sci. Rep. 2021, 11, 17098. [Google Scholar] [CrossRef]
- Cochrane, K.; De Young, C.; Soto, D.; Bahri, T. (Eds.) Climate Change Implications for Fisheries and Aquaculture: Overview of Current Scientific Knowledge; FAO Fisheries and Aquaculture Technical Paper. No. 530; FAO: Rome, Italy, 2009; 212p. [Google Scholar]
- Gitz, V.; Meybeck, A.; Lipper, L.; Young, C.D.; Braatz, S. Climate Change and Food Security: Risks and Responses; Food and Agriculture Organization of the United Nations (FAO) Report; FAO: Rome, Italy, 2016; Volume 110, pp. 2–4. [Google Scholar]
- Pirozzi, I.; Southgate, P.C.; Lucas, J.S. Aquaculture Systems Design. In Aquaculture: Farming Aquatic Animals and Plants, 3rd ed.; Lucas, J.S., Southgate, P.C., Pirozzi, I., Tucker, S.C., Eds.; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2019; pp. 41–62. [Google Scholar]
- Abdulhai, H.; Ghomi, M.K.S. Rainbow trout culture in Iran: Development and concerns. Aquac Asia 2005, 10, 34–38. [Google Scholar]
- Climate change and European aquatic resources (CERES). Case study: Rainbow trout in north-west Europe. In European Union’s Horizon 2020 Research and Innovation Program; No 678193; CERES: Hamburg, Germany, 2019. [Google Scholar]
- Wenger, S.J.; Isaak, D.J.; Luce, C.H.; Neville, H.M.; Fausch, K.D.; Dunham, J.B.; Dauwalter, D.C.; Young, M.K.; Elsner, M.M.; Rieman, B.E.; et al. Flow regime, temperature, and biotic interactions drive differential declines of trout species under climate change. Proc. Natl. Acad. Sci. USA 2011, 108, 14175–14180. [Google Scholar] [CrossRef]
- David, L.H.; Pinho, S.M.; Agostinho, F.; Costa, J.I.; Portella, M.C.; Keesman, K.J.; Garcia, F. Sustainability of urban aquaponics farms: An emergy point of view. J. Clean. Prod. 2022, 333, 129896. [Google Scholar] [CrossRef]
- Farrant, D.N.; Frank, K.L.; Larsen, A.E. Reuse and recycle: Integrating aquaculture and agricultural systems to increase production and reduce nutrient pollution. Sci. Total Environ. 2021, 785, 146859. [Google Scholar] [CrossRef]
- Martsikalis, P.; Gkafas, G.A.; Apostolidis, A.P.; Exadactylos, A. Genetic Structure Profile of Rainbow Trout (Oncorhynchus mykiss) Farmed Strains in Greece. Turk. J. Fish. Aquat. Sci. 2014, 14, 749–757. [Google Scholar] [CrossRef]
- European Market Observatory for Fisheries and Aquaculture Products (EUMOFA). Freshwater Aquaculture in Europe; Publications Office of the European Union: Luxembourg, 2021; pp. 1–83. [Google Scholar]
- European Market Observatory for Fisheries and Aquaculture Products (EUMOFA). Portion Trout in the Europ; Publications Office of the European Union: Luxembourg, 2021; pp. 1–62. [Google Scholar]
- Bozoglu, M.; Ceyhan, V.; Cinemre, A.H.; Demiryürek, K.; Kilic, O. Evaluation of different trout farming systems and some policy issues in the Black Sea region, Turkey. J. Appl. Sci. 2006, 6, 2882–2888. [Google Scholar] [CrossRef]
- D’Orbcastel, E.R.; Blancheton, J.P.; Boujard, T.; Aubin, J.; Moutounet, Y.; Przybyla, C.; Belaud, A. Comparison of two methods for evaluating waste of a flow through trout farm. J. Aquac. 2008, 274, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Cowx, I.G.; Nunn, A.D.; Harvey, J.P. Quantitative sampling of 0-group fish populations in large lowland rivers: Point abundance sampling by electric fishing versus micromesh seine netting. Arch. Für Hydrobiol. 2001, 151, 369–382. [Google Scholar] [CrossRef]
- FAO. Oncorhynchus Mykiss: Cultured Aquatic Species Information Programme; Cowx, I.G., Ed.; Fisheries and Aquaculture Division: Rome, Italy, 2022. [Google Scholar]
- European Market Observatory for Fisheries and Aquaculture Products (EUMOFA). The European Fish Market; Publications Office of the European Union: Luxembourg, 2021; pp. 1–111. [Google Scholar]
- European Aquaculture Production Report (FEAP). Fish Farming Production in Europe, 2014–2019; FEAP: Brussels, Belgium, 2020; pp. 2–48. [Google Scholar]
- Eurostat Database. Available online: https://ec.europa.eu/eurostat/data/database (accessed on 13 August 2022).
- Cai, A.; Yan, J.N.X.; Leung, P.S. Benchmarking Species Diversification in Global Aquaculture; Food & Agriculture Organization: Rome, Italy, 2022; Volume 605. [Google Scholar]
- Apc advanced planning—Consulting. Report on the Marketing of Aquaculture Species Produced in GREECE, Part A; European Union: Athens, Greece, 2016; pp. 1–91. [Google Scholar]
- Crawford, S.S.; Muir, A.M. Global introductions of salmon and trout in the genus Oncorhynchus: 1870–2007. Fish Biol. Fish. 2008, 18, 313–344. [Google Scholar] [CrossRef]
- Stanković, D.; Crivelli, A.J.; Snoj, A. Rainbow Trout in Europe: Introduction, Naturalization, and Impacts. Rev. Fish. Sci. Aquac. 2015, 23, 39–71. [Google Scholar] [CrossRef]
- Framian, B.V. Review of the EU aquaculture sector and results of costs and earnings survey. Final Rep. 2009, 6, 69. [Google Scholar]
- Economidis, P.S.; Dimitriou, E.; Pagoni, R.; Michaloudi, E.; Natsis, L. Introduced and translocated fish species in the inland waters of Greece. Fish. Manag. Ecol. 2000, 7, 239–250. [Google Scholar] [CrossRef]
- Copp, G.H.; Garthwaite, R.; Gozlan, R.E. Risk identification and assessment of non-native freshwater fishes: Concepts and perspectives on protocols for the UK. J. Appl. Ichthyol. 2005, 21, 371–373. [Google Scholar] [CrossRef]
- Welcomme, R.L.; Bartley, D.M. Current approaches to the enhancement of fisheries. Fish. Manag. Ecol. 1998, 5, 351–382. [Google Scholar] [CrossRef]
- Behnke, R.J. Comment: First Documented Case of Anadromy in a Population of Introduced Rainbow Trout in Patagonia, Argentina. Trans. Am. Fish. Soc. 2002, 131, 582–585. [Google Scholar] [CrossRef]
- FAO. Management of the Aquaponic Systems; FAO: Rome, Italy, 2015. [Google Scholar]
- Gall, G.A.E.; Crandell, P.A. The Rainbow trout. Aquaculture 1992, 100, 1–10. [Google Scholar] [CrossRef]
- Stout, M. Aquaponic Gardening: Discover the Dual Benefits of Raising Fish and Plants Together (Idiot’s Guides), Illustrated ed.; Alpha Publishing: New York, NY, USA, 2013; pp. 1–352. [Google Scholar]
- Appleford, P.; Lucas, S.J.; Southgate, C.P. Aquaculture: Farming Aquatic Animals and Plants; Second Condition; Lucas, S.J., Southgate, C.P., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2012; pp. 18–50. [Google Scholar]
- Birolo, M.; Bordignon, F.; Trocino, A.; Fasolato, L.; Pascual, A.; Godoy, S.; Nicoletto, C.; Maucieri, C.; Xiccato, G. Effects of stocking density on the growth and flesh quality of rainbow trout (Oncorhynchus mykiss) reared in a low-tech aquaponic system. Aquaculture 2020, 529, 735653. [Google Scholar] [CrossRef]
- Naumowicz, K.; Pajdak, J.; Terech-Majewska, E.; Szarek, J. Intracohort cannibalism and methods for its mitigation in cultured freshwater fish. Rev Fish Biol Fish. 2017, 27, 193–208. [Google Scholar]
- Molony, B. Environmental Requirements and Tolerances of Rainbow Trout (Oncorhynchus mykiss) and Brown Trout (Salmo trutta) with Special Reference to Western Australia: A Review; Department of Fisheries Perth, Department of Fisheries, Government of Western Australia: Perth, Australia, 2001; Volume 130, pp. 1–28.
- Avkhimovich, D. Effect of Water Quality on Rainbow Trout Performance Water Oxygen Level in Commercial Trout Farm. Bachelor’s Thesis, Mikkely University of Applied Sciences, Mikkely, Finland, 2013. [Google Scholar]
- Möck, A.; Peters, G. Lysozyme activity in rainbow trout, Oncorhynchus mykiss (Walbaum), stressed by handling, transport and water pollution. J. Fish Biol. 1990, 37, 873–885. [Google Scholar] [CrossRef]
- Islam, J.M.; Kunzmann, A.; Slater, J.M. Responses of aquaculture fish to climate change induced extreme temperatures: A review. J. World Aquac. Soc. 2021, 53, 314–366. [Google Scholar] [CrossRef]
- Leberg, P.L. Influence of genetic variability on population growth: Implications for conservation. J. Fish Biol. 1990, 37, 193–195. [Google Scholar] [CrossRef]
- Gjerde, B.; Gunnes, K.; Gjerdem, T. Effect of inbreeding on survival and growth in rainbow trout. Aquaculture 1983, 34, 327–332. [Google Scholar] [CrossRef]
- Su, G.S.; Liljedahl, L.E.; Gall, G.A.E. Effects of inbreeding on growth and reproductive traits in rainbow trout (Oncorhynchus mykiss). Aquaculture 1996, 142, 139–148. [Google Scholar] [CrossRef]
- Wang, S.; Hard, J.J.; Utter, F. Salmonid inbreeding: A review. Rev. Fish Biol. Fish. 2002, 11, 301–319. [Google Scholar] [CrossRef]
- Frankham, R. Genetics and extinction. Biol. Conserv. 2005, 126, 131–140. [Google Scholar] [CrossRef]
- Paul, K.; D’ambrosio, J.; Phocas, F. Temporal and region-specific variations in genome-wide inbreeding effects on female size and reproduction traits of rainbow trout. Evol. Appl. 2022, 15, 645–662. [Google Scholar] [CrossRef]
- Kause, A.; Ritola, O.; Paananen, T.; Wahlroos, H.; Mäntysaari, E.A. Genetic trends in growth, sexual maturity and skeletal deformations, and rate of inbreeding in a breeding programme for rainbow trout (Oncorhynchus mykiss). Aquaculture 2005, 247, 177–187. [Google Scholar] [CrossRef]
- Ferguson, M.M.; Drahushchak, L.R. Disease resistance and enzyme heterozygosity in rainbow trout. Heredity 1990, 64, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R. Stress and adaptation in conservation genetics. J. Evol. Biol. 2005, 18, 750–755. [Google Scholar] [CrossRef]
- Papageorgiou, N. Trout and Its Rearing; University Studios Press: Thessaloniki, Greece, 1985; pp. 10–50. (in Greek) [Google Scholar]
- FAO. Small-Scale Aquaponic Food Production—Integrated Fish and Plant Farming; FAO: Rome, Italy, 2014. [Google Scholar]
- Ultsch, G.R.; Ott, M.E.; Heisler, N. Standard metabolic rate, critical oxygen tension, and aerobic scope for spontaneous activity of trout (Salmo gairdneri) and carp (Cyprinus carpio) in acidified water. Comp. Biochem. Physiol. 1980, 67, 329–335. [Google Scholar] [CrossRef]
- Sae-Lim, P.; Kause, A.; Mulder, H.A.; Olesen, I. Breeding and genetics symposium: Climate change and selective breeding in aquaculture. J. Anim. Sci. 2017, 95, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, Y.H.; Robaina, L.; Pirhonen, J.; Mente, E.; Domínguez, D.; Parisi, G. Fish Welfare in Aquaponic Systems: Its Relation to Water Quality with an Emphasis on Feed and Faeces—A Review. Water 2017, 9, 13. [Google Scholar] [CrossRef]
- Davidson, J.; Good, C.; Barrows, F.T.; Welsh, C.; Kenney, P.B.; Summerfelt, S.T. Comparing the effects of feeding a grain- or a fish meal-based diet on water quality, waste production, and rainbow trout Oncorhynchus mykiss performance within low exchange water recirculating aquaculture systems. Aquac. Eng. 2014, 52, 45–57. [Google Scholar] [CrossRef]
- Gjedrem, T.; Robinson, N.; Rye, M. The importance of selective breeding in aquaculture to meet future demands for animal protein: A review. Aquaculture 2012, 350–353, 117–121. [Google Scholar] [CrossRef]
- United Nations. The World Population Prospects: 2015 Revision; 2015. Available online: http://www.un.org/en/development/desa/publications/world-population-prospects-2015-revision.html (accessed on 16 November 2016).
- De Silva, S.S.; Soto, D. Climate change and aquaculture: Potential impacts, adaptation and mitigation. In Climate Change Implications for Fisheries and Aquaculture: Overview of Current Scientific Knowledge; FAO Fisheries and Aquaculture Technical paper 530; FAO: Rome, Italy, 2009; pp. 151–212. [Google Scholar]
- Kutty, M.N. Would Food Crisis, FAO Alert and India. World Aquac. 2010, 41, 6–7. [Google Scholar]
- FAO. FAO Yearbook 2014: Fishery and Aquaculture Statistics; FAO: Rome, Italy, 2016; pp. 1–105. [Google Scholar]
- Dülger, N.; Kumlu, M.; Türkmen, S.; Ölçülü, A.; Tufan Eroldoĝan, O.; Asuman Yilmaz, H.; Öçal, N. Thermal tolerance of European Sea Bass (Dicentrarchus labrax) juveniles acclimated to three temperature levels. J. Therm. Biol. 2012, 37, 79–82. [Google Scholar] [CrossRef]
- Reid, G.K.; Gurney-Smith, H.J.; Marcogliese, D.J.; Knowler, D.; Benfey, T.; Garber, A.F.; Forster, I.; Chopin, T.; Brewer-Dalton, K.; Moccia, R.D.; et al. Climate change and aquaculture: Considering biological response and resources. Aquac. Environ. Interact. 2019, 11, 569–602. [Google Scholar] [CrossRef]
- Aranda, I.; Castro, L.; Alía, R.; Pardos, J.A.; Gil, L. Low temperature during winter elicits differential responses among populations of the Mediterranean evergreen cork oak (Quercus suber). Tree Physiol. 2005, 25, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Besson, M.; Vandeputte, M.; van Arendon, J.A.M.A.M.; Aubin, J.; de Boer, I.J.M.J.M.; Quillet, E.; Komen, H. Influence of water temperature on the economic value of growth rate in fish farming: The case of sea bass (Dicentrarchus labrax) cage farming in the Mediterranean. J. Aquac. 2016, 462, 47–55. [Google Scholar] [CrossRef]
- Llorente, I.; Luna, L. The competitive advantages arising from different environmental conditions in seabream, Sparus aurata, production in the Mediterranean Sea. J. World Aquac. Soc. 2013, 44, 611–627. [Google Scholar] [CrossRef]
- Choi, Y.W.; Campbell, D.J.; Aldridge, J.C.; Eltahir, E.A.B. Near-term regional climate change over Bangladesh. Clim. Dyn. 2021, 57, 3055–3073. [Google Scholar] [CrossRef]
- Dastagir, M.R. Modeling recent climate change induced extreme events in Bangladesh: A review. Weather Clim. Extrem. 2015, 7, 49–60. [Google Scholar] [CrossRef]
- Thuy, N.T.T.; Giang, N.T.H.; Hoai, H.T.T.; Van Dan, T. Impact of climate change on aquaculture in Phu Vang district, Thua Thien Hue province, Vietnam; Agriculture and Development Discussion Paper Series 2017-3; Southeast Asian Regional Center for Graduate Study and Research in Agriculture (SEARCA): Los Baños, Philippines, 2017; pp. 1–51. [Google Scholar]
- Hobday, A.J.; Spillman, C.M.; Eveson, J.P.; Hartog, J.R.; Zhang, M.X.; Brodie, S. A framework for combining seasonal forecasts and climate projections to aid risk management for fisheries and aquaculture. Front. Mar. Sci. 2018, 5, 137. [Google Scholar] [CrossRef]
- McCoy, D.; McManus, M.A.; Kotubetey, K.; Kawelo, A.H.; Young, C.; D’Andrea, B.; Ruttenberg, K.C.; Alegado, R.A. Large-scale climatic effects on traditional Hawaiian fishpond aquaculture. PLoS ONE 2017, 12, e0187951. [Google Scholar]
- Hamon, K.G. Report on Minimising Economic Losses, Opportunities and Challenges for Aquaculture in EUROPE; CERES Deliverable 4.2. 2019, pp. 1–151. Available online: https://ec.europa.eu/research/participants/documents/downloadPublic?documentIds=080166e5c7cfc5cc&appId=PPGMS (accessed on 29 August 2022).
- Roychowdhury, P.; Aftabuddin, M.; Pati, M.K. Thermal stress altered growth performance and metabolism and induced anaemia and liver disorder in Labeo rohita. Aquac. Res. 2020, 51, 1406–1414. [Google Scholar] [CrossRef]
- Rosa, R.; Marques, A.; Nunes, M.L. Impact of climate change in Mediterranean aquaculture. Rev. Aquac. 2012, 4, 163–177. [Google Scholar] [CrossRef]
- Rosa, R.; Marques, A.; Nunes, M.L. Mediterranean aquaculture in a changing climate. In The Mediterranean Sea: Its history and Present Challenges; Springer: Dodrecht, The Netherlands, 2014; Volume 9789400767, pp. 605–616. [Google Scholar]
- Doubleday, Z.A.; Clarke, S.M.; Li, X.; Pecl, G.T.; Ward, T.M.; Battaglene, S.; Frusher, S.; Gibbs, P.J.; Hobday, A.J.; Hutchinson, N.; et al. Assessing the risk of climate change to aquaculture: A case study from south-east Australia. Aquac. Environ. Interact. 2013, 3, 163–175. [Google Scholar] [CrossRef]
- Roberts, S.D.; Van Ruth, P.D.; Wilkinson, C.; Bastianello, S.S.; Bansemer, M.S. Marine heatwave, harmful algae blooms and an extensive fish kill event during 2013 in South Australia. Front. Mar. Sci. 2019, 6, 610. [Google Scholar] [CrossRef]
- Rountrey, A.N.; Coulson, P.G.; Meeuwig, J.J.; Meekan, M. Water temperature and fish growth: Otoliths predict growth patterns of a marine fish in a changing climate. Glob. Change Biol. 2014, 20, 2450–2458. [Google Scholar] [CrossRef] [PubMed]
- Vornanen, M. The temperature dependence of electrical excitability in fish hearts. J. Exp. Biol. 2016, 219, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.A.; Schreck, C.B.; Ewing, R.D.; Hemmingsen, A.R.; Patiño, R. Changes in plasma cortisol during stress and smoltification in Coho Salmon, Oncorhynchus kisutch. Gen. Comp. Endocrinol. 1985, 59, 468–471. [Google Scholar] [CrossRef]
- Heath, A.G.; Iwama, G.K.; Pickering, A.D.; Sumpter, J.P.; Schreck, C.B. Fish stress and health in aquaculture. Estuaries 1998, 21, 501. [Google Scholar] [CrossRef]
- Schreck, C.B.; Tort, L. The concept of stress in fish. In Fish Physiology; Academic Press: Cambridge, MA, USA, 2016; Volume 35, pp. 1–34. [Google Scholar]
- Crawshaw, L.I. Physiological and behavioral reactions of fishes to temperature change. J. Fish. Res. Board Can. 1977, 34, 730–734. [Google Scholar] [CrossRef]
- Li, A.J.; Leung, P.T.Y.; Bao, V.W.W.; Lui, G.C.S.; Leung, K.M.Y. Temperature-dependent physiological and biochemical responses of the marine medaka Oryzias melastigma with consideration of both low and high thermal extremes. J. Therm. Biol. 2015, 54, 98–105. [Google Scholar] [CrossRef]
- Van den Burg, E.H.; Peeters, R.R.; Verhoye, M.; Meek, J.; Flik, G.; Van der Linden, A. Brain responses to ambient temperature fluctuations in fish: Reduction of blood volume and initiation of a whole-body stress response. J. Neurophysiol. 2005, 93, 2849–2855. [Google Scholar] [CrossRef]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef]
- Faught, E.; Hernandez-Perez, J.; Wilson, J.M.; Vijayan, M.M. Stress in response to environmental changes. In Climate Change and Non-Infectious Fish Disorders; CABI: Wallingford, UK, 2020; pp. 136–162. [Google Scholar]
- Feidantsis, K.; Pörtner, H.O.; Giantsis, I.A.; Michaelidis, B. Advances in understanding the impacts of global warming on marine fishes farmed offshore: Sparus aurata as a case study. J. Fish Biol. 2021, 98, 1509–1523. [Google Scholar] [CrossRef] [PubMed]
- Little, A.G.; Loughland, I.; Seebacher, F. What do warming waters mean for fish physiology and fisheries. J. Fish Biol. 2020, 97, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, M.; Clota, F.; Sadoul, B.; Blanc, M.O.; Blondeau-Bidet, E.; Bégout, M.L.; Cousin, X.; Geffroy, B. Low temperature has opposite effects on sex determination in a marine fish at the larval/postlarval and juvenile stages. Ecol. Evol. 2020, 10, 13825–13835. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.J.; Kunzmann, A.; Slater, M.J. Extreme winter cold-induced osmoregulatory, metabolic, and physiological responses in European seabass (Dicentrarchus labrax) acclimatized at different salinities. Sci. Total Environ. 2021, 771, 145202. [Google Scholar] [CrossRef]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef]
- Dockray, J.J.; Reid, S.D.; Wood, C.M. Effects of elevated summer temperatures and reduced pH on metabolism and growth of juvenile rainbow trout (Oncorhynchus mykiss) on unlimited ration. Can. J. Fish. Aquat. Sci. 1996, 53, 2752–2763. [Google Scholar] [CrossRef]
- Linton, T.K.; Reid, S.D.; Wood, C.M. The metabolic costs and physiological consequences to juvenile rainbow trout of a simulated summer warming scenario in the presence and absence of sublethal ammonia. Trans. Am. Fish. Soc. 1997, 126, 259–272. [Google Scholar] [CrossRef]
- Morgan, I.J.; McDonald, D.G.; Wood, C.M. The cost of living for freshwater fish in a warmer, more polluted world. Glob. Change Biol. 2001, 7, 345–355. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Bonga, W.; Sjoerd, E. The stress response in fish. Physiol. Rev. 1977, 77, 591–625. [Google Scholar] [CrossRef]
- Reid, S.G.; Bernier, N.J.; Perry, S.F. The adrenergic stress response in fish: Control of catecholamine storage and release. Comp. Biochem. Physiol. —C Pharmacol. Toxicol. Endocrinol. 1998, 120, 1–27. [Google Scholar] [CrossRef]
- Weyts, F.A.A.; Cohen, N.; Flik, G.; Verburg-van Kemenade, B.M.L. Interactions between the immune system and the hypothalamo-pituitary-interrenal axis in fish. Fish Shellfish Immunol. 1999, 9, 1–20. [Google Scholar] [CrossRef]
- Chang, C.H.; Huang, J.J.; Yeh, C.Y.; Tang, C.H.; Hwang, L.Y.; Lee, T.H. Salinity effects on strategies of glycogen utilization in livers of euryhaline milkfish (Chanos chanos) under hypothermal stress. Front. Physiol. 2019, 9, 81. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Berdal, B.; Blust, R.; Brix, O.; Colosimo, A.; De Wachter, B.; Giuliani, A.; Johansen, T.; Fischer, T.; Knust, R.; et al. Climate induced temperature effects on growth performance, fecundity and recruitment in marine fish: Developing a hypothesis for cause and effect relationships in Atlantic cod (Gadus morhua) and common eelpout (Zoarces viviparus). Cont. Shelf Res. 2001, 21, 1975–1997. [Google Scholar] [CrossRef]
- Seebacher, F.; Post, E. Climate change impacts on animal migration. Clim. Change Responses 2015, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Servili, A.; Canario, A.V.M.; Mouchel, O.; Muñoz-Cueto, J.A. Climate change impacts on fish reproduction are mediated at multiple levels of the brain-pituitary-gonad axis. Gen. Comp. Endocrinol. 2020, 291, 113439. [Google Scholar] [CrossRef] [PubMed]
- King, H.; Pankhurst, N. Ovarian growth and plasma sex steroid and vitellogenin profiles during vitellogenesis in Tasmanian female Atlantic salmon (Salmo salar). J. Aquac. 2003, 219, 797–813. [Google Scholar] [CrossRef]
- King, H.R.; Pankhurst, N.W. Effect of short-term temperature reduction on ovulation and LHRHa responsiveness in female Atlantic salmon (Salmo salar) maintained at elevated water temperatures. J. Aquac. 2004, 238, 421–436. [Google Scholar] [CrossRef]
- Pankhurst, N.W.; King, H.R. Temperature and salmonid reproduction: Implications for aquaculture. J. Fish Biol. 2010, 76, 69–85. [Google Scholar] [CrossRef]
- Zarski, D.; Horváth, A.; Bernáth, G.; Krejszeff, S.; Radoczi, J.; Palinska-Zarska, K.; Bokor, Z.; Kupren, K.; Urbányi, B. Stimulation of ovulation and spermiation. In Controlled Reproduction of Wild Eurasian Perch; Springer: Cham, Switzerland, 2017; pp. 33–40. [Google Scholar]
- Dadras, H.; Dzyuba, V.; Golpour, A.; Xin, M.; Dzyuba, B. In vitro antioxidant enzyme activity and sperm motility at different temperatures in sterlet Acipenser ruthenus and rainbow trout Oncorhynchus mykiss. Fish Physiol. Biochem. 2019, 45, 1791–1800. [Google Scholar] [CrossRef]
- Amiel, J.J.; Bao, S.; Shine, R. The effects of incubation temperature on the development of the cortical forebrain in a lizard. Anim. Cogn. 2017, 20, 117–125. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, S. The neurobiology of climate change. Sci. Nat. 2018, 105, 11. [Google Scholar] [CrossRef] [PubMed]
- Pallotta, M.M.; Turano, M.; Ronca, R.; Mezzasalma, M.; Petraccioli, A.; Odierna, G.; Capriglione, T. Brain gene expression is influenced by incubation temperature during leopard gecko (Eublepharis macularius) development. J. Exp. Zool. —Part B Mol. Dev. Evol. 2017, 328, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, J.G.; McCormick, S.D. Upper thermal limits of growth in brook trout and their relationship to stress physiology. J. Exp. Biol. 2017, 220, 3976–3987. [Google Scholar] [CrossRef] [PubMed]
- Giffard-Mena, I.; Lorin-Nebel, C.; Charmantier, G.; Castille, R.; Boulo, V. Adaptation of the sea-bass (Dicentrarchus labrax) to fresh water: Role of aquaporins and Na+/K+-ATPases. Comp. Biochem. Physiol. —Part A Mol. Integr. Physiol. 2008, 150, 332–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas-Chacoff, L.; Regish, A.M.; Weinstock, A.; McCormick, S.D. Effects of elevated temperature on osmoregulation and stress responses in Atlantic salmon Salmo salar smolts in fresh water and seawater. J. Fish Biol. 2018, 93, 550–559. [Google Scholar] [CrossRef]
- Smith, S.; Bernatchez, I.; Beheregaray, I.B. RNA-seq analysis reveals extensive transcriptional plasticity to temperature stress in a freshwater fish species. BMC Genom 2013, 14, 375. [Google Scholar] [CrossRef]
- Madeira, D.; Narciso, L.; Cabral, H.N.; Vinagre, C.; Diniz, M.S. HSP70 production patterns in coastal and estuarine organisms facing increasing temperatures. J. Sea Res. 2012, 73, 137–147. [Google Scholar] [CrossRef]
- Maulvault, A.L.; Barbosa, V.; Alves, R.; Custodio, A.; Anacleto, P.; Repolho, T.; Pousão Ferreira, P.; Rosa, R.; Marques, A.; Diniz, M. Ecophysiological responses of juvenile seabass (Dicentrarchus labrax) exposed to increased temperature and dietary methylmercury. Sci. Total Environ. 2017, 586, 551–558. [Google Scholar] [CrossRef]
- Reyes-Lopez, F.E.; Aerts, J.; Vallejos-Vidal, E.; Ampe, B.; Dierckens, K.; Tort, L.; Bossier, P. Modulation of innate immune-related genes and glucocorticoid synthesis in gnotobiotic full-sibling European sea bass (Dicentrarchus labrax) larvae challenged with Vibrio anguillarum. Front. Immunol. 2018, 9, 914. [Google Scholar] [CrossRef]
- Donaldson, M.R.; Cooke, S.J.; Patterson, D.A.; Macdonald, J.S. Cold shock and fish. J. Fish Biol. 2008, 73, 1491–1530. [Google Scholar] [CrossRef]
- Nakano, K.; Iwama, G.K. The 70-kDa heat shock protein response in two intertidal sculpins, Oligocottus maculosus and O. snyderi: Relationship of hsp70 and thermal tolerance. Comp. Biochem. Physiol. —Part A Mol. Integr. Physiol. 2002, 133, 79–94. [Google Scholar] [CrossRef]
- Werner, I.; Viant, M.R.; Rosenblum, E.S.; Gantner, A.S.; Tjeerdema, R.S.; Johnson, M.L. Cellular responses to temperature stress in steelhead trout (Onchorynchus mykiss) parr with different rearing histories. Fish Physiol. Biochem. 2006, 32, 261–273. [Google Scholar] [CrossRef]
- Werner, I.; Smith, T.B.; Feliciano, J.; Johnson, M.L. Heat shock proteins in juvenile steelhead reflect thermal conditions in the Navarro river watershed, California. Trans. Am. Fish. Soc. 2005, 134, 399–410. [Google Scholar] [CrossRef]
- Sopinka, N.M.; Donaldson, M.R.; O’Connor, C.M.; Suski, C.D.; Cooke, S.J. Stress indicators in fish. Fish Physiol. 2016, 35, 405–462. [Google Scholar]
- Yamashita, M.; Yabu, T.; Ojima, N. Stress protein HSP70 in fish. Aqua-BioScience Monogr. 2010, 3, 111–141. [Google Scholar] [CrossRef]
- Eissa, N.; Wang, H.P. Transcriptional stress responses to environmental and husbandry stressors in aquaculture species. Rev. Aquac. 2016, 8, 61–88. [Google Scholar] [CrossRef]
- Almroth, B.C.; Asker, N.; Wassmur, B.; Rosengren, M.; Jutfelt, F.; Gräns, A.; Sundell, K.; Axelsson, M.; Sturve, J.; Sturve, J. Warmer water temperature results in oxidative damage in an Antarctic fish, Bald notothen. J. Exp. Mar. Biol. Ecol. 2015, 468, 130–137. [Google Scholar] [CrossRef]
- Madeira, D.; Vinagre, C.; Diniz, M.S. Are fish in hot water? Effects of warming on oxidative stress metabolism in the commercial species Sparus aurata. Ecol. Indic. 2016, 63, 324–331. [Google Scholar] [CrossRef]
- Benítez-Dorta, V.; Caballero, M.J.; Betancor, M.B.; Manchado, M.; Tort, L.; Torrecillas, S.; Zamorano, M.J.; Izquierdo, M.; Montero, D. Effects of thermal stress on the expression of glucocorticoid receptor complex linked genes in Senegalese sole (Solea senegalensis): Acute and adaptive stress responses. Gen. Comp. Endocrinol. 2017, 252, 173–185. [Google Scholar] [CrossRef]
- Araújo, J.E.; Madeira, D.; Vitorino, R.; Repolho, T.; Rosa, R.; Diniz, M. Negative synergistic impacts of ocean warming and acidification on the survival and proteome of the commercial sea bream, Sparus aurata. J. Sea Res. 2018, 139, 50–61. [Google Scholar] [CrossRef]
- Kyprianou, T.D.; Pörtner, H.O.; Anestis, A.; Kostoglou, B.; Feidantsis, K.; Michaelidis, B. Metabolic and molecular stress responses of gilthead seam bream Sparus aurata during exposure to low ambient temperature: An analysis of mechanisms underlying the winter syndrome. J. Comp. Physiol. B 2010, 180, 1005–1018. [Google Scholar] [CrossRef]
- Kamunde, C.; Sappal, R.; Melegy, T.M. Brown seaweed (AquaArom) supplementation increases food intake and improves growth, antioxidant status and resistance to temperature stress in Atlantic salmon, Salmo salar. PLoS ONE 2019, 14, e0219792. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Ye, S.; Bureau, D.P.; Liu, H.; Yin, J.; Mou, Z.; Lin, H.; Hao, F. Global metabolic responses of the lenok (Brachymystax lenok) to thermal stress. Comparative Biochemistry and Physiology—Part D Genom. Proteom. 2019, 29, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Zhang, F.; Wang, T.; Zheng, X.; Li, Y.; Huang, B.; Zhang, C. Physiological and morphological changes in Turbot (Psetta maxima) gill tissue during waterless storage. J. Aquac. 2019, 508, 30–35. [Google Scholar] [CrossRef]
- Pickering, A.D.D. Growth and stress in fish production. Genet. Aquac. 1993, 111, 51–63. [Google Scholar] [CrossRef]
- Barton, B.A.; Peter, R.E. Plasma cortisol stress response in fingerling rainbow trout, Salmo gairdneri Richardson, to various transport conditions, anaesthesia, and cold shock. J. Fish Biol. 1982, 20, 39–51. [Google Scholar] [CrossRef]
- Sappal, R.; MacDougald, M.; Stevens, D.; Fast, M.D.; Kamunde, C. Copper alters the effect of temperature on mitochondrial bioenergetics in rainbow trout, Oncorhynchus mykiss. Arch. Environ. Contam. Toxicol. 2014, 66, 430–440. [Google Scholar] [CrossRef]
- Portner, H.O. Climate variations and the physiological basis of temperature dependent biogeography: Systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2002, 132, 739–761. [Google Scholar] [CrossRef]
- Portner, H.O.; Peck, M.A. Climate change impacts on fish and fisheries: Towards a cause and effect understanding. J. Fish Biol. 2010, 77, 1745–1779. [Google Scholar] [CrossRef] [PubMed]
- Iftikar, F.I.; Hickey, A.J. Do mitochondria limit hot fish hearts? Understanding the role of mitochondrial function with heat stress in Notolabrus celidotus. PLoS ONE 2013, 8, e64120. [Google Scholar] [CrossRef] [PubMed]
- Blier, P.U.; Lemieux, H.; Pichaud, N. Holding our breath in our modern world: Will mitochondria keep the pace with climate changes. Can. J. Zool. 2014, 92, 591–601. [Google Scholar] [CrossRef]
- Guderley, H. Mitochondria and temperature. In Encyclopedia of Fish Physiology: Energetics, Interactions with the Environment, Lifestyles, and Applications; Farrell, A.P., Ed.; Academic Press: New York, NY, USA, 2011; pp. 1709–1716. [Google Scholar]
- Ficke, A.D.; Myrick, C.A.; Hansen, L.J. Potential impacts of global climate change on freshwater fisheries. Rev. Fish Biol. Fish. 2007, 17, 581–613. [Google Scholar] [CrossRef]
- Doney, S.C.; Ruckelshaus, M.; Duffy, J.E.; Barry, J.P. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef]
- Makrinos, D.L.; Bowden, T.J. Natural environmental impacts on teleost immune function. Fish Shellfish Immunol. 2016, 53, 50–57. [Google Scholar] [CrossRef]
- Wright, R.K.; Cooper, E.L. Temperature effects on ectotherm immune responses. Dev. Comp. Immunol. 1981, 5, 117–122. [Google Scholar] [CrossRef]
- Bowden, T.J. Modulation of the immune system of fish by their environment. Fish Shellfish Immunol. 2008, 25, 373–383. [Google Scholar] [CrossRef]
- Pascoli, F.; Lanzano, G.S.; Negrato, E.; Poltronieri, C.; Trocino, A.; Radaelli, G.; Bertotto, D. Seasonal effects on hematological and innate immune parameters in sea bass Dicentrarchus labrax. Fish Shellfish Immunol. 2011, 31, 1081–1087. [Google Scholar] [CrossRef]
- Yada, T.; Tort, L. Stress and disease resistance: Immune system and Immunoendocrine interactions. Fish Physiol. 2016, 35, 365–403. [Google Scholar]
- Bailey, C.; Segner, H.; Casanova-Nakayama, A.; Wahli, T. Who needs the hotspot? The effect of temperature on the fish host immune response to Tetracapsuloides bryosalmonae the causative agent of proliferative kidney disease. Fish Shellfish Immunol. 2017, 63, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, Y.; Liu, Z.; Kang, Y.; Wang, J. Transcriptomic responses to heat stress in rainbow trout Oncorhynchus mykiss head kidney. Fish Shellfish Immunol. 2018, 82, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.P.; Liu, Z.; Zhou, Y.J.; Wang, Y.J.; Huang, J.Q.; Li, Y.J.; Kang, Y.J.; Wang, J.F.; Liu, X.X. Effects of Chronic Heat Stress on Part of Serum Non-specific Immunity Parameters in Rainbow Trout (Oncorhynchus mykiss). J. Agric. Biotechnol. 2017, 25, 1078–1085. [Google Scholar]
- Zhou, T.; Gui, L.; Liu, M.; Li, W.; Hu, P.; Duarte, D.F.C.; Niu, H.; Chen, L. Transcriptomic responses to low temperature stress in the Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2019, 84, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Hafs, A.W.; Mazik, P.M.; Kenney, P.B.; Silverstein, J.T. Impact of carbon dioxide level, water velocity, strain, and feeding regimen on growth and fillet attributes of cultured rainbow trout (Oncorhynchus mykiss). J. Aquac. 2012, 350–353, 46–53. [Google Scholar] [CrossRef]
- Yáñez, J.M.; Xu, P.; Carvalheiro, R.; Hayes, B. Genomics applied to livestock and aquaculture breeding. Evol. Appl. 2022, 15, 517. [Google Scholar] [CrossRef]
- Adams, O.A.; Zhang, Y.; Gilbert, M.H.; Lawrence, C.S.; Snow, M.; Farrell, A.P. An unusually high upper thermal acclimation potential for rainbow trout. Conserv. Physiol. 2022, 10, coab101. [Google Scholar] [CrossRef]
- Mulder, H.A.; Sae-Lim, P.; Kause, A.; Olesen, I. Selective breeding in aquaculture for future environments under climate change. In Proceedings of the FAO International Symposium on “The Role of Agricultural Biotechnologies in Sustainable Food Systems and Nutrition”, Rome, Italy, 15–17 February 2016; pp. 45–46. [Google Scholar]
- Abisha, R.; Krishnani, K.K.; Sukhdhane, K.; Verma, A.K.; Brahmane, M.; Chadha, N.K. Sustainable development of climate-resilient aquaculture and culture-based fisheries through adaptation of abiotic stresses: A review. J. Water Clim. Change 2022, 13, 2671–2689. [Google Scholar] [CrossRef]
- Rharrhour, H.; Wariaghli, F.; Goddek, S.; Sadik, M.; El, A. Towards sustainable food productions in Morocco: Aquaponics. E3S Web Conf. 2022, 337, 03004. [Google Scholar] [CrossRef]
- Wiens, G.D.; LaPatra, S.E.; Welch, T.J.; Evenhuis, J.P.; Rexroad III, C.E.; Leeds, T.D. On-farm performance of rainbow trout (Oncorhynchus mykiss) selectively bred for resistance to bacterial cold water disease: Effect of rearing environment on survival phenotype. Aquaculture 2013, 388, 128–136. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- World Development Report. Agriculture for Development; World Bank: Washington, DC, USA, 2008. [Google Scholar]
- Rivera-Ferre, M.; Ortega-Cerdà, M.; Baumgärtner, J. Rethinking. Study and Management of Agricultural Systems for Policy Design. Sustainability 2013, 5, 3858–3875. [Google Scholar] [CrossRef]
- Velten, S.; Leventon, J.; Jager, N.; Newig, J. What Is Sustainable Agriculture: A Systematic Review. Sustainability 2015, 7, 7833–7865. [Google Scholar] [CrossRef]
- Ebeling, J.M.; Timmons, M.B. Recirculating Aquaculture; Cayuga Aqua Ventures: Ithaca, NY, USA, 2010. [Google Scholar]
- Ebeling, J.M.; Timmons, M.B. Recirculating Aquaculture Systems. In Aquaculture Production Systems; Tidwell, J.H., Ed.; John Willey& Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Kingler, D.; Naylor, R. Searching for Solutions in Aquaculture: Charting a Sustainable Course. Annu. Rev. Environ. Resour. 2012, 37, 247–276. [Google Scholar]
- Tyson, R.C.; Treadwell, D.D.; Simonne, E.H. Opportunities and Challenges to Sustainability in Aquaponic Systems. HorTechnology 2011, 21, 6–13. [Google Scholar] [CrossRef]
- Ridler, N.; Wowchuk, M.; Robinson, B.; Barrington, K.; Chopin, T.; Robinson, S.; Page, F.; Reid, G.; Szemerda, M.; Sewuster, J.; et al. Integrated Multi-Trophic Aquaculture (IMTA): A potential strategic choice for farmers. Aquac. Econ. Manag. 2007, 11, 1–13. [Google Scholar] [CrossRef]
- Reid, G.K.; Liutkus, M.; Robinson, S.M.C.; Chopin, T.R.; Blair, T.; Lander, T.; Mullen, J.; Page, F.; Moccia, R.D. A review of the biophysical properties of salmonid faeces: Implications for aquaculture waste dispersal models and integrated multi-trophic aquaculture. Aquac. Res. 2009, 40, 257–273. [Google Scholar] [CrossRef]
- Ecolife Conservation. Introduction to Aquaponics; Ecolife Conservation: Escondido, CA, USA, 2017; pp. 2–24. [Google Scholar]
- Kaltsis, I. Aquaculture a Perfect Ecosystem for the Development of Ornamental Fish and Plants. Bachelor’s Thesis, Faculty of Agricultural Technology and Food and Nutrition Technology, Department of Fisheries and Aquaculture Technology, Mesologgi, Greece, 2014. [Google Scholar]
- Martins, C.I.M.; Edinga, E.H.; Verdegema, M.C.J.; Heinsbroeka, L.T.N.; Schneider, O.; Blanchetond, J.P.; Roque d’Orbcastel, E.; Verretha, J.A.J. New developments in recirculating aquaculture systems in Europe: A perspective on environmental sustainability. Agric. Eng. 2010, 43, 83–93. [Google Scholar] [CrossRef]
- Nelson, R.L.; Pade, J.S. Aquaponic equipment the clarifier. Aquaponics J. 2007, 4, 30–31. [Google Scholar]
- Tesi, R. Colture Protette: Ortoflorovivaismo in Ambiente Mediterraneo; Il sole 24 ore Edagricole: Bologna, Italy, 2008. [Google Scholar]
- Malorgio, F.; Incrocci, L.; Pardossi, A. La Tecnica della Coltivazione Fuori Suolo; Universita di Pisa: Pisa, Italy, 2005; pp. 1–143. [Google Scholar]
- Hochmuth, G.J.; Hanlon, E.A. Commercial Vegetable Fertilization Principles; University of Florida, Soil Water Science Department: Gainesville, FL, USA, 2010. [Google Scholar]
- Maucieri, C.; Nicoletto, C.; Zanin, G.; Xiccato, G.; Borin, M.; Sambo, P. Composition and quality traits of vegetables grown in a low-tech aquaponic system at different fish stocking densities. J. Sci. Food Agric. 2020, 100, 4310–4318. [Google Scholar] [CrossRef]
- Al Tawaha, A.R.; Wahab, P.E.M.; Binti Jaafar, H.; Zuan, A.T.K.; Hassan, M.Z. Effects of Fish Stocking Density on Water Quality, Growth Performance of Tilapia (Oreochromis niloticus) and Yield of Butterhead Lettuce (Lactuca sativa) Grown in Decoupled Recirculation Aquaponic Systems. J. Ecol. Eng. 2021, 22, 8–19. [Google Scholar] [CrossRef]
- Maucieri, C.; Nicoletto, C.; Zanin, G.; Birolo, M.; Xiccato, G.; Sambo, P.; Borin, M. Nitrogen budget in recirculating aquaponic systems with different fish stocking density. Ital. J. Agron. 2020, 15, 239–245. [Google Scholar] [CrossRef]
- Adler, P.R.; Harper, J.K.; Takeda, F.; Summerfelt, S.T. Economic analysis of an aquaponic system for the integrated production of rainbow trout and plants. Int. J. Recirc. Aquac. 2000, 1, 15–34. [Google Scholar] [CrossRef]
- McMurtry, M.R.; Nelson, P.V.; Sanders, D.C.; Hodges, L. Sand culture of vegetables using recirculated aquacultural effluents. Appl. Agric. Res. 1990, 5, 280–284. [Google Scholar]
- McMurtry, M.R.; Sanders, D.C.; Cure, J.D.; Hodson, R.G. Effects of biofilter/culture tank volume ratios on productivity of a recirculating fish/vegetable co-culture system. J. Appl. Aquac. 1997, 7, 33–51. [Google Scholar] [CrossRef]
- Goddek, S.; Joyce, A.; Kotzen, B.; Burnell, G.M. Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Springer Nature: Berlin/Heidelberg, Germany, 2019; 619p. [Google Scholar]
- Papoutsoglou, S.E. Fish Diet; Publications Stamoulis A: Athens, Greece, 2008; 976p. [Google Scholar]
- Olesen, I.; Myhr, A.I.; Rosendal, G.K. Sustainable aquaculture: Are we getting there? Ethical perspectives on salmon farming. J. Agric. Environ. Ethics 2011, 24, 381–408. [Google Scholar] [CrossRef]
- Somerville, C.; Cohen, M.; Pantanella, E.; Stankus, A.; Lovatelli, A. Small-scale aquaponic food production. Integrated fish and plant farming. In FAO Fisheries and Aquaculture; Technical Paper. No. 589.; FAO: Rome, Italy, 2014; 262p. [Google Scholar]
- Miličić, V.; Thorarinsdottir, R.; Dos Santos, M.; Turnšek Hančić, M. Commercial Aquaponics Approaching the European Market: To Consumers’ Perceptions of Aquaponics Products in Europe. Water 2017, 9, 80. [Google Scholar] [CrossRef]
- Al-Hafedh, Y.S.; Alam, A.; Beltagi, M.S. Food production and water conservation in a recirculating aquaponic system in Saudi Arabia at different ratios of fish feed to plants. J. World Aquac. Soc. 2008, 39, 510–520. [Google Scholar] [CrossRef]
- Pappa, V.A.; Kapsis, P.; Mente, E.; Berillis, P. Aquaponics Software in Greece. J. Fish. Sci. 2017, 11, 1–4. [Google Scholar] [CrossRef]
- Stathopoulou, P.; Berillis, P.; Levizou, E.; Sakellariou-Makrantonaki, M.; Kormas, A.K.; Aggelaki, A.; Kapsis, P.; Vlahos, N.; Mente, E. Aquaponics: A mutually beneficial relationship of fish, plants and bacteria. In Proceedings of the 3rd International Congress on Applied Ichthyology & Aquatic Environment, Volos, Greece, 8–11 November 2018; pp. 8–11. [Google Scholar]
- Krastanova, M.; Sirakov, I.; Ivanova-Kirilova, S.; Yarkov, D.; Orozova, P. Aquaponic systems: Biological and technological parameters. Biotechnol. Biotechnol. Equip. 2022, 36, 305–316. [Google Scholar] [CrossRef]
- Cretu, M.; Dediu, L.; Coadă, M.T.; Rîmniceanu, C.; Plăcintă, S.; Stroe, M.D.; Vasilean, I. Comparative study on the growth and development of thyme and basil herbs in aquaponic system and hydroponic system. Anim. Sci. 2022, 1, 573–589. [Google Scholar]
- Mihailovic-Stanojevic, N.; Belščak-Cvitanović, A.; Grujić-Milanović, J.; Ivanov, M.; Jovović, D.; Bugarski, D.; Miloradović, Z. Antioxidant and antihypertensive activity of extract from Thymus serpyllum L. in experimental hypertension. Plant Foods Hum. Nutr. 2013, 68, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Grespan, R.; Aguiar, R.P.; Giubilei, F.N.; Fuso, R.R.; Damião, M.J.; Silva, E.L.; Mikcha, J.G.; Hernandes, L.; Amado, C.B.; Cuman, R.K.N. Hepatoprotective effect of pretreatment with Thymus vulgaris essential oil in experimental model of acetaminophen-induced injury. Evid. -Based Complementary Altern. Med. 2014, 2014, 954136. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; De Martino, L.; Melone, A.; De Feo, V.; Nazzaro, F.; Coppola, R. Preservation of Chicken Breast Meat Treated with Thyme and Balm Essential Oils. J. Food Sci. 2010, 75, 528–535. [Google Scholar] [CrossRef]
- Sandulachi, E.; Macari, A.; Ghendov-Mosanu, A.; Cojocari, D.; Sturza, R. Antioxidant and Antimicrobial Activity of Basil, Thyme and Tarragon 580 Used in Meat Products. Adv. Microbiol. 2021, 11, 591–606. [Google Scholar] [CrossRef]
- Abdel-Rahim, M.M.; Awad, Y.M.; Abdallah, Y.A.; Radwan, S.M. Effects of four medicinal plants on the bioeconomic analysis and water-use efficiency of Nile tilapia, Oreochromis niloticus fry nursed under a small-scale aquaponics system. Aquac. Aquar. Conserv. Legis. 2019, 12, 851–866. [Google Scholar]
- Knaus, U.; Wenzel, L.C.; Appelbaum, S.; Palm, H.W. Aquaponics (sl) Production of spearmint (Mentha spicata) with African catfish (Clarias gariepinus) in Northern Germany. Sustainability 2020, 12, 8717. [Google Scholar] [CrossRef]
- Körner, O.; Gutzmann, E.; Kledal, P.R. A dynamic model simulating the symbiotic effects in aquaponic systems. In Proceedings of the International Symposium on New Technologies and Management for Greenhouses-GreenSys2015, Evora, Portugal, 19–23 July 2015; Volume 1170, pp. 309–316. [Google Scholar]
- Rakocy, J.E. Aquaponics-Integrating Fish and Plant Culture. Aquac. Prod. Syst. 2012, 1, 343–386. [Google Scholar]
- Siapatis, K.; Galanis, I. Aquaponic System: The Combination of Fish Culture and Hydroponic of Plants. Diploma Thesis, Department of Agricultural Science and Aquaculture, University of Thessaly, Volos, Greece, 2018; pp. 2–50. [Google Scholar]
- Schmautz, Z.; Loeu, F.; Liebisch, F.; Graber, A.; Mathis, A.; Bulc, T.G.; Junge, R. Tomato productivity and quality in aquaponics: Comparison of three hydroponic methods. Water 2016, 8, 533. [Google Scholar] [CrossRef]
- Alessio, G.; Allegrucci, G.; Angle, G.; Arlati, G.; Baldrati, R.; Ballestrazzi, P.; Belvedere, C.P.; Beraldo, D.; Berton, C.; Boglione, P.; et al. Acquacoltura Responsabile: Verso le Produzioni Acquatiche del Terzo Millennio; FAO: Rome, Italy, 2001. [Google Scholar]
- Fronte, B.; Galliano, G.; Bibbiani, C. From freshwater to marine aquaponic: New opportunities for marine fish species production. In Proceedings of the Conference VIVUS—On Agriculture, Environmentalism, Horticulture and Floristics, Food Production and Processing and Nutrition. With Knowledge and Experience to New Entrepreneurial Opportunities, Biotechnical Centre Naklo, Slovenia, 20–21 April 2016; pp. 514–521. [Google Scholar]
- Boroujerdnia, M.; Ansari, N.A. Effect of Different Levels of Nitrogen Fertilizer and Cultivars on Growth, Yield and Yield Components of Romaine Lettuce (Lactuca sativa L.). Middle East. Russ. J. Plant Sci. Biotechnol. 2007, 1, 47–53. [Google Scholar]
- Rakocy, J.E.; Masser, M.P.; Losordo, T.M. Recirculating Aquaculture Tank Production Systems: Aquaponics—Integrating Fish and Plant Culture; Publication no. 454.; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2006. [Google Scholar]
- Maucieri, C.; Nicoletto, C.; Junge, R.; Schmautz, Z.; Sambo, P.; Borin, M. Hydroponic systems and water management in aquaponics: A review. Ital. J. Agron. 2018, 13, 1012. [Google Scholar] [CrossRef]
- Walker, R.L.; Burns, I.G.; Moorby, J. Responses of plant growth rate to nitrogen supply: A comparison of relative addition and N interruption treatments. J. Exp. Bot. 2001, 52, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Forchino, A.A.; Lourguioui, H.; Brigolin, D.; Pastres, R. Aquaponics and sustainability: The comparison of two different aquaponic techniques using the Life Cycle Assessment (LCA). Aquac. Eng. 2017, 77, 80–88. [Google Scholar] [CrossRef]
- Lennard, W.A. Aquaponic Integration of Murray Cod (Maccullochella peelii peelii) Aquaculture and Lettuce (Lactuca sativa) Hydroponics. Ph.D. Thesis, School of Applied Sciences, Department of Biotechnology and Environmental Biology, Royal Melbourne Institute of Technology, Melbourne, VIC, Australia, 2006. [Google Scholar]
- Pantanella, J.E. Nutrition and Quality of Aquaponic Systems. Ph.D. Thesis, Università degli studi della Tuscia, Viterbo, Italy, 2012. [Google Scholar]
- Graber, A.; Junge, R. Aquaponic systems: Nutrient recycling from fish wastewater by vegetable production. Desalination 2009, 246, 147–156. [Google Scholar] [CrossRef]
- Bittsanszky, A.; Uzinger, N.; Mathis, A.; Gunlai, G. Nutrient supply of plants in aquaponic systems. Ecocycles 2016, 2, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Delaide, B.; Goddek, S.; Gott, J.; Soyeurt, H.; Haissam Jijakli, M.; Lalman, J.; Junge, R. Lettuce (Lactuca sativa L. var. Sucrine) growth performance in complemented aquaponic solution outperforms hydroponics. Water 2016, 8, 467. [Google Scholar] [CrossRef]
- Rakocy, J.E.; Shultz, R.C.; Bailey, D.S.; Thoman, E.S. Aquaponic production of tilapia and basil: Comparing a batch and staggered cropping system. In Proceedings of the South Pacific Soilless Culture Conference-SPSCC 648, Palmerston North, New Zealand, 10–13 February 2003; Volume 648, pp. 63–69. [Google Scholar]
- Licamele, J. Biomass Production and Nutrient Dynamics in an Aquaponics System. Ph.D. Thesis, Department of Agriculture and Biosystems Engineering, University of Arizona, Tuscon, AZ, USA, 2009. [Google Scholar]
- Liedl, B.E.; Cummins, M.; Young, A.; Williams, M.L. Hydroponic lettuce production using liquid effluent from poultry waste bioremediation as a nutrient source. In Proceedings of the VII International Symposium on Protected Cultivation in Mild Winter Climates: Production, Pest Management and Global Competition 659, Kissimmee, FL, USA, 23–27 March 2004; pp. 721–728. [Google Scholar]
- Calfo, A.; Williams, C. Book of Coral Propagation, 2nd ed.; Reef Gardering for Aquarist; Reading Trees: Oxford, UK, 2007; Volume 1, 250p. [Google Scholar]
- Buzby, K.M.; West, T.P.; Waterland, N.L.; Lin, L.S. Remediation of flow-through trout raceway effluent via aquaponics. N. Am. J. Aquac. 2017, 79, 53–60. [Google Scholar] [CrossRef]
- Velichkova, K.; Sirakov, I.; Stoyanova, S.; Staykov, Y. Cultivation of lettuce (Lactuca sativa L.) and rainbow trout (Oncorhynchus mykiss W.) in the aquaponic recirculation system. J. Cent. Eur. Agric. 2019, 20, 967–973. [Google Scholar] [CrossRef]
- Endut, A.; Jusoh, A.; Ali, N.; Wan Nik, W.B. Nutrient removal from aquaculture wastewater by vegetable production in aquaponics recirculation system. Desalination Water Treat. 2011, 32, 422–430. [Google Scholar] [CrossRef]
- Khater, E.S.G.; Bahnasawy, A.H.; Shams, A.E.H.S.; Hassaan, M.S.; Hassan, Y.A. Utilization of effluent fish farms in tomato cultivation. Ecol. Eng. 2015, 83, 199–207. [Google Scholar] [CrossRef]
- Bordignon, F.; Sturaro, E.; Trocino, A.; Birolo, M.; Xiccato, G.; Berton, M. Comparative life cycle assessment of rainbow trout (Oncorhynchus mykiss) farming at two stocking densities in a low-tech aquaponic system. Aquaculture 2022, 556, 738264. [Google Scholar] [CrossRef]
- Petrea, Ş.M.; Cristea, V.; Dediu, L.; Contoman, M.; Cretu, M.; Antache, A.; Coadă, M.T.; Bandi, A.C. A Study of Phosphorus and Calcium Dynamics in an Integrated Rainbow Trout and Spinach (Nores variety) Aquaponic System with Different Crop Densities. Sci. Pap. Anim. Sci. Biotechnol. 2014, 47, 196–206. [Google Scholar]
- Bordignon, F.; Gasco, L.; Birolo, M.; Trocino, A.; Caimi, C.; Ballarin, C.; Bortoletti, M.; Nicoletto, C.; Maucieri, C.; Xiccato, G. Performance and fillet traits of rainbow trout (Oncorhynchus mykiss) fed different levels of Hermetia illucens meal in a low-tech aquaponic system. Aquaculture 2022, 546, 737279. [Google Scholar] [CrossRef]
- Sirakov, I.; Velichkova, K.; Slavcheva-Sirakova, D. The effect of yarrow (Achillea millefolium) supplemented diet on growth performance, biochemical blood parameters and meat quality of rainbow trout (Oncorhynchus mykiss W.) and growth of lettuce (Lactuca sativa) cultivated in aquaponic recirculation system. J. Hyg. Eng. Des. 2019, 28, 28–32. [Google Scholar]
- Velichkova, K.; Sirakov, I.; Valkova, E. The effect of sweet flag (Acorus calamus L.) supplemented diet on growth performance, biochemical blood parameters and meat quality of rainbow trout (Oncorhynchus mykiss W.) and growth of lettuce (Lactuca sativa L.) cultivated in aquaponic recirculation system. Aquac. Aquar. Conserv. Legis. 2020, 13, 3840–3848. [Google Scholar]
- Petrea, Ş.M.; Cristea, V.; Dediu, L.; Contoman, M.; Lupoae, P.; Mocanu, M. Vegetable Production in an Integrated Aquaponic System with Rainbow Trout and Spinach. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Anim. Sci. Biotechnol. 2013, 70, 45–54. [Google Scholar]
- Khalil, S. Growth performance, nutrients and microbial dynamic in aquaponics systems as affected by water temperature. Eur. J. Hortic. Sci. 2018, 83, 388–394. [Google Scholar] [CrossRef]
- Zainal, A.G.; Yulianto, H.; Yanfika, H. Financial benefits of the environmentally friendly aquaponic media system. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Changsha, China, 18–20 September 2020; IOP Publishing: Bristol, UK, 2020; Volume 739, pp. 1–8. [Google Scholar]
- Kaiser, F.; Harbach, H. InnoFish-innovative adaptation of integrated aquaculture in an established extensive fish farm. AACL Bioflux 2022, 15, 873–877. [Google Scholar]
- Love, D.C.; Fry, J.P.; Genello, L.; Hill, E.S.; Frederick, J.A.; Li, X.; Semmens, K. An international survey of aquaponics practitioners. PLoS ONE 2014, 9, e102662. [Google Scholar] [CrossRef]
- Love, D.C.; Fry, J.P.; Li, X.; Hill, E.S.; Genello, L.; Semmens, K.; Thompson, R.E. Commercial aquaponics production and profitability: Findings from an international survey. Aquaculture 2015, 435, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Villarroel, M.; Junge, R.; Komives, T.; König, B.; Plaza, I.; Bittsánszky, A.; Joly, A. Survey of aquaponics in Europe. Water 2016, 8, 468. [Google Scholar] [CrossRef]
- Goddek, S.; Delaide, B.; Mankasingh, U.; Ragnarsdottir, K.V.; Jijakli, H.; Thorarinsdottir, R. Challenges of sustainable and commercial aquaponics. Sustainability 2015, 7, 4199–4224. [Google Scholar] [CrossRef]
- Palm, H.W.; Bissa, K.; Knaus, U. Significant factors affecting the economic sustainability of closed aquaponic systems. Part II: Fish and plant growth. Aquac. Aquar. Conserv. Legis. 2014, 7, 162–175. [Google Scholar]
- Maucieri, C.; Nicoletto, C.; Zanin, G.; Birolo, M.; Trocino, A.; Sambo, P.; Borin, M.; Xiccato, G. Effect of stocking density of fish on water quality and growth performance of European Carp and leafy vegetables in a low-tech aquaponic system. PLoS ONE 2019, 14, e0217561. [Google Scholar] [CrossRef]
- Hussain, T.; Verma, A.K.; Tiwari, V.K.; Prakash, C.; Rathore, G.; Shete, A.P.; Saharan, N. Effect of water flow rates on growth of Cyprinus carpio var. Koi (Cyprinus carpio L., 1758) and spinach plant in aquaponic system. Aquac. Int. 2015, 23, 369–384. [Google Scholar] [CrossRef]
- Alcarraz, Q.E.W.; Tapia, L.O.; Alcarraz, Q.Y.M. Microbiological analysis of lettuce (Lactuca sativa L.) grown in an aquaponic and hydroponic system. Net J. Agric. Sci. 2019, 7, 50–55. [Google Scholar]
- Hussain, T.; Verma, A.K.; Tiwari, V.K.; Prakash, C.; Rathore, G.; Shete, A.P.; Nuwansi, K.K.T. Optimizing koi carp, Cyprinus carpio var. Koi (Linnaeus, 1758), stocking density and nutrient recycling with spinach in an aquaponic system. J. World Aquac. Soc. 2014, 45, 652–661. [Google Scholar] [CrossRef]
- Rayhan, M.Z.; Rahman, M.A.; Hossain, M.A.; Akter, T.; Akter, T. Effect of stocking density on growth performance of monosex tilapia (Oreochromis niloticus) with Indian spinach (Basella alba) in a recirculating aquaponic system. Int. J. Environ. Agric. Biotechnol. 2018, 3, 239073. [Google Scholar] [CrossRef]
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasdravanidis, C.; Alvanou, M.V.; Lattos, A.; Papadopoulos, D.K.; Chatzigeorgiou, I.; Ravani, M.; Liantas, G.; Georgoulis, I.; Feidantsis, K.; Ntinas, G.K.; et al. Aquaponics as a Promising Strategy to Mitigate Impacts of Climate Change on Rainbow Trout Culture. Animals 2022, 12, 2523. https://doi.org/10.3390/ani12192523
Vasdravanidis C, Alvanou MV, Lattos A, Papadopoulos DK, Chatzigeorgiou I, Ravani M, Liantas G, Georgoulis I, Feidantsis K, Ntinas GK, et al. Aquaponics as a Promising Strategy to Mitigate Impacts of Climate Change on Rainbow Trout Culture. Animals. 2022; 12(19):2523. https://doi.org/10.3390/ani12192523
Chicago/Turabian StyleVasdravanidis, Christos, Maria V. Alvanou, Athanasios Lattos, Dimitrios K. Papadopoulos, Ioanna Chatzigeorgiou, Maria Ravani, Georgios Liantas, Ioannis Georgoulis, Konstantinos Feidantsis, Georgios K. Ntinas, and et al. 2022. "Aquaponics as a Promising Strategy to Mitigate Impacts of Climate Change on Rainbow Trout Culture" Animals 12, no. 19: 2523. https://doi.org/10.3390/ani12192523
APA StyleVasdravanidis, C., Alvanou, M. V., Lattos, A., Papadopoulos, D. K., Chatzigeorgiou, I., Ravani, M., Liantas, G., Georgoulis, I., Feidantsis, K., Ntinas, G. K., & Giantsis, I. A. (2022). Aquaponics as a Promising Strategy to Mitigate Impacts of Climate Change on Rainbow Trout Culture. Animals, 12(19), 2523. https://doi.org/10.3390/ani12192523











