The Colour, Composition and Eating Quality of Beef from Late- or Early-Maturing Suckler Bulls Finished at Pasture with or without Concentrate Supplementation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management and Sampling Procedures
2.2. Slaughter, Sampling Procedures, pH and Colour Measurement
2.3. Meat Chemical Analysis, Instrumental Texture and Sensory Evaluation
2.4. Perimysium Connective Tissue Isolation
2.5. Pyridinoline Quantification
2.6. Ehrlich Chromogen Crosslink Concentration
2.7. Sensory Assessment
2.8. Statistical Analysis
3. Results
3.1. Animal Growth and Carcass Characteristics
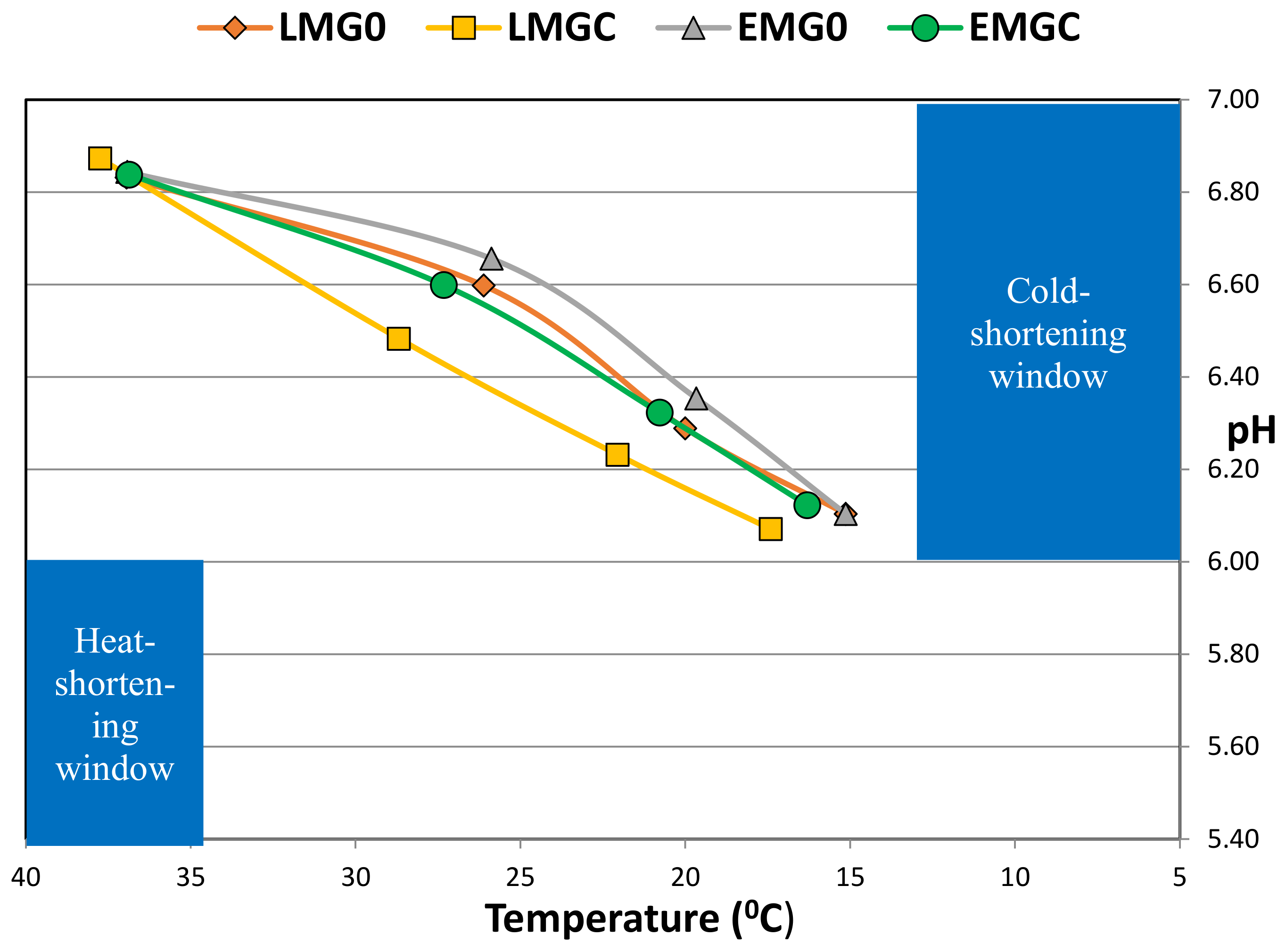
3.2. Post-Mortem pH and Temperature
3.3. Muscle Colour
3.4. Muscle Composition
3.5. WBSF and Sensory Characteristics
3.6. Correlation Analysis
4. Discussion
4.1. Context
4.2. Meat pH and Colour
4.3. Intramuscular Fat Concentration
4.4. Eating Quality
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Jensen, K.L.; Clark, C.D.; Lambert, D.M. Consumer willingness to pay for beef grown using climate friendly production practices. Food Policy 2016, 64, 93–106. [Google Scholar] [CrossRef]
- Lenighan, Y.M.; Nugent, A.P.; Moloney, A.P.; Monahan, F.J.; Walton, J.; Flynn, A.; Roche, H.M.; McNulty, B.A. A modelling approach to investigate the impact of consumption of three different beef compositions on human dietary fat intakes. Pub. Health Nutr. 2020, 23, 2373–2383. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, E.G.; Crosson, P.; McGee, M. Finishing male cattle from the beef suckler herd. Ir. Grass. Assoc. J. 2011, 45, 131–146. [Google Scholar]
- Kenny, D.; Murphy, C.P.; Sleator, R.D.; Judge, M.M.; Evans, R.D.; Berry, D.P. Animal-level factors associated with the achievement of desirable specifications in Irish beef carcasses graded using the EUROP classification system. J. Anim. Sci. 2020, 98, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.; Wilson, S.S.; McElhinney, C.K.; Monahan, F.J.; McGee, M.; O’Sullivan, M.G.; O’Riordan, E.G.; Kerry, J.P.; Moloney, A.P. Suckler bulls slaughtered at 15 months of age: Effect of different production systems on the fatty acid profile and selected quality characteristics of Longissimus thoracis. Foods 2019, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Moloney, A.P.; O’ Riordan, E.G.; McGee, M.; O’Sullivan, M.G.; Kerry, J.P.; Wilson, S.S.; Monahan, F.J.; Kelly, A.K.; McMenamin, K.; Moran, L. Carcass characteristics, colour and eating quality of beef from late maturing suckler bulls finished at pasture or indoors on a high concentrate ration. Anim. Prod. Sci. 2022, 62, 590–600. [Google Scholar] [CrossRef]
- Regan, M. Grass-Based Production Systems for Suckler-Bred Male Cattle. Ph.D. Thesis, University College Dublin/National University of Ireland, Dublin, Ireland, 2018; 142p. [Google Scholar]
- Moloney, A.P.; Picard, B.; Moran, L. The effect of extended post-mortem ageing on the Warner–Brazler shear force of Longissimus thoracis from beef heifers from two sire breeds, slaughtered at 20 or 25 mo of age. Ir. J. Agric. Food Res. 2020, 59, 206–214. [Google Scholar] [CrossRef]
- Holman, B.W.B.; van de Ven, R.J.; Mao, Y.; Coombs, C.E.O.; Hopkins, D.L. Using instrumental (CIE and reflectance) measures to predict consumers’ acceptance of beef colour. Meat Sci. 2017, 127, 57–62. [Google Scholar] [CrossRef]
- Mezgebo, G.B.; Monahan, F.J.; McGee, M.; O’Riordan, E.G.; Picard, B.; Richardson, R.I.; Moloney, A.P. Biochemical and organoleptic characteristics of muscle from early and late maturing bulls in different production systems. Animal 2017, 11, 1636–1644. [Google Scholar] [CrossRef]
- Moran, L.; O’Sullivan, M.G.; Kerry, J.P.; Picard, B.; McGee, M.; O’Riordan, E.G.; Moloney, A.P. Effect of a grazing period prior to finishing on a high concentrate diet on meat quality from bulls and steers. Meat Sci. 2017, 125, 76–83. [Google Scholar] [CrossRef]
- Shackelford, S.D.; Wheeler, T.L.; Koohmaraie, M. Relationship between shear force and trained sensory panel tenderness ratings of 10 major muscles from Bos indicus and Bos taurus cattle. J. Anim. Sci. 1995, 73, 3333–3340. [Google Scholar] [CrossRef] [PubMed]
- Kolar, K. Colorimetric determination of hydroxyproline as measure of collagen content in meat and meat products: NMKL collaborative study. J. Assoc. Off. Anal. Chem. 1990, 73, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Huynh, M.B.; Morin, C.; Carpentier, G.; Garcia-Filipe, S.; Talhas-Perret, S.; Barbier-Chassefière, V.; van Kuppevelt, T.H.; Martelly, I.; Albanese, P.; Papy-Garcia, D. Age-related changes in rat myocardium involve altered capacities of glycosaminoglycans to potentiate growth factor functions and heparan sulfate-altered sulfation. J. Biol. Chem. 2012, 287, 11363–11373. [Google Scholar] [CrossRef] [PubMed]
- Bank, R.A.; Beekman, B.; Verzijl, N.; de Roos, J.A.; Sakkee, A.N.; TeKoppele, J.M. Sensitive fluorimetric quantitation of pyridinium and pentosidine crosslinks in biological samples in a single high-performance liquid chromatographic run. J. Chrom. B Biomed. Sci. Applic. 1997, 703, 37–44. [Google Scholar] [CrossRef]
- Roy, B.; Sedgewick, G.; Aalhus, J.; Basarab, J.; Bruce, H. Modification of mature non-reducible collagen cross-link concentrations in bovine m. gluteus medius and semitendinosus with steer age at slaughter, breed cross and growth promotants. Meat Sci. 2015, 110, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Horgan, D.J.; Jones, P.N.; King, N.L.; Kurth, L.B.; Kuypers, R. The relationship between animal age and the thermal stability and cross-link content of collagen from five goat muscles. Meat Sci. 1991, 29, 251–262. [Google Scholar] [CrossRef]
- Stone, H.; Bleibaum, R.N.; Thomas, H.A. Affective testing. In Sensory Evaluation Practices; Stone, H., Bleibaum, R.N., Thomas, H.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 291–325. [Google Scholar]
- Stone, H.; Bleibaum, R.N.; Thomas, H.A. Test strategy and design of experiments. In Sensory Evaluation Practices; Stone, H., Bleibaum, R.N., Thomas, H.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 117–157. [Google Scholar]
- American Meat Science Association. Research Guidelines for Cookery, Sensory Evaluation and Instrumental Tenderness Measurements of Fresh Meat; National Livestock and Meat Board: Chicago, IL, USA, 2005. [Google Scholar]
- Bord Bia. Grass Fed Beef Standard. 2020. Available online: https://www.bordbia.ie/globalassets/bordbia2020/farmers--growers/grass-fed-standard/grass-fed-beef-standard.pdf (accessed on 20 June 2022).
- Bonny, S.P.F.; Pethick, D.W.; Legrand, I.; Wierzbicki, J.; Allen, P.; Farmer, L.J.; Polkinghorne, R.L.; Hocquette, J.-F.; Gardner, G.E. European conformation and fat scores have no relationship with eating quality. Animal 2016, 10, 996–1006. [Google Scholar] [CrossRef]
- Judge, M.M.; Conroy, S.; Hegarty, P.J.; Cromie, A.R.; Fanning, R.; Kelly, D.; Crofton, E.; Berry, D.P. Eating quality of the longissimus thoracis muscle in beef cattle—Contributing factors to the underlying variability and associations with performance traits. Meat Sci. 2021, 172, 108371. [Google Scholar] [CrossRef]
- Ashmore, C.R.; Carroll, F.; Doerr, L.; Tompkins, G.; Stokes, H.; Parker, W. Experimental prevention of dark-cutting meat. J. Anim. Sci. 1973, 36, 33–36. [Google Scholar] [CrossRef]
- Viljoen, H.F.; De Kock, H.L.; Webb, E.C. Consumer acceptability of dark, firm and dry (DFD) and normal pH beef steaks. Meat Sci. 2002, 61, 181–185. [Google Scholar] [CrossRef]
- Thompson, J.M. Managing meat tenderness. Meat Sci. 2002, 62, 295–308. [Google Scholar] [CrossRef]
- Dunne, P.; Monahan, F.J.; Moloney, A.P. Current perspectives on the darker beef often reported from extensively-managed cattle: Does physical activity play a significant role? Livest. Sci. 2011, 142, 1–22. [Google Scholar] [CrossRef]
- Acciaro, M.; Decandia, M.; Giovanetti, V.; Manca, C.; Dimauro, C.; Rassu, S.P.G.; Addis, M.; Molle, G. Effects of grass- and concentrate-based finishing systems on the quality of meat from the M. longissimus thoracis of young Sarda bulls. Anim. Prod. Sci. 2021, 81, 807–820. [Google Scholar] [CrossRef]
- Moran, L.; Wilson, S.S.; O’Sullivan, M.G.; McGee, M.; O’Riordan, E.G.; Monahan, F.J.; Kerry, J.P.; Moloney, A.P. Quality of three muscles from suckler bulls finished on concentrates and slaughtered at 16 months of age or slaughtered at 19 months of age from two production systems. Animal 2020, 14, 2203–2211. [Google Scholar] [CrossRef]
- Cafferky, J.; Hamill, R.M.; Allen, P.; O’Doherty, J.V.; Cromie, A.; Sweeney, T. Effect of breed and gender on meat quality of M. longissimus thoracis et lumborum muscle from crossbred beef bulls and steers. Foods 2019, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Cuvelier, C.; Clinquart, A.; Hocquette, J.F.; Cabaraux, J.F.; Dufrasne, I.; Istasse, L.; Hornick, J.L. Comparison of composition and quality traits of meat from young finishing bulls from Belgian Blue, Limousin and Aberdeen Angus breeds. Meat Sci. 2006, 74, 522–531. [Google Scholar] [CrossRef]
- Ripoll, G.; Albertí, P.; Panea, B.; Failla, S.; Hocquette, J.-F.; Dunner, S.; Sañudo, C.; Olleta, J.L.; Christensen, M.; Ertbjerg, P.; et al. Colour variability of beef in young bulls from fifteen European breeds. Int. J. Food Sci.Technol. 2018, 53, 2777–2785. [Google Scholar] [CrossRef]
- Christensen, M.; Ertbjerg, P.; Failla, S.; Sañudo, C.; Richardson, R.I.; Nute, G.R.; Olleta, J.L.; Panea, B.; Albertí, P.; Juárez, M.; et al. Relationship between collagen characteristics, lipid content and raw and cooked texture of meat from young bulls of fifteen European breeds. Meat Sci. 2011, 87, 61–65. [Google Scholar] [CrossRef]
- Ellies-Oury, M.P.; Lee, A.; Jacob, H.; Hocquette, J.F. Meat consumption–what French consumers feel about the quality of beef? Ital. J. Anim. Sci. 2019, 18, 646–656. [Google Scholar] [CrossRef]
- O’Quinn, T.G.; Legako, J.F.; Brooks, J.C.; Miller, M.F. Evaluation of the contribution of tenderness, juiciness, and flavor to the overall consumer beef eating experience. Trans. Anim. Sci. 2018, 2, 26–36. [Google Scholar] [CrossRef]
- Liu, J.; Ellies-Oury, M.-P.; Chriki, S.; Legrand, I.; Pogorzelski, G.; Wierzbicki, J.; Farmer, L.; Troy, D.; Polkinghorne, R.; Hocquette, J.-F. Contributions of tenderness, juiciness and flavour liking to overall liking of beef in Europe. Meat Sci. 2020, 168, 108190. [Google Scholar] [CrossRef] [PubMed]
- Maltin, C.A.; Sinclair, K.D.; Warriss, P.; Grant, C.M.; Porter, A.D.; Delday, M.I.; Warkup, C.C. The effects of age at slaughter, genotype and finishing system on the biochemical properties, muscle fibre type characteristics and eating quality of bull beef from suckled calves. Anim. Sci. 1998, 66, 341–348. [Google Scholar] [CrossRef]
- Dubost, A.; Micol, D.; Meunier, B.; Lethias, C.; Listrat, A. Relationships between structural characteristics of bovine intramuscular connective tissue assessed by image analysis and collagen and proteoglycan content. Meat Sci. 2013, 93, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T. Role of extracellular matrix in development of skeletal muscle and postmortem aging of meat. Meat Sci. J. 2015, 109, 48–55. [Google Scholar] [CrossRef]
- Sinclair, K.D.; Cuthbertson, A.; Rutter, A.; Franklin, M.F. The effects of age at slaughter, genotype and finishing system on the organleptic properties and texture of bull beef from suckled calves. Anim. Sci. 1998, 66, 329–340. [Google Scholar] [CrossRef]
- Sevane, N.; Nute, G.; Sanudo, C.; Cortes, O.; Canon, J.; Williams, J.L. Muscle lipid composition in bulls from 15 European breeds. Livest. Sci. 2014, 160, 1–11. [Google Scholar] [CrossRef]
- Bureš, D.; Bartoň, L.; Zahrádková, R.; Teslík, V.; Krejčová, M. Chemical composition, sensory characteristics and fatty acid profile of muscle from Aberdeen Angus, Charolais, Simmental, and Hereford bulls. Czech J. Anim. Sci. 2006, 51, 279–284. [Google Scholar] [CrossRef]
- Conanec, A.; Campo, M.; Richardson, R.I.; Ertbjerg, P.; Failla, S.; Panea, B.; Chavent, M.; Saracco, J.; Williams, J.L.; Ellies-Oury, M.-P.; et al. Has breed any effect on beef sensory quality? Livest. Sci. 2021, 250, 104548. [Google Scholar] [CrossRef]
- Blanco, M.; Casasus, I.; Ripoll, G.; Panea, B.; Alberti, P.; Joy, M. Lucerne grazing compared with concentrate-feeding slightly modifies carcass and meat quality of young bulls. Meat Sci. 2010, 84, 545–552. [Google Scholar] [CrossRef]
- Yuksel, S.; Yanar, M.; Aksu, M.I.; Kopuzulu, S.; Kaban, G.; Sezgin, E.; Oz, F. Effects of different finishing systems on carcass traits, fatty acid composition and beef quality characteristics of young Eastern Anatolian Red bulls. Trop. Anim. Health Prod. 2012, 44, 1521–1528. [Google Scholar] [CrossRef]
- Conroy, P.M.; O’Sullivan, M.G.; Hamill, R.M.; Kerry, J.P. Sensory capability of young, middle–aged and elderly Irish assessors to identify beef steaks of varying texture. Meat Sci. 2017, 132, 125–130. [Google Scholar] [CrossRef] [PubMed]

| Breed Type (BT) | Late Maturing | Early Maturing | Mean | ||
| Concentrates (C, g/kg) | 0 | 400 | 0 | 400 | |
| Lightness 1 | |||||
| 1 h 2 | 41.9 | 41.8 | 41.9 | 41.2 | 41.70 |
| 24 h | 44.1 | 43.9 | 43.8 | 43.4 | 43.80 |
| Mean | 43.0 | 42.9 | 42.8 | 42.3 | |
| s.e.d. | 0.64 | ||||
| Significance 3 | |||||
| BT | NS | ||||
| C | NS | ||||
| BT*C | NS | ||||
| Time | *** | ||||
| Time*BT | NS | ||||
| Time*C | NS | ||||
| Time*BT*C | NS | ||||
| Redness 1 | |||||
| 1 h | 12.9 | 12.3 | 13.0 | 12.7 | 12.7 |
| 24 h | 15.4 | 15.3 | 14.8 | 15.0 | 15.1 |
| Mean | 14.1 | 13.8 | 13.9 | 13.9 | |
| s.e.d. | 0.47 | ||||
| Significance | |||||
| BT | NS | ||||
| C | NS | ||||
| BT*C | NS | ||||
| Time | *** | ||||
| Time*BT | 0.078 | ||||
| Time*C | NS | ||||
| Time*BT*C | NS | ||||
| Yellowness 1 | |||||
| 1 h | 9.4 | 8.8 | 9.4 | 8.9 | 9.1 |
| 24 h | 12.5 | 12.2 | 12.2 | 12.2 | 12.3 |
| Mean | 11.0 | 10.5 | 10.8 | 10.6 | |
| s.e.d. | 0.36 | ||||
| Significance | |||||
| BT | NS | ||||
| C | NS | ||||
| BT*C | NS | ||||
| Time | *** | ||||
| Time*BT | NS | ||||
| Time*C | NS | ||||
| Time*BT*C | NS | ||||
| Breed Type (BT) | Late Maturing | Early Maturing | Mean | ||
| Concentrates (C, g/kg) | 0 | 400 | 0 | 400 | |
| Chroma 1 | |||||
| 1 h | 16.0 | 15.1 | 16.0 | 15.5 | 15.7 |
| 24 h | 19.9 | 19.6 | 19.2 | 19.4 | 19.5 |
| Mean | 17.9 | 17.3 | 17.6 | 17.5 | |
| s.e.d. | 0.55 | ||||
| Significance | |||||
| BT | NS | ||||
| C | NS | ||||
| BT*C | NS | ||||
| Time | *** | ||||
| Time*BT | NS | ||||
| Time*C | NS | ||||
| Time*BT*C | NS | ||||
| Hue 1 | |||||
| 1 h | 36.0 | 35.6 | 35.8 | 35.0 | 35.6 |
| 24 h | 39.4 | 38.6 | 39.5 | 39.2 | 39.2 |
| Mean | 37.7 | 37.1 | 37.7 | 37.1 | |
| s.e.d. | 0.68 | ||||
| Significance | |||||
| BT | NS | ||||
| C | NS | ||||
| BT*C | NS | ||||
| Time | *** | ||||
| Time*BT | 0.056 | ||||
| Time*C | NS | ||||
| Time*BT*C | NS | ||||
| Breed Type (BT) | Late Maturing | Early Maturing | S.e.d. | Significance 1 | ||||
|---|---|---|---|---|---|---|---|---|
| Concentrates (C, g/kg) | 0 | 400 | 0 | 400 | BT | C | BT*C | |
| pH | 5.52 | 5.51 | 5.50 | 5.51 | 0.023 | NS | NS | NS |
| Composition (g/kg muscle) | ||||||||
| Lipid | 1.8 | 2.1 | 4.7 | 6.4 | 1.86 | ** | NS | NS |
| Protein | 229.3 | 229.4 | 230.6 | 228.2 | 16.2 | NS | NS | NS |
| Moisture | 752.5 | 749.3 | 749.6 | 753.6 | 3.7 | NS | NS | NS |
| Soluble collagen (g/kg muscle) | 0.50 | 0.48 | 0.56 | 0.56 | 0.058 | NS | NS | NS |
| Insoluble collagen (g/kg muscle) | 4.61 | 4.37 | 4.57 | 4.85 | 0.439 | NS | NS | NS |
| Total collagen (g/kg muscle) | 5.11 | 4.85 | 5.13 | 5.40 | 0.481 | NS | NS | NS |
| Collagen solubility (g/kg) | 97.3 | 97.9 | 109.9 | 105.3 | 7.03 | * | NS | NS |
| Proteoglycans (mg/kg muscle) | 12.47 | 13.38 | 13.2 | 14.75 | 1.688 | NS | NS | NS |
| Proteoglycans (g/kg collagen) | 2.54 | 2.78 | 2.65 | 2.93 | 0.387 | NS | NS | NS |
| Perimysial collagen (PC) (g/kg muscle) | 3.01 | 3.44 | 3.52 | 4.16 | 0.226 | *** | ** | NS |
| Pyridinoline (mol/mol PC collagen) | 0.36 | 0.34 | 0.33 | 0.35 | 0.019 | NS | NS | NS |
| Pyridinoline (mmol/kg muscle) | 3.58 | 3.96 | 3.85 | 4.86 | 0.340 | * | ** | NS |
| Ehrlich chromogen (mol/mol PC collagen) | 0.39 | 0.37 | 0.37 | 0.39 | 0.013 | NS | NS | NS |
| Ehrlich chromogen (mmol/kg muscle) | 3.90 | 4.30 | 4.31 | 5.39 | 0.348 | ** | ** | NS |
| Breed Type (BT) | Late Maturing | Early Maturing | S.e.d. | Significance 1 | ||||
|---|---|---|---|---|---|---|---|---|
| Concentrates (C, g/kg) | 0 | 400 | 0 | 400 | BT | C | BT*C | |
| WBSF (N) | 37.4 | 43.3 | 37.1 | 38.4 | 2.46 | NS | * | NS |
| Cook loss (g/kg) | 292.6 | 296.1 | 293.4 | 303.2 | 8.58 | NS | NS | NS |
| Sensory characteristics 2 | ||||||||
| Tenderness | 4.07 | 3.74 | 4.48 | 3.92 | 0.218 | 0.058 | ** | NS |
| Flavour | 4.10 | 4.03 | 4.32 | 4.09 | 0.187 | NS | NS | NS |
| Firmness | 5.12 | 5.38 | 5.02 | 5.40 | 0.229 | NS | 0.053 | NS |
| Texture | 3.78 | 3.73 | 4.27 | 3.85 | 0.229 | 0.067 | NS | NS |
| Acceptability | 3.95 | 3.78 | 4.34 | 3.99 | 0.234 | 0.080 | NS | NS |
| WBSF | Tenderness | Flavour | Firmness | Texture | Acceptability | |
|---|---|---|---|---|---|---|
| Fat score | 0.01 | −0.01 | −0.01 | 0.09 | 0.05 | −0.04 |
| IMF (g/kg muscle) | −0.17 | 0.05 | 0.06 | −0.15 | 0.09 | 0.09 |
| Collagen (g/kg muscle) | −0.21 | −0.08 | −0.03 | 0.04 | −0.05 | 0.02 |
| Collagen solubility (g/kg) | 0.16 | 0.10 | −0.01 | 0.04 | 0.11 | 0.02 |
| Proteoglycans (mg/kg muscle) | 0.05 | −0.13 | −0.10 | 0.10 | 0.02 | −0.07 |
| Proteoglycans (g/kg collagen) | 0.15 | −0.04 | −0.07 | 0.07 | 0.11 | 0.03 |
| Perimysium collagen (g/kg muscle) | 0.08 | −0.12 | −0.01 | 0.01 | −0.04 | −0.04 |
| Pyridinoline (mol/mol perimysium collagen) | −0.08 | 0.17 | −0.01 | −0.01 | 0.02 | 0.02 |
| Pyridinoline (mmol/kg muscle) | 0.01 | −0.03 | −0.08 | 0.09 | −0.07 | −0.09 |
| Ehrlich chromogen (mol/mol perimysium collagen) | −0.06 | −0.15 | −0.09 | 0.09 | −0.16 | −0.06 |
| Ehrlich chromogen (mmol/kg muscle) | −0.01 | −0.19 | −0.10 | 0.12 | −0.11 | −0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moloney, A.P.; Wilson, S.S.; Siphambili, S.; Moran, L.; O’Riordan, E.G.; O’Sullivan, M.G.; Kerry, J.P.; Monahan, F.J.; McGee, M. The Colour, Composition and Eating Quality of Beef from Late- or Early-Maturing Suckler Bulls Finished at Pasture with or without Concentrate Supplementation. Animals 2022, 12, 2417. https://doi.org/10.3390/ani12182417
Moloney AP, Wilson SS, Siphambili S, Moran L, O’Riordan EG, O’Sullivan MG, Kerry JP, Monahan FJ, McGee M. The Colour, Composition and Eating Quality of Beef from Late- or Early-Maturing Suckler Bulls Finished at Pasture with or without Concentrate Supplementation. Animals. 2022; 12(18):2417. https://doi.org/10.3390/ani12182417
Chicago/Turabian StyleMoloney, Aidan P., Shannon S. Wilson, Sibhekiso Siphambili, Lara Moran, Edward G. O’Riordan, Maurice G. O’Sullivan, Joseph P. Kerry, Frank J. Monahan, and Mark McGee. 2022. "The Colour, Composition and Eating Quality of Beef from Late- or Early-Maturing Suckler Bulls Finished at Pasture with or without Concentrate Supplementation" Animals 12, no. 18: 2417. https://doi.org/10.3390/ani12182417






