Reporting of Freshwater Cyanobacterial Poisoning in Terrestrial Wildlife: A Systematic Map
Abstract
:Simple Summary
Abstract
1. Introduction
2. Protocol
2.1. Literature Sources and Search Terms
2.2. Literature Search and Inclusion Criteria
- Literature reporting cyanotoxin poisonings in terrestrial wildlife, including reptiles, amphibians, mammals, birds, and aerial insects with aquatic larval stages. Cyanobacterial poisonings in other populations, such as aquatic species, domestic species, and humans, were excluded.
- Literature discussing terrestrial wildlife exposure to cyanobacterial species and toxins, including direct or indirect exposure and including chronic or acute intoxication. Literature discussing poisonings due to experimental exposure to toxins was excluded from this review.
- Literature discussing algal poisoning in the context of coastal, marine, or estuarine waters (HABs) was only included if terrestrial wildlife species were affected. In these instances, non-cyanobacterial algal species and algal toxins may be implicated. Due to the focus of the review, it is relevant to include these cases in investigating surveillance techniques for terrestrial wildlife poisoning.
- Grey literature was not actively searched for evidence of poisonings but was not excluded if captured by the literature search.
- We included only literature in English, but limitations regarding this are acknowledged, as cyanoHABs may occur anywhere in the world and be reported in any language. Due to resource and time constraints, we were not able to include any non-English language studies. Any texts excluded due to language were recorded.
- All study types except for reviews were included.
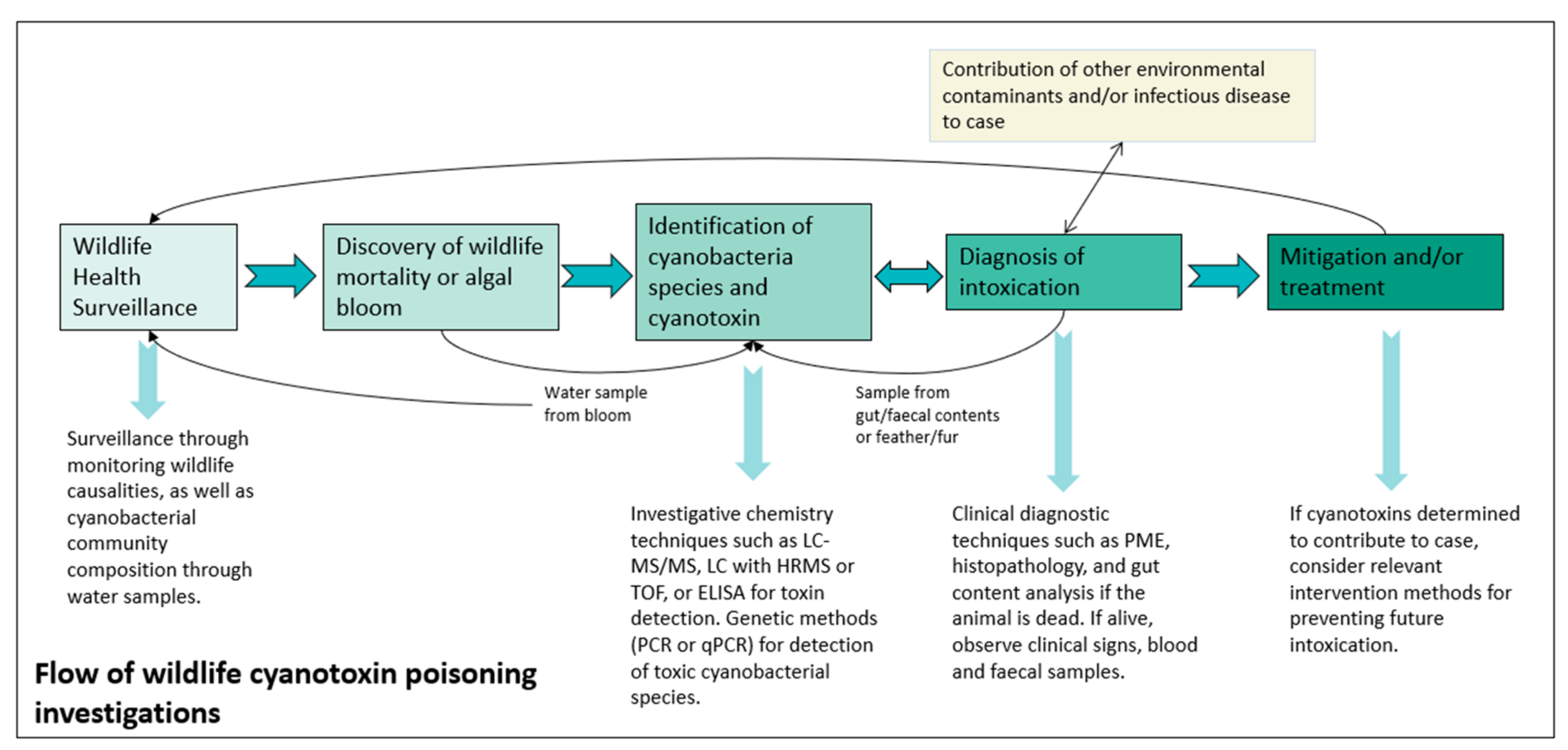
2.3. Recommended Method of Diagnosis and Investigation
2.4. Data Extraction
2.5. Data Synthesis and Visualisation
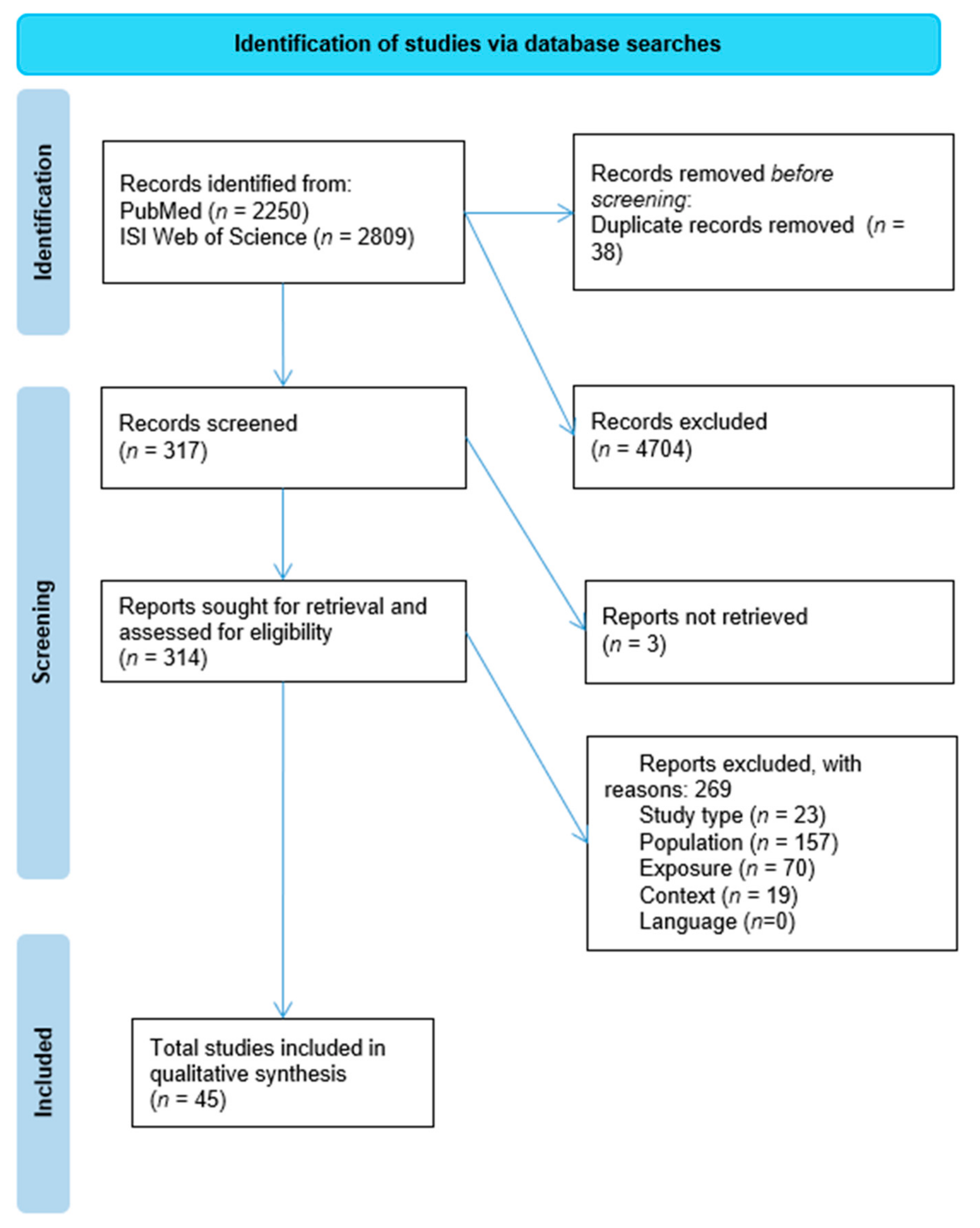
3. Results
3.1. Eligibility of Studies
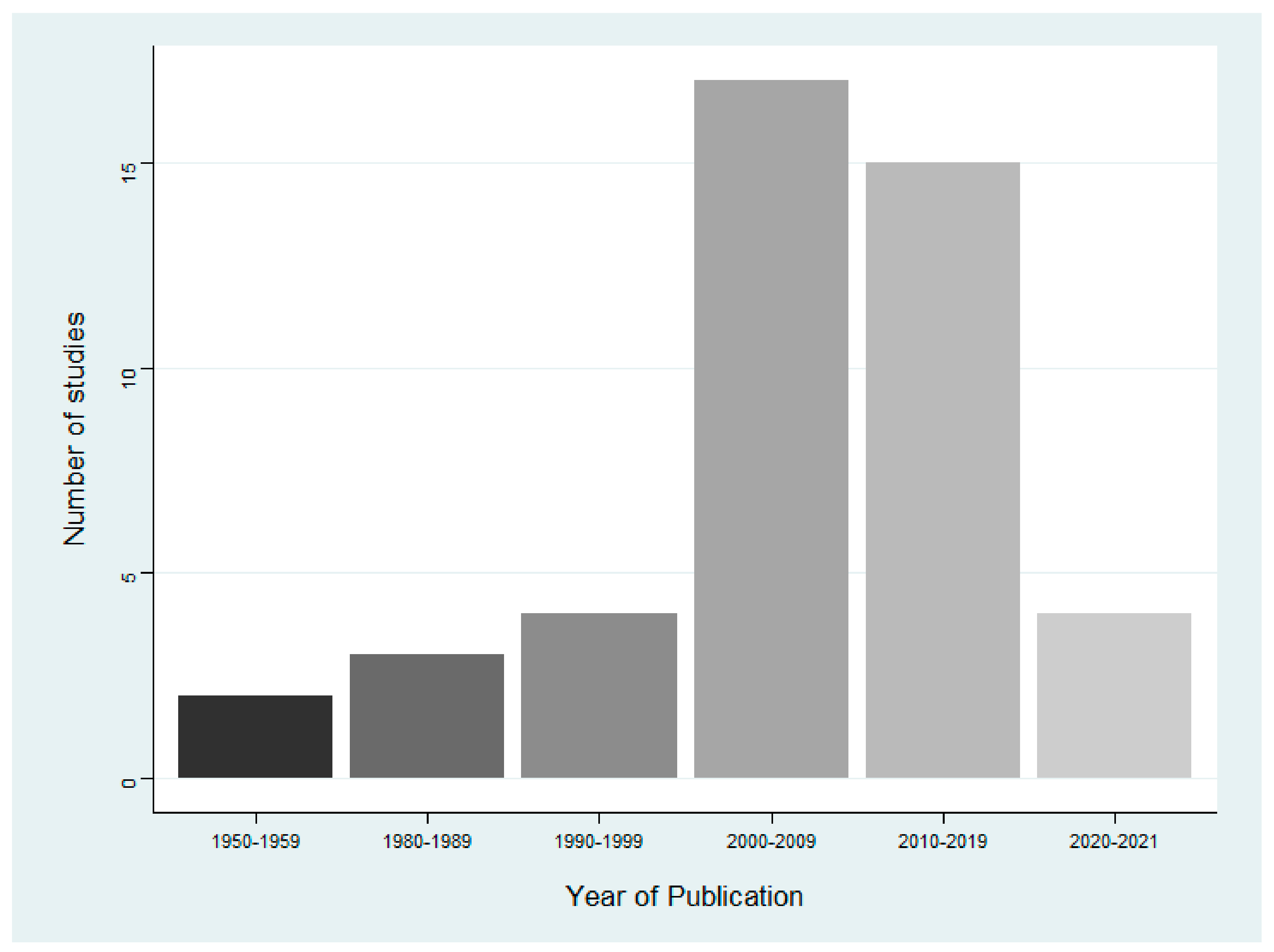
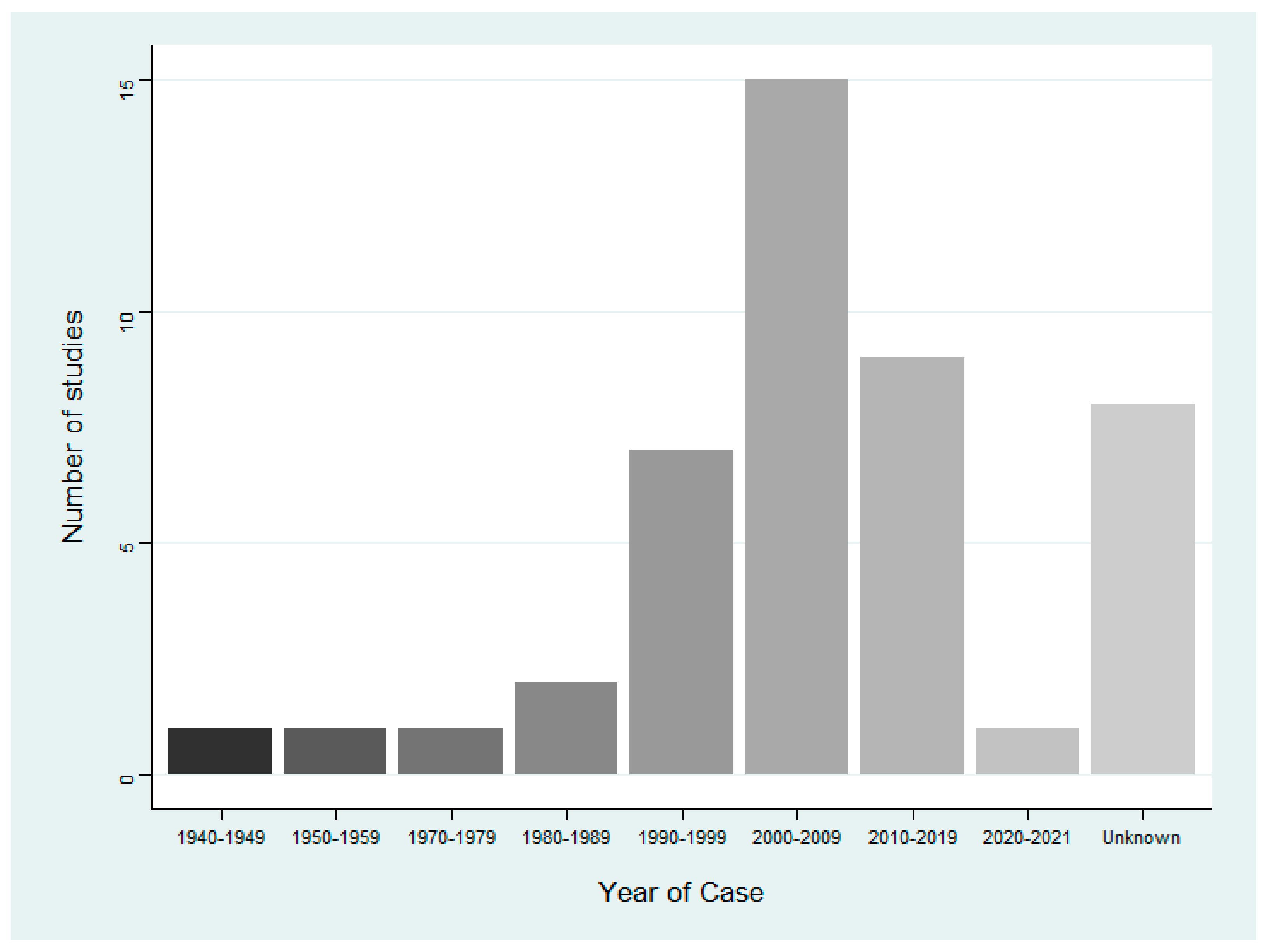
3.2. Characteristics of Included Studies
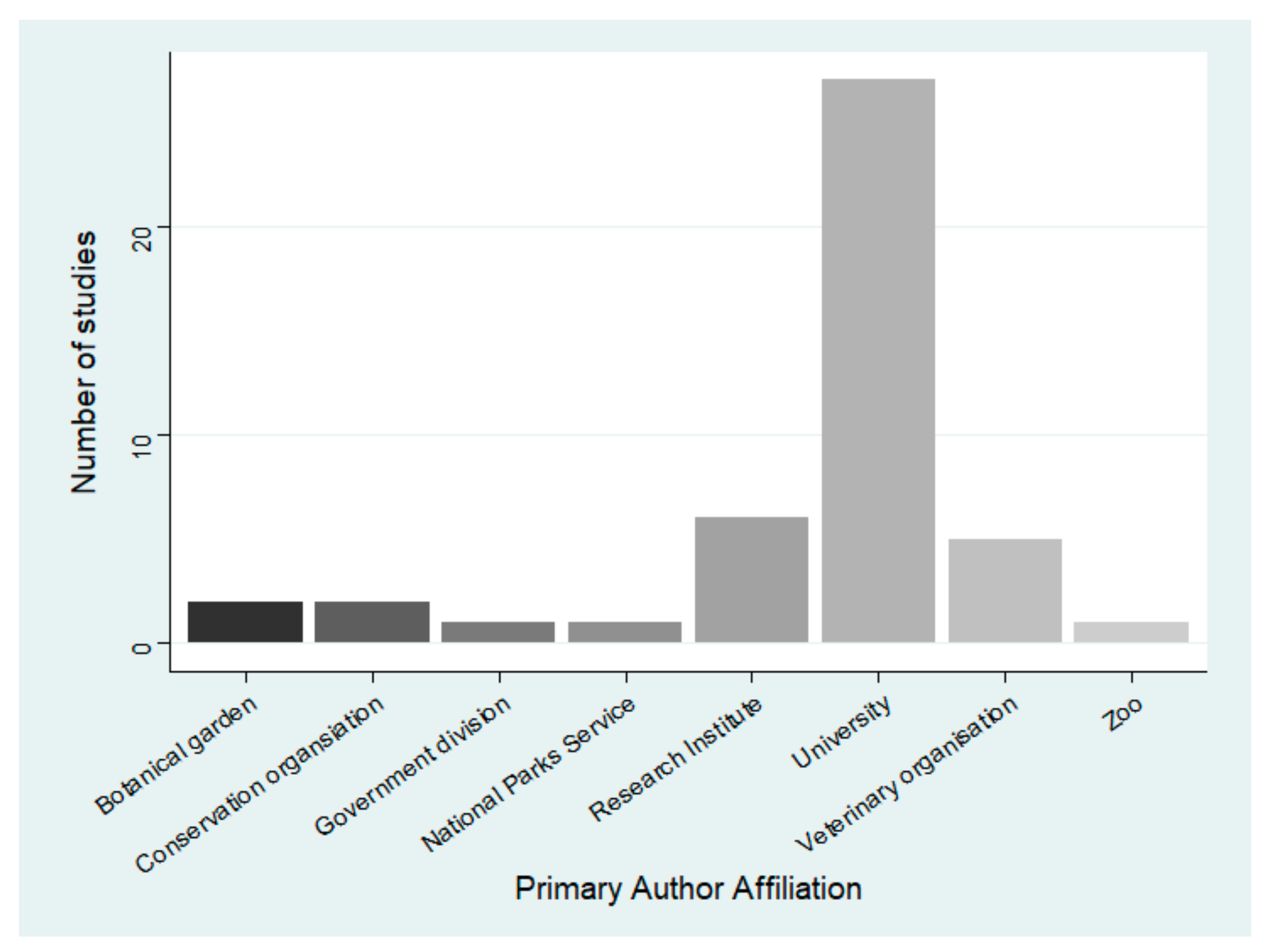
3.3. Lead Author Affiliation
3.4. Cyanobacterial Species and Toxins
3.5. Animal Species Involved and Exposure
3.6. Surveillance and Diagnostics
4. Discussion
4.1. Invasive Cyanobacteria
4.2. Co-Occurrence of Cyanotoxins and Environmental Contaminants
4.3. Methods of Surveillance and Diagnosis
4.4. Improving Investigations
4.5. Review Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Full Reference | Summary |
|---|---|
| Soll, MD* & Williams, M. Mortality of a white rhinoceros (Ceratotherium simum) suspected to be associated with the blue-green alga Microcystis aeruginosa. Journal of the South African Veterinary Association 1985, 56(1), pp. 49–51. | Death of three reintroduced white rhinos Ceratotherium simum in South Africa from toxic Microcystis aeruginosa. |
| Pybus, M.J., Hobson, D.P. and Onderka, D.K. Mass mortality of bats due to probable blue-green algal toxicity. Journal of Wildlife Diseases 1986, 22(3), pp. 449–450. | Mass mortality of bats (Myotis spp. and Lasiurus sp.) due to Anabaena flos-aquae in Alberta, Canada. |
| Banack, S.A. and Cox, P.A. Biomagnification of cycad neurotoxins in flying foxes: implications for ALS-PDC in Guam. Neurology 2003, 61(3), pp. 387–389. | Accumulation of the toxin BMAA within Guam flying fox Pteropus tokudae tissues. |
| Krienitz, L., Ballot, A., Kotut, K., Wiegand, C., Pütz, S., Metcalf, J.S., Codd, G.A. and Stephan, P. Contribution of hot spring cyanobacteria to the mysterious deaths of Lesser Flamingos at Lake Bogoria, Kenya. FEMS microbiology ecology 2003, 43(2), pp. 141–148. https://doi.org/10.1016/S0168–6496(02)00387–2. | Mass mortality of lesser flamingos Phoeniconaias minor in Kenya due to toxins within cyanobacterial hot spring mats. |
| Lopez-Rodas, V., Maneiro, E., Lanzarot, M.P., Perdigones, N. and Costas, E. Mass wildlife mortality due to cyanobacteria in the Doñana National Park, Spain. The Veterinary Record 2008, 162(10), p. 317. | Mass mortality of 47 species of waterfowl due to Microcystis aeruginosa bloom in Doñana National Park, Spain. |
| Nasri, H., El Herry, S. and Bouaïcha, N. First reported case of turtle deaths during a toxic Microcystis spp. bloom in Lake Oubeira, Algeria. Ecotoxicology and Environmental Safety 2008, 71(2), pp. 535–544. https://doi.org/10.1016/j.ecoenv.2007.12.009. | Mortality of turtles due to toxic Microcystis spp. bloom in Algeria. |
| Bidigare, R.R., Christensen, S.J., Wilde, S.B. and Banack, S.A. Cyanobacteria and BMAA: possible linkage with avian vacuolar myelinopathy (AVM) in the south-eastern United States. Amyotrophic Lateral Sclerosis 2009, 10(sup2), pp. 71–73. | Bird mortalities due to toxic epiphytic cyanobacterial species (order Stigonematales) in Southeastern USA. |
| Oberholster, P.J., Myburgh, J.G., Govender, D., Bengis, R. and Botha, A.M. Identification of toxigenic Microcystis strains after incidents of wild animal mortalities in the Kruger National Park, South Africa. Ecotoxicology and Environmental Safety 2009, 72(4), pp. 1177–1182. https://doi.org/10.1016/j.ecoenv.2008.12.014. | Mortality of megaherbivores in Kruger National Park, South Africa, by microcystins. Bloom formation arose from eutrophication due to high numbers of hippopotami in dams. |
| Woller-Skar, M.M., Jones, D.N., Luttenton, M.R. and Russell, A.L. Microcystin detected in little brown bats (Myotis lucifugus). The American Midland Naturalist 2015, 174(2), pp. 331–334 | Bioaccumulation of microcystins in little brown bats Myotis lucifugus consuming mayfly larvae in Michigan, USA. |
| Moy, N.J., Dodson, J., Tassone, S.J., Bukaveckas, P.A. and Bulluck, L.P. Biotransport of algal toxins to riparian food webs. Environmental science & technology 2016, 50(18), pp. 10007–10014. https://doi.org/10.1021/acs.est.6b02760. | Biotransfer of microcystins in a riparian food web, from mayfly larvae to prothonotary warblers Protonotaria citrea in Virginia, USA. |
| Refsnider, J.M., Garcia, J.A., Holliker, B., Hulbert, A.C., Nunez, A. and Streby, H.M. Effects of harmful algal blooms on stress levels and immune functioning in wetland-associated songbirds and reptiles. Science of The Total Environment 2021, 788, p. 147790. https://doi.org/10.1016/j.scitotenv.2021.147790. | Accumulation of microcystins and stress levels in reptiles and songbirds exposed to harmful algal blooms in Ohio, USA. |
| Bengis, R., Govender, D., Lane, E., Myburgh, J., Oberholster, P., Buss, P., Prozesky, L. and Keet, D. Eco-epidemiological and pathological features of wildlife mortality events related to cyanobacterial bio-intoxication in the Kruger National Park, South Africa. Journal of the South African Veterinary Association 2016, 87(1), pp. 1–9. https://doi.org/10.4102/jsava.v87i1.1391. | Mortality of megaherbivores in Kruger National Park, South Africa, by microcystins. Bloom formation arose from eutrophication due to high numbers of hippopotami in dams. |
| Masango, M.G., Myburgh, J.G., Labuschagne, L., Govender, D., Bengis, R.G. and Naicker, D. Assessment of Microcystis bloom toxicity associated with wildlife mortality in the Kruger National Park, South Africa. Journal of Wildlife Diseases 2010, 46(1), pp. 95–102. https://doi.org/10.7589/0090–3558-46.1.95. | Mortality of megaherbivores in Kruger National Park, South Africa, by microcystins. Bloom formation arose from eutrophication due to high numbers of hippopotami in dams. |
| Cox, P.A., Banack, S.A. and Murch, S.J. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proceedings of the National Academy of Sciences 2003, 100(23), pp. 13380–13383. https://doi.org/10.1073/pnas.2235808100. | Accumulation of the toxin BMAA within Guam flying fox Pteropus tokudae tissues. |
| van Aarde, R.J., Pimm, S.L., Guldemond, R., Huang, R. and Maré, C. The 2020 elephant die-off in Botswana. PeerJ 2021, 9, p. e10686. https://doi.org/10.7717/peerj.10686. | Mortality of African savanna elephants Loxodonta africanawith in Botswana, with cyanotoxins as a proposed diagnosis. |
| Matsunaga, H., Harada, K.I., Senma, M., Ito, Y., Yasuda, N., Ushida, S. and Kimura, Y. Possible cause of unnatural mass death of wild birds in a pond in Nishinomiya, Japan: sudden appearance of toxic cyanobacteria. Natural Toxins 1999, 7(2),pp. 81–84. https://doi.org/10.1002/(SICI)1522–7189(199903/04)7:2%3C81::AID-NT44%3E3.0.CO;2-O. | Mortality of spot-billed ducks Anas zonorhyncha in Japan due to a Microcystis aeruginosa bloom. |
| Lugomela, C., Pratap, H.B. and Mgaya, Y.D. Cyanobacteria blooms—A possible cause of mass mortality of Lesser Flamingos in Lake Manyara and Lake Big Momela, Tanzania. Harmful Algae 2006, 5(5), pp. 534–541. https://doi.org/10.1016/j.hal.2005.10.001. | Lesser flamingo Phoeniconaias minor mass mortality due to toxic Arthrospira fusiformis in Tanzania. |
| Wirsing, B., Hoffmann, L., Heinze, R., Klein, D., Daloze, D., Braekman, J.C. and Weckesser, J. First report on the identification of microcystin in a water bloom collected in Belgium. Systematic and applied microbiology 1998, 21(1), pp. 23–27. https://doi.org/10.1016/S0723–2020(98)80004–0. | Bird deaths associated with a toxic Microcystis aeruginosa bloom in Belgium. |
| Sherlock, I.R., James, K.J., Caudwell, F.B. and MacKintosh, C. First identification of microcystins in Irish lakes aided by a new derivatisation procedure for electrospray mass spectrometric analysis. Natural toxins 1997, 5(6), pp. 247–254. https://doi.org/10.1002/(SICI)1522–7189(1997)5:6%3C247::AID-NT5%3E3.0.CO;2-N. | Terrestrial wildlife deaths in Ireland associated with microcystins. |
| Craighead, D., Metcalf, J.S., Banack, S.A., Amgalan, L., Reynolds, H.V. and Batmunkh, M. Presence of the neurotoxic amino acids β-N-methylamino-L-alanine (BMAA) and 2, 4-diamino-butyric acid (DAB) in shallow springs from the Gobi Desert. Amyotrophic Lateral Sclerosis 2009, 10(sup2), pp. 96–100. https://doi.org/10.3109/17482960903278469. | Presence of BMAA and DAB in desert pools of the Gobi Desert, a water source for the endangered Gobi bear Ursus arctos isabellinus. |
| Takahashi, T., Umehara, A. and Tsutsumi, H. Diffusion of microcystins (cyanobacteria hepatotoxins) from the reservoir of Isahaya Bay, Japan, into the marine and surrounding ecosystems as a result of large-scale drainage. Marine pollution bulletin 2014, 89(1–2), pp. 250–258. https://doi.org/10.1016/j.marpolbul.2014.09.052. | Microcystins in the Isahaya Bay reservoir, Japan, and detection of toxins within aquatic insects and their predators. |
| Papadimitriou, T., Katsiapi, M., Vlachopoulos, K., Christopoulos, A., Laspidou, C., Moustaka-Gouni, M. and Kormas, K. Cyanotoxins as the “common suspects” for the Dalmatian pelican (Pelecanus crispus) deaths in a Mediterranean reconstructed reservoir. Environmental pollution 2018, 234, pp. 779–787. https://doi.org/10.1016/j.envpol.2017.12.022. | Intoxication from microcystins and saxitoxins in dalmatian pelicans Pelicanus crispus in Karla Reservoir, Greece, causing death. |
| Handeland, K. and Østensvik, Ø. Microcystin poisoning in roe deer (Capreolus capreolus). Toxicon 2010, 56(6), pp. 1076–1078. https://doi.org/10.1016/j.toxicon.2010.06.023. | Hepatotoxicosis in Norwegian wild roe deer from microcystin. |
| Gkelis, S., Lanaras, T. and Sivonen, K. The presence of microcystins and other cyanobacterial bioactive peptides in aquatic fauna collected from Greek freshwaters. Aquatic toxicology 2006, 78(1), pp. 32–41. https://doi.org/10.1016/j.aquatox.2006.02.001. | Presence of microcystin in frog tissues in Greece. |
| Henriksen, P., Carmichael, W.W., An, J. and Moestrup, Ø. Detection of an anatoxin-a (s)-like anticholinesterase in natural blooms and cultures of cyanobacteria/blue-green algae from Danish lakes and in the stomach contents of poisoned birds. Toxicon 1997, 35(6), pp. 901–913. https://doi.org/10.1016/S0041–0101(96)00190–0. | Toxic Anabaena spp. found in stomach contents of wild birds in Denmark. |
| Alonso-Andicoberry, C., García-Viliada, L., Lopez-Rodas, V. and Costas, E. Catastrophic mortality of flamingos in a Spanish national park caused by cyanobacteria. The Veterinary Record 2002, 151(23), p.706. https://doi.org/10.1136/vr.151.23.706. | Greater flamingo Phoenicopterus ruber mortality in Doñana National Park, Spain. |
| Carmichael, W.W. and Li, R.H. Cyanobacteria toxins in the Salton Sea. Saline systems 2006, 2(1), pp. 1–13. doi: 10.1186/1746–1448-2–5 | Eared grebe Podiceps nigricollis mortalities in the Salton Sea due to toxic cyanobacterial blooms. |
| Straubinger-Gansberger, N., Gruber, M., Kaggwa, M.N., Lawton, L., Oduor, S.O. and Schagerl, M. Sudden flamingo deaths in Kenyan Rift Valley lakes. Wildlife Biology 2014, 20(3), pp. 185–189. https://doi.org/10.2981/wlb.00018. | Lesser flamingo deaths in Kenya, suspected cause of toxic Arthrospira fusiformis strains. |
| Metcalf, J.S., Banack, S.A., Kotut, K., Krienitz, L. and Codd, G.A. Amino acid neurotoxins in feathers of the Lesser Flamingo, Phoeniconaias minor. Chemosphere 2013, 90(2), pp. 835–839. https://doi.org/10.1016/j.chemosphere.2012.09.094. | BMAA and DAB within feathers of lesser flamingos in Kenya. |
| Metcalf, J.S., Morrison, L.F., Krienitz, L., Ballot, A., Krause, E., Kotut, K., Pütz, S., Wiegand, C., Pflugmacher, S. and Codd, G.A. Analysis of the cyanotoxins anatoxin-a and microcystins in Lesser Flamingo feathers. Toxicological & Environmental Chemistry 2006, 88(1), pp. 159–167. https://doi.org/10.1080/02772240500491604. | Microcystin found in feathers of lesser flamingo carcasses that died from algal poisoning. |
| Chen, J., Zhang, D., Xie, P., Wang, Q. and Ma, Z. Simultaneous determination of microcystin contaminations in various vertebrates (fish, turtle, duck and water bird) from a large eutrophic Chinese lake, Lake Taihu, with toxic Microcystis blooms. Science of the Total Environment 2009, 407(10), pp. 3317–3322. https://doi.org/10.1016/j.scitotenv.2009.02.005. | Accumulation of microcystins within turtle and bird livers from Lake Taihu, China. |
| Wilde, S.B., Murphy, T.M., Hope, C.P., Habrun, S.K., Kempton, J., Birrenkott, A., Wiley, F., Bowerman, W.W. and Lewitus, A.J. Avian vacuolar myelinopathy linked to exotic aquatic plants and a novel cyanobacterial species. Environmental Toxicology: An International Journal 2005, 20(3), pp. 348–353. https://doi.org/10.1002/tox.2011. | Epiphytic species of cyanobacteria (order Stigonematales) linked to fatal disease in American wild birds. |
| Doster, E., Chislock, M.F., Roberts, J.F., Kottwitz, J.J. and Wilson, A.E Recognition of an important water quality issue at zoos: prevalence and potential threat of toxic cyanobacteria. Journal of Zoo and Wildlife Medicine 2014, pp. 165–168. | Yellow-bellied slider Trachemys scripta scripta deaths due to microcystin poisoning in a zoo in Alabama, USA. |
| McCain, S., Sim, R.R., Howerth, E.W., Aschenbroich, S., Kirejczyk, S.G., McHale, B., Jerry, C., Kottwitz, J.J., Wilson, A.E. and McManamon, R. Myonecrosis and death due to presumed microcystin toxicosis in american white pelicans (pelecanus erythrorhyncos). Journal of Zoo and Wildlife Medicine 2020, 51(2), pp. 407–415. https://doi.org/10.1638/2019–0117. | Microcystin poisoning of zoo-housed American white pelicans Pelecanus erythrorhynchos at Birmingham Zoo, USA. |
| Rose, E.T. Toxic algae in Iowa lakes. In Proceedings of the Iowa Academy of Science 1953 (Vol. 60, No. 1, pp. 738–746). | Mass mortalities of waterfowl, gulls, and rodents in Iowa Lakes, Iowa, due to harmful algal blooms. |
| Buttke, D.E., Walker, A., Huang, I.S., Flewelling, L., Lankton, J., Ballmann, A.E., Clapp, T., Lindsay, J. and Zimba, P.V. Green tree frog (Hyla cinerea) and ground squirrel (Xerospermophilus spilosoma) mortality attributed to inland brevetoxin transport at Padre Island National Seashore, Texas, USA, 2015. Journal of wildlife diseases 2018, 54(1), pp. 142–146. https://doi.org/10.7589/2017–01-018. | Green tree frog Hyla cinerea and ground squirrel Xerospermophilus spilosoma mortality due to rainwater contaminated with cyanotoxins. |
| Chen, J. and Xie, P. Accumulation of hepatotoxic microcystins in freshwater mussels, aquatic insect larvae and oligochaetes in a large, shallow eutrophic lake (Lake Chaohu) of subtropical China. Fresen. Environ. Bull 2008, 17, pp. 849–854. | Accumulation of microcystins in insect larva Chironomus sp. in Lake Chaohu, China. |
| Xue, Q., Su, X., Steinman, A.D., Cai, Y., Zhao, Y. and Xie, L. Accumulation of microcystins in a dominant Chironomid Larvae (Tanypus chinensis) of a large, shallow and eutrophic Chinese lake, Lake Taihu. Scientific reports 2016, 6(1), pp. 1–10. https://doi.org/10.1038/srep31097. | Accumulation of microcystin in larvae of Tanypus chinensis in Lake Taihu, China. |
| Trout-Haney, J.V. and Cottingham, K.L. Microcystins in planktonic and benthic food web components from Greenlandic lakes. Ecosphere 2021, 12(6), p.e03539. https://doi.org/10.1002/ecs2.3539. | Microcystins found within tissues of chironomid larvae found in Greenlandic lakes. |
| Nonga, H.E., Sandvik, M., Miles, C.O., Lie, E., Mdegela, R.H., Mwamengele, G.L., Semuguruka, W.D. and Skaare, J.U. Possible involvement of microcystins in the unexplained mass mortalities of Lesser Flamingo (Phoeniconaias minor Geoffroy) at Lake Manyara in Tanzania. Hydrobiologia 2011, 678(1), pp. 167–178. https://doi.org/10.1007/s10750–011-0844–8. | Contribution of microcystin intoxication in lesser flamingo mortality in Tanzania. |
| Willén, E., Ahlgren, G., Tilahun, G., Spoof, L., Neffling, M.R. and Meriluoto, J. Cyanotoxin production in seven Ethiopian Rift Valley lakes. Inland Waters 2011, 1(2), pp. 81–91. https://doi.org/10.5268/IW-1.2.391. | The potential implication of microcystins in mortality of wild and domestic animals in Ethiopia. |
| O’donoghue, J.G. and Wilton, G.S. Algal poisoning in Alberta. Canadian Journal of Comparative Medicine and Veterinary Science, 1951, 15(8), p.193. | Livestock and waterfowl poisonings in Alberta, Canada. |
| Mahmood, N.A., Carmichael, W.W. and Pfahler, D. Anticholinesterase poisonings in dogs from a cyanobacterial(blue-green algae) bloom dominated by Anabaena flos-aquae. American Journal of Veterinary Research, 1988, 49(4), pp. 500–503. | Poisoning and mortality of a muskrat and great blue heron in South Dakota, USA. |
| Moy, N.J., Dodson, J., Tassone, S.J., Bukaveckas, P.A. and Bulluck, L.P. Biotransport of algal toxins to riparian food webs. Environmental Science & Technology, 2016, 50(18), pp. 10007–10014. | Accumulation of microcystins in prothonotary warblers due to their aquatic prey (i.e., mayflies) diet in Chesapeake Bay, USA. |
| Reifel, K.M., McCoy, M.P., Rocke, T.E., Tiffany, M.A., Hurlbert, S.H. and Faulkner, D.J. Possible importance of algal toxins in the Salton Sea, California. Hydrobiologia, 2002, 473(1), pp. 275–292. | Implication of cyanotoxins in eared grebe deaths in the Salton Sea, California, USA. |
References
- Paerl, H.W.; Huisman, J.M. Climate: Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Hudnell, H.K.; Paul, V.J. Chapter 11: Global warming and cyanobacterial harmful algal blooms. Cyanobacterial Harmful Algal Bloom. State Sci. Res. Needs 2008, 619, 239–257. [Google Scholar]
- Gobler, C.J.; Doherty, O.M.; Hattenrath-Lehmann, T.K.; Griffith, A.W.; Kang, Y.; Litaker, R.W. Ocean warming since 1982 has expanded the niche of toxic algal blooms in the North Atlantic and North Pacific oceans. Proc. Natl. Acad. Sci. USA 2017, 114, 4975–4980. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Sukenik, A.; Quesada, A.; Salmaso, N. Global expansion of toxic and non-toxic cyanobacteria: Effect on ecosystem functioning. Biodivers. Conserv. 2015, 24, 889–908. [Google Scholar] [CrossRef]
- Paerl, H.W.; Fulton, R.S.; Moisander, P.H.; Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci. World J. 2001, 1, 76–113. [Google Scholar] [CrossRef]
- Sellner, K.G.; Doucette, G.J.; Kirkpatrick, G.J. Harmful algal blooms: Causes, impacts and detection. J. Ind. Microbiol. Biotechnol. 2003, 30, 383–406. [Google Scholar] [CrossRef]
- Holland, A.; Kinnear, S. Interpreting the possible ecological role(s) of cyanotoxins: Compounds for competitive advantage and/or physiological aide? Mar. Drugs 2013, 11, 2239–2258. [Google Scholar] [CrossRef]
- Lampert, W. Toxicity of the blue-green Microcystis aeruginosa: Effective defense mechanism against grazing pressure by Daphnia. Verh. Intern. Ver. Limnol. 1981, 21, 1436–1440. [Google Scholar]
- Kirk, K.L.; Gilbert, J.J. Variation in the herbivore response to chemical defenses: Zooplankton foraging on toxic cyanobacteria. Ecology 1992, 73, 2208–2217. [Google Scholar] [CrossRef]
- Campos, A.; Vasconcelos, V. Molecular mechanisms of microcystin toxicity in animal cells. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef] [PubMed]
- Craig, M.; Holmes, C.F. Freshwater hepatotoxins: Microcystin and nodularin, mechanisms of toxicity and effects on health. In Food Science and Technology; Marcel Dekker: New York, NY, USA, 2000; pp. 643–672. [Google Scholar]
- Fawell, J.K.; Mitchell, R.E.; Everett, D.J.; Hill, R.E. The toxicity of cyanobacterial toxins in the mouse: I microcystin-LR. Hum. Exp. Toxicol. 1999, 18, 162–167. [Google Scholar] [CrossRef]
- Fiore, M.F.; de Lima, S.T.; Carmichael, W.W.; McKinnie, S.M.; Chekan, J.R.; Moore, B.S. Guanitoxin, re-naming a cyanobacterial organophosphate toxin. Harmful Algae 2020, 92, 101737. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, R.M.; Chen, C.H.; Reynolds, C.S.; Meghal, S.K.; Koeppe, R.E. The ‘neurotoxicity’of L-2, 4-diaminobutyric acid. Biochem. J. 1968, 106, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Delcourt, N.; Claudepierre, T.; Maignien, T.; Arnich, N.; Mattei, C. Cellular and Molecular Aspects of the β-N-Methylamino-l-alanine (BMAA) Mode of Action within the Neurodegenerative Pathway: Facts and Controversy. Toxins 2017, 10, 6. [Google Scholar] [CrossRef]
- Cruz-Aguado, R.; Winkler, D.; Shaw, C.A. Lack of behavioral and neuropathological effects of dietary β-methylamino-L-alanine (BMAA) in mice. Pharmacol. Biochem. Behav. 2006, 84, 294–299. [Google Scholar] [CrossRef]
- Karamyan, V.T.; Speth, R.C. Animal models of BMAA neurotoxicity: A critical review. Life Sci. 2008, 82, 233–246. [Google Scholar] [CrossRef]
- Nunn, P.B.; Ponnusamy, M. β-N-Methylaminoalanine (BMAA): Metabolism and metabolic effects in model systems and in neural and other tissues of the rat in vitro. Toxicon 2009, 54, 85–94. [Google Scholar] [CrossRef]
- Chiu, A.S.; Gehringer, M.M.; Welch, J.H.; Neilan, B.A. Does α-amino-β-methylaminopropionic acid (BMAA) play a role in neurodegeneration? Int. J. Environ. Res. Public Health 2011, 8, 3728–3746. [Google Scholar] [CrossRef]
- Chernoff, N.; Hill, D.J.; Diggs, D.L.; Faison, B.D.; Francis, B.M.; Lang, J.R.; Larue, M.M.; Le, T.T.; Loftin, K.A.; Lugo, J.N.; et al. A critical review of the postulated role of the non-essential amino acid, β-N-methylamino-L-alanine, in neurodegenerative disease in humans. J. Toxicol. Environ. Health Part B 2017, 20, 183–229. [Google Scholar] [CrossRef]
- Lopez-Rodas, V.; Maneiro, E.; Lanzarot, M.P.; Perdigones, N.; Costas, E. Mass wildlife mortality due to cyanobacteria in the Doñana National Park, Spain. Vet. Rec. 2008, 162, 317. [Google Scholar] [CrossRef] [PubMed]
- Masango, M.G.; Myburgh, J.G.; Labuschagne, L.; Govender, D.; Bengis, R.G.; Naicker, D. Assessment of Microcystis bloom toxicity associated with wildlife mortality in the Kruger National Park, South Africa. J. Wildl. Dis. 2010, 46, 95–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metcalf, J.S.; Codd, G.A. Co-occurrence of cyanobacteria and cyanotoxins with other environmental health hazards: Impacts and implications. Toxins 2020, 12, 629. [Google Scholar] [CrossRef] [PubMed]
- Straubinger-Gansberger, N.; Lawton, L.; Gruber, M.; Kaggwa, M.N.; Oduor, S.O.; Schagerl, M. Sudden flamingo deaths in Kenyan Rift Valley lakes. Wildl. Biol. 2014, 20, 185–189. [Google Scholar] [CrossRef]
- Murphy, T.; Lawson, A.; Nalewajko, C.; Murkin, H.; Ross, L.; Oguma, K.; McIntyre, T. Algal toxins—Initiators of avian botulism? Environ. Toxicol. Int. J. 2000, 15, 558–567. [Google Scholar] [CrossRef]
- Gochfeld, M.; Gochfeld, D.J.; Minton, D.; Murray, B.G.; Pyle, P.; Seto, N.; Smith, D.; Burger, J. Metals in feathers of bonin petrel, Christmas shearwater, wedge-tailed shearwater, and red-tailed tropicbird in the Hawaian Islands, northern Pacific. Environ. Monit. Assess. 1999, 59, 343–358. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Li, R.H. Cyanobacteria toxins in the Salton Sea. Saline Syst. 2006, 2, 5. [Google Scholar] [CrossRef]
- Reifel, K.M.; McCoy, M.P.; Rocke, T.E.; Tiffany, M.A.; Hurlbert, S.H.; Faulkner, D.J. Possible importance of algal toxins in the Salton Sea, California. Hydrobiologia 2002, 473, 275–292. [Google Scholar] [CrossRef]
- Zhang, D.; Xie, P.; Liu, Y.; Qiu, T. Transfer, distribution and bioaccumulation of microcystins in the aquatic food web in Lake Taihu, China, with potential risks to human health. Sci. Total Environ. 2009, 407, 2191–2199. [Google Scholar] [CrossRef]
- Ferrão-Filho, A.D.S.; Kozlowsky-Suzuki, B. Cyanotoxins: Bioaccumulation and effects on aquatic animals. Mar. Drugs 2011, 9, 2729–2772. [Google Scholar] [CrossRef]
- Drobac, D.; Tokodi, N.; Simeunović, J.; Baltić, V.; Stanić, D.; Svirčev, Z. Human exposure to cyanotoxins and their effects on health. Arh. Za Hig. Rada I Toksikol. 2013, 64, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Woller-Skar, M.M.; Jones, D.N.; Luttenton, M.R.; Russell, A.L. Microcystin detected in little brown bats (Myotis lucifugus). Am. Midl. Nat. 2015, 174, 331–334. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc. Natl. Acad. Sci. USA 2003, 100, 13380–13383. [Google Scholar] [CrossRef] [PubMed]
- Stewart, I.; Seawright, A.A.; Shaw, G.R. Cyanobacterial poisoning in livestock, wild mammals and birds—An overview. Adv. Exp. Med. Biol. 2008, 619, 613–637. [Google Scholar] [CrossRef]
- Carmichael, W. A world overview—One-hundred-twenty-seven years of research on toxic cyanobacteria—Where do we go from here? Cyanobacterial Harmful Algal Bloom. State Sci. Res. Needs 2008, 105–125. [Google Scholar]
- Prosser, P.; Nattrass, C.; Prosser, C. Rate of removal of bird carcasses in arable farmland by predators and scavengers. Ecotoxicol. Environ. Saf. 2008, 71, 601–608. [Google Scholar] [CrossRef]
- Guimarães, M.; Doherty, P.F.; Munguía-Steyer, R. Strengthening population inference in herpetofaunal studies by addressing detection probability. South Am. J. Herpetol. 2014, 9, 1–8. [Google Scholar] [CrossRef]
- Kiesecker, J.M.; Blaustein, A.R.; Belden, L.K. Complex causes of amphibian population declines. Nature 2001, 410, 681–684. [Google Scholar] [CrossRef]
- Wang, H.; Xu, C.; Liu, Y.; Jeppesen, E.; Svenning, J.C.; Wu, J.; Zhang, W.; Zhou, T.; Wang, P.; Nangombe, S.; et al. From unusual suspect to serial killer: Cyanotoxins boosted by climate change may jeopardize megafauna. Innovation 2021, 2, 100092. [Google Scholar] [CrossRef]
- Collaboration for Environmental Evidence. Guidelines for systematic review and evidence synthesis in environmental management. Environ. Evid. 2018, 4, 1–82. Available online: https://environmentalevidence.org/wp-content/uploads/2014/06/Review-guidelines-version-4.2-final.pdf (accessed on 14 December 2021).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Wohlin, C. Guidelines for snowballing in systematic literature studies and a replication in software engineering. In Proceedings of the 18th International Conference on Evaluation and Assessment in Software Engineering, London, UK, 13–14 May 2014; pp. 1–10. [Google Scholar] [CrossRef]
- Kaushik, R.; Balasubramanian, R. Methods and approaches used for detection of cyanotoxins in environmental samples: A review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1349–1383. [Google Scholar] [CrossRef]
- Beasley, V.R.; Dahlem, A.M.; Cook, W.O.; Valentine, W.M.; Lovell, R.A.; Hooser, S.B.; Harada, K.I.; Suzuki, M.; Carmichael, W.W. Diagnostic and clinically important aspects of cyanobacterial (blue-green algae) toxicoses. J. Vet. Diagn. Investig. 1989, 1, 359–365. [Google Scholar] [CrossRef] [Green Version]
- Ouellette, A.; Wilhelm, S. Toxic cyanobacteria: The evolving molecular toolbox. Front. Ecol. Environ. 2003, 1, 359–366. [Google Scholar] [CrossRef]
- Sundaravadivelu, D.; Sanan, T.T.; Venkatapathy, R.; Mash, H.; Tettenhorst, D.; DAnglada, L.; Frey, S.; Tatters, A.O.; Lazorchak, J. Determination of Cyanotoxins and Prymnesins in Water, Fish Tissue, and Other Matrices: A Review. Toxins 2022, 14, 213. [Google Scholar] [CrossRef]
- Gaget, V.; Lau, M.; Sendall, B.; Froscio, S.; Humpage, A.R. Cyanotoxins: Which detection technique for an optimum risk assessment? Water Res. 2017, 118, 227–238. [Google Scholar] [CrossRef]
- Sanseverino, I.; António, D.C.; Loos, R.; Lettieri, T. Cyanotoxins: Methods and approaches for their analysis and detection. Centre JR Ed. 2017. [Google Scholar]
- Nicholson, B.C.; Burch, M.D. Evaluation of Analytical Methods for Detection and Quantification of Cyanotoxins in Relation to Australian Drinking Water Guidelines; National Health and Medical Research Council of Australia: Canberra, Australia, 2001; p. 57. [Google Scholar]
- Young, N.; Sharpe, R.A.; Barciela, R.; Nichols, G.; Davidson, K.; Berdalet, E.; Fleming, L.E. Marine harmful algal blooms and human health: A systematic scoping review. Harmful Algae 2020, 98, 101901. [Google Scholar] [CrossRef]
- Backer, L.C.; Miller, M. Sentinel animals in a one health approach to harmful cyanobacterial and algal blooms. Vet. Sci. 2016, 3, 8. [Google Scholar] [CrossRef]
- Wood, R. Acute animal and human poisonings from cyanotoxin exposure—A review of the literature. Environ. Int. 2016, 91, 276–282. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Wickham, H. vggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016; ISBN 978–3-319–24277-4. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation. 2022. Available online: https://dplyr.tidyverse.org (accessed on 14 December 2021).
- Haddaway, N.R.; Feierman, A.; Grainger, M.J.; Gray, C.T.; Tanriver-Ayder, E.; Dhaubanjar, S.; Westgate, M.J. EviAtlas: A tool for visualising evidence synthesis databases. Environ. Evid. 2019, 8, 22. [Google Scholar] [CrossRef]
- O’donoghue, J.G.; Wilton, G.S. Algal poisoning in Alberta. Can. J. Comp. Med. Vet. Sci. 1951, 15, 193. [Google Scholar] [PubMed]
- Neilan, B.A.; Saker, M.L.; Fastner, J.; Törökné, A.; Burns, B. Phylogeography of the invasive cyanobacterium Cylindrospermopsis raciborskii. Mol. Ecol. 2003, 12, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef]
- Sukenik, A.; Hadas, O.; Kaplan, A.; Quesada, A. Invasion of Nostocales (cyanobacteria) to subtropical and temperate freshwater lakes–physiological, regional, and global driving forces. Front. Microbiol. 2012, 3, 86. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, G.; Leunert, F.; Cirés, S.; Jöhnk, K.D.; Rücker, J.; Nixdorf, B.; Wiedner, C. Competitiveness of invasive and native cyanobacteria from temperate freshwaters under various light and temperature conditions. J. Plankton Res. 2010, 32, 1009–1021. [Google Scholar] [CrossRef]
- Kaštovský, J.; Hauer, T.; Mareš, J.; Krautová, M.; Bešta, T.; Komárek, J.; Desortová, B.; Heteša, J.; Hindáková, A.; Houk, V.; et al. A review of the alien and expansive species of freshwater cyanobacteria and algae in the Czech Republic. Biol. Invasions 2010, 12, 3599–3625. [Google Scholar] [CrossRef]
- Litchman, E. Invisible invaders: Non-pathogenic invasive microbes in aquatic and terrestrial ecosystems. Ecol. Lett. 2010, 13, 1560–1572. [Google Scholar] [CrossRef]
- Wilde, S.B.; Johansen, J.R.; Wilde, H.D.; Jiang, P.; Bartelme, B.; Haynie, R.S. Aetokthonos hydrillicola gen. et snov.: Epiphytic cyanobacteria on invasive aquatic plants implicated in Avian Vacuolar Myelinopathy. Phytotaxa 2014, 181, 243–260. [Google Scholar] [CrossRef]
- Kokociński, M.; Akçaalan, R.; Salmaso, N.; Stoyneva-Gärtner Mand Sukenik, A. Expansion of alien and invasive cyanobacteria. Handb. Cyanobacterial Monit. Cyanotoxin Anal. 2016, 28–39. [Google Scholar]
- Kim, I.S.; Park, H.K.; Kim, Y.J. Development of genus-specific PCR primers for molecular monitoring of invasive nostocalean cyanobacteria. Int. J. Environ. Res. Public Health 2021, 18, 5703. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Xiao, P.; Yang, J.R.; Chen, H.; Jin, L.; Yang, Y.; Lin, T.F.; Willis, A.; Yang, J. Precision early detection of invasive and toxic cyanobacteria: A case study of Raphidiopsis raciborskii. Harmful Algae 2021, 110, 102125. [Google Scholar] [CrossRef] [PubMed]
- Wilde, S.B.; Murphy, T.M.; Hope, C.P.; Habrun, S.K.; Kempton, J.; Birrenkott, A.; Wiley, F.; Bowerman, W.W.; Lewitus, A.J. Avian vacuolar myelinopathy linked to exotic aquatic plants and a novel cyanobacterial species. Environ. Toxicol. Int. J. 2005, 20, 348–353. [Google Scholar] [CrossRef]
- Doblin, M.A.; Coyne, K.J.; Rinta-Kanto, J.M.; Wilhelm, S.W.; Dobbs, F.C. Dynamics and short-term survival of toxic cyanobacteria species in ballast water from NOBOB vessels transiting the Great Lakes—Implications for HAB invasions. Harmful Algae 2007, 6, 519–530. [Google Scholar] [CrossRef]
- Wilk-Woźniak, E.; Najberek, K. Towards clarifying the presence of alien algae in inland waters—Can we predict places of their occurrence? Biologia 2013, 68, 838–844. [Google Scholar] [CrossRef]
- Dvořák, P.; Poulíčková, A.; Hašler, P.; Belli, M.; Casamatta, D.A.; Papini, A. Species concepts and speciation factors in cyanobacteria, with connection to the problems of diversity and classification. Biodivers. Conserv. 2015, 24, 739–757. [Google Scholar] [CrossRef]
- Gaysina, L.A.; Johansen, J.R.; Saraf, A.; Allaguvatova, R.Z.; Pal, S.; Singh, P. Roholtiella volcanica sp. nov. A New Species of Cyanobacteria from Kamchatkan Volcanic Soils. Diversity 2022, 14, 620. [Google Scholar] [CrossRef]
- Al-Sammak, M.A.; Hoagland, K.D.; Cassada, D.; Snow, D.D. Co-occurrence of the cyanotoxins BMAA, DABA and anatoxin-a in Nebraska reservoirs, fish, and aquatic plants. Toxins 2014, 6, 488–508. [Google Scholar] [CrossRef]
- Al-Sammak, M.A.; Hoagland, K.D.; Snow, D.D.; Cassada, D. Methods for simultaneous detection of the cyanotoxins BMAA, DABA, and anatoxin-a in environmental samples. Toxicon 2013, 76, 316–325. [Google Scholar] [CrossRef]
- Berny, P. Pesticides and the intoxication of wild animals. J. Vet. Pharmacol. Ther. 2007, 30, 93–679. [Google Scholar] [CrossRef]
- Pain, D.J.; Mateo, R.; Green, R.E. Effects of lead from ammunition on birds and other wildlife: A review and update. Ambio 2019, 48, 935–953. [Google Scholar] [CrossRef] [PubMed]
- Rattner, B.A. History of wildlife toxicology. Ecotoxicology 2009, 18, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Gkelis, S.; Lanaras, T.; Sivonen, K. The presence of microcystins and other cyanobacterial bioactive peptides in aquatic fauna collected from Greek freshwaters. Aquat. Toxicol. 2006, 78, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Ibelings, B.W.; Bruning, K.; De Jonge, J.; Wolfstein, K.; Pires, L.D.; Postma, J.; Burger, T. Distribution of microcystins in a lake foodweb: No evidence for biomagnification. Microb. Ecol. 2005, 49, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Su, X.; Steinman, A.D.; Cai, Y.; Zhao, Y.; Xie, L. Accumulation of microcystins in a dominant Chironomid Larvae (Tanypus chinensis) of a large, shallow and eutrophic Chinese lake, Lake Taihu. Sci. Rep. 2016, 6, 31097. [Google Scholar] [CrossRef] [Green Version]
- Krienitz, L.; Ballot, A.; Kotut, K.; Wiegand, C.; Pütz, S.; Metcalf, J.S.; Codd, G.A.; Stephan, P. Contribution of hot spring cyanobacteria to the mysterious deaths of Lesser Flamingos at Lake Bogoria, Kenya. FEMS Microbiol. Ecol. 2003, 43, 141–148. [Google Scholar] [CrossRef]
- Pennycott, T.; Young, F.M.; Metcalf, J.S.; Codd, G.A. Necrotic enteritis in mute swans associated with cyanobacterial toxins. Vet. Rec. 2004, 154, 575–576. [Google Scholar]
- Krienitz, L.; Ballot, A.; Casper, P.; Codd, G.A.; Kotut, K.; Metcalf, J.S.; Morrison, L.F.; Pflugmacher, S.; Wiegand, C. Contribution of toxic cyanobacteria to massive deaths of Lesser Flamingos at saline-alkaline lakes of Kenya. Int. Ver. Für Theor. Und Angew. Limnol. Verh. 2005, 29, 783–786. [Google Scholar] [CrossRef]
- Matsunaga, H.; Harada, K.I.; Senma, M.; Ito, Y.; Yasuda, N.; Ushida, S.; Kimura, Y. Possible cause of unnatural mass death of wild birds in a pond in Nishinomiya, Japan: Sudden appearance of toxic cyanobacteria. Nat. Toxins 1999, 7, 81–84. [Google Scholar] [CrossRef]
- Ibelings, B.W.; Backer, L.C.; Kardinaal, W.E.A.; Chorus, I. Current approaches to cyanotoxin risk assessment and risk management around the globe. Harmful Algae 2014, 40, 63–74. [Google Scholar] [CrossRef]
- Nasri, H.; El Herry, S.; Bouaïcha, N. First reported case of turtle deaths during a toxic Microcystis s bloom in Lake Oubeira, Algeria. Ecotoxicol. Environ. Saf. 2008, 71, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Kakuschke, A.; Prange, A. The influence of metal pollution on the immune system a potential stressor for marine mammals in the North Sea. Int. J. Comp. Psychol. 2007, 20, 179–193. [Google Scholar]
- Gilberston, M.K. Immuno suppression in the Northern Leopard Frog (Rana pipens) induced by pesticide exposure. Environ. Toxicol. Chem. 2003, 22, 101–110. [Google Scholar] [CrossRef]
- Sanderfoot, O.V.; Holloway, T. Air pollution impacts on avian species via inhalation exposure and associated outcomes. Environ. Res. Lett. 2017, 12, 083002. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Morrison, L.F.; Krienitz, L.; Ballot, A.; Krause, E.; Kotut, K.; Pütz, S.; Wiegand, C.; Pflugmacher, S.; Codd, G.A. Analysis of the cyanotoxins anatoxin-a and microcystins in Lesser Flamingo feathers. Toxicol. Environ. Chem. 2006, 88, 159–167. [Google Scholar] [CrossRef]
- Nehring, S. Mortality of dogs associated with a mass development of Nodularia spumigena (Cyanophyceae) in a brackish lake at the German North Sea coast. J. Plankton Res. 1993, 15, 867–872. [Google Scholar] [CrossRef]
- Halderen, A.; Rharding, W.; Wessels, J.C.; Schneider, D.; Pheine, E.; Van, J.; Merwe, D.; Fourie, J. Cyanobacterial (blue-green algae) poisoning of livestock. S. Afr. Vet. Ver. 1995, 66, 260–264. [Google Scholar]
- Ryser-Degiorgis, M. Wildlife health investigations: Needs, challenges and recommendations. BMC Vet. Res. 2013, 9, 223. [Google Scholar] [CrossRef] [Green Version]
- Brookes, J.; Burch, M.; Grützmacher, G.; Klitzke, S. Managing cyanotoxin risks at the drinking-water offtake. In Toxic Cyanobacteria in Water; Taylor & Francis: Oxford, UK, 2021; p. 563. [Google Scholar]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Taylor & Francis: Oxford, UK, 2021; p. 858. [Google Scholar]
- Ho, J.C.; Michalak, A.M. Challenges in tracking harmful algal blooms: A synthesis of evidence from Lake Erie. J. Great Lakes Res. 2015, 41, 317–325. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, D.; Xie, P.; Wang, Q.; Ma, Z. Simultaneous determination of microcystin contaminations in various vertebrates (fish, turtle, duck and water bird) from a large eutrophic Chinese lake, Lake Taihu, with toxic Microcystis blooms. Sci. Total Environ. 2009, 407, 3317–3322. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J. Current techniques for detecting and monitoring algal toxins and causative harmful algal blooms. J. Environ. Anal. Chem. 2015, 2, 2380–2391. [Google Scholar] [CrossRef]
- Baker, L.; Sendall, B.C.; Gasser, R.B.; Menjivar, T.; Neilan, B.A.; Jex, A.R. Rapid, multiplex-tandem PCR assay for automated detection and differentiation of toxigenic cyanobacterial blooms. Mol. Cell. Probes 2013, 27, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, F.F.; Madramootoo, C.A.; Jabaji, S. Development and application of a multiplex qPCR technique to detect multiple microcystin-producing cyanobacterial genera in a Canadian freshwater lake. J. Appl. Phycol. 2014, 26, 1675–1726. [Google Scholar] [CrossRef]
- Pacheco, A.B.F.; Guedes, I.A.; Azevedo, S.M. Is qPCR a reliable indicator of cyanotoxin risk in freshwater? Toxins 2016, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (EPA). Available online: https://www.epa.gov/ground-water-and-drinking-water/detection-methods-cyanotoxins (accessed on 16 August 2022).
- Lawson, B.; Neimanis, A.; Lavazza, A.; López-Olvera, J.R.; Tavernier, P.; Billinis, C.; Duff, J.P.; Mladenov, D.T.; Rijks, J.M.; Savić, S.; et al. How to start up a national wildlife health surveillance programme. Animals 2021, 11, 2543. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D. HABs in a changing world: A perspective on harmful algal blooms, their impacts, and research and management in a dynamic era of climactic and environmental change. In Harmful algae 2012: Proceedings of the 15th International Conference on Harmful Algae: 29 October–2 November 2012, CECO, Changwon, Gyeongnam, Korea; Hak Gyoon, K., Beatriz, R., Gustaaf, M., Hallegraeff, C., Kyu Lee, M., Eds.; NIH Public Access: Bethesda, MD, USA, 2012; Volume 3. [Google Scholar]
- Tebbs, E.J.; Remedios, J.J.; Harper, D.M. Remote sensing of chlorophyll-a as a measure of cyanobacterial biomass in Lake Bogoria, a hypertrophic, saline-alkaline, flamingo lake, using Landsat EMT+. Remote Sens. 2013, 135, 92–106. [Google Scholar] [CrossRef]
- Carvalho, G.A.; Minnett, P.J.; Fleming, L.E.; Banzon, V.F.; Baringer, W. Satellite remote sensing of harmful algal blooms: A new multi-algorithm method for detecting the Florida Red Tide (Karenia brevis). Harmful Algae 2010, 9, 440–448. [Google Scholar] [CrossRef]
- Backer, L.C.; Manassaram-Baptiste, D.; LePrell, R.; Bolton, B. Cyanobacteria and algae blooms: Review of health and environmental data from the harmful algal bloom-related illness surveillance system (HABISS) 2007–2011. Toxins 2015, 7, 1048–1064. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.M.; Schaeffer, B.A.; Darling, J.A.; Urquhart, E.A.; Johnston, J.M.; Ignatius, A.R.; Myer, M.H.; Loftin, K.A.; Werdell, P.J.; Stumpf, R. Satellite monitoring of cyanobacterial harmful algal bloom frequency in recreational waters and drinking water sources. Ecol. Indic. 2017, 80, 84–95. [Google Scholar] [CrossRef]
- Binding, C.E.; Greenberg, T.A.; McCullough, G.; Watson, S.B.; Page, E. An analysis of satellite-derived chlorophyll and algal bloom indices on Lake Winnipeg. J. Great Lakes Res. 2018, 44, 436–446. [Google Scholar] [CrossRef]
- Alcantara, E.; Coimbra, K.; Ogashawara, I.; Rodrigues, T.; Mantovani, J.; Rotta, L.H.; Park, E.; Cunha, D.G.F. A satellite-based investigation into the algae bloom variability in large water supply urban reservoirs during COVID-19 lockdown. Remote Sens. Appl. Soc. Environ. 2021, 23, 100555. [Google Scholar] [CrossRef]
- Legleiter, C.J.; King, T.V.; Carpenter, K.D.; Hall, N.C.; Mumford, A.C.; Slonecker, T.; Graham, J.L.; Stengel, V.G.; Simon, N.; Rosen, B.H. Spectral mixture analysis for surveillance of harmful algal blooms (SMASH): A field-, laboratory-, and satellite-based approach to identifying cyanobacteria genera from remotely sensed data. Remote Sens. Environ. 2022, 279, 113089. [Google Scholar] [CrossRef]
- Li, X.; Savkin, A.V. Networked unmanned aerial vehicles for surveillance and monitoring: A survey. Future Internet 2021, 13, 174. [Google Scholar] [CrossRef]
- Trout-Haney, J.V.; Cottingham, K.L. Microcystins in planktonic and benthic food web components from Greenlandic lakes. Ecosphere 2021, 12, e03539. [Google Scholar] [CrossRef]
- Foss, A.J.; Aubel, M.T.; Gallagher, B.; Mettee, N.; Miller, A.; Fogelson, S.B. Diagnosing Microcystin Intoxication of Canines: Clinicopathological Indications, Pathological Characteristics, and Analytical Detection in Postmortem and Antemortem Samples. Toxins 2019, 11, 456. [Google Scholar] [CrossRef]
- Kotut, K.; Krienitz, L. Does the potentially toxic cyanobacterium Microcystis exist in the soda lakes of East Africa? Hydrobiologia 2011, 664, 219–225. [Google Scholar] [CrossRef]
- Papadimitriou, T.; Katsiapi, M.; Vlachopoulos, K.; Christopoulos, A.; Laspidou, C.; Moustaka-Gouni, M.; Kormas, K. Cyanotoxins as the “common suspects” for the Dalmatian pelican (Pelecanus crispus) deaths in a Mediterranean reconstructed reservoir. Environ. Pollut. 2018, 234, 779–787. [Google Scholar] [CrossRef]
- Bengis, R.; Govender, D.; Lane, E.; Myburgh, J.; Oberholster, P.; Buss, P.; Prozesky, L.; Keet, D. Eco-epidemiological and pathological features of wildlife mortality events related to cyanobacterial bio-intoxication in the Kruger National Park, South Africa. J. S. Afr. Vet. Assoc. 2016, 87, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Oberholster, P.J.; Myburgh, J.G.; Govender, D.; Bengis, R.; Botha, A.M. Identification of toxigenic Microcystis strains after incidents of wild animal mortalities in the Kruger National Park, South Africa. Ecotoxicol. Environ. Saf. 2009, 72, 1177–1182. [Google Scholar] [CrossRef]
- Amano, T.; González-Varo, J.P.; Sutherland, W.J. Languages Are Still a Major Barrier to Global Science. PLoS Biol. 2016, 14, e2000933. [Google Scholar] [CrossRef]
| Category | Coding Variable | Description |
|---|---|---|
| Meta-data | Reference | Author, date, title, and publisher |
| Author affiliation | What organisation the authors are affiliated with (veterinary institute, university, government, conservation organisation, etc.) | |
| Date of publication | Year of publication | |
| Date of poisoning event/study | Year of event | |
| Study type | Experimental, observational, survey, review, etc. | |
| Location of study | Country and continent of study | |
| Outcome | The outcome resulted in death or recovery | |
| Case type | Chronic or acute intoxication | |
| Methods of reporting and diagnosis | Cyanobacterial species | Species of cyanobacteria involved in event |
| Toxin | Toxin involved in event | |
| Identification of toxin and cyanobacterial species | Investigative chemistry techniques used to identify cyanobacterial species and toxin (high-performance liquid chromatography, enzyme-linked immunosorbent assay, protein phosphatase inhibition assay, bioassay, mass spectrometry, etc.) | |
| Presence of a bloom | Was a cyanobacterial bloom present? (yes/no) | |
| Environmental conditions | The environmental conditions present (hot and dry, windy, prolonged sunshine, etc.) | |
| Bloom frequency | The frequency in which blooms are occurring | |
| Environmental contaminants | The environmental contaminants present at time of poisoning (sewage, fertiliser, heavy metals, pesticides, etc.) | |
| Diagnosis | Was an official diagnosis made in the case? (yes/no) | |
| Diagnostic methods used | Types of methods used to reach a diagnosis (post-mortem examination, histopathology, blood chemistry, clinical signs, stomach/faecal contents, etc.) | |
| Recommended method of investigation | Did the investigation follow our recommended method? (yes/no) | |
| Exposure pathway | How was the animal exposed to the toxin (ingestion, inhalation, transplacental transfer, transdermal uptake, etc.)? | |
| Number of carcasses | Number of carcasses discovered post-poisoning event | |
| Animal species | Animal species involved in the poisoning event | |
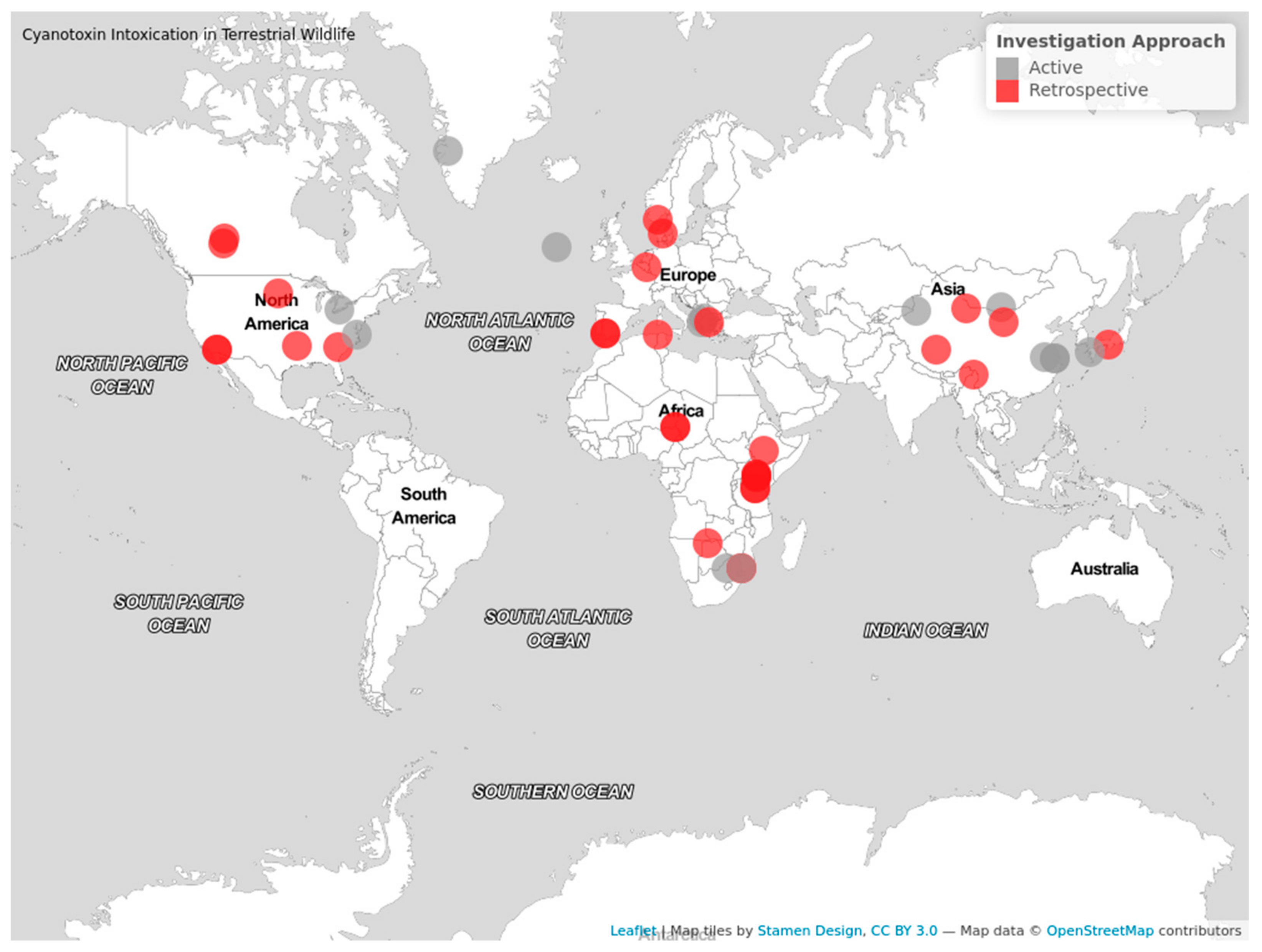
| Investigation approach | Was the investigation active or retrospective? | |
| Mitigation | What mitigation method was employed after or during the event (treatment, water treatment, monitoring, moving animals, etc.)? |
| Study Characteristic | Narrative Summary 1 |
|---|---|
| Study type | The majority of papers were case reports, encompassing 71.1% of all included papers. Field observations, notes, surveillance studies, and epidemiological studies made up 15.4%, 4.4%, 4.4%, and 4.4% of included papers, respectively. |
| Location of incidents | North America, Africa, and Europe were the continents where cases were reported the most, contributing to 31.1%, 28.9%, and 22.2%, respectively. Cases in South Africa contributed to 30.8% of all African cases. The lowest number of cases occurred within Asia, contributing to 17.8% of cases. No cases of terrestrial wildlife were reported from Australasia or South America. |
| Presence of a harmful cyanobacterial bloom | Harmful cyanobacterial blooms were present in 64.4% of cases. When a bloom was present, the cause of the bloom was unknown in 51.7% of cases. Benthic cyanobacterial mats were also present in a number of cases and were the cause of intoxication in 8.9% of cases, all of which were lesser flamingo Phoeniconaias minor mortalities. |
| Cyanotoxin | Microcystin equivalents accounted for 64.4% of all cases included in this review. Of these, 56.9% were able to be identified to the variant level, which included MC-LR a, MC-RR b, MC-YR c, MC-LF d, and MC-LA e, with MC-LR being the most common. Anatoxin-a accounted for 8.9% of all cases, and guanitoxin accounted for 2.2% of cases. Other toxins contributing to mortality, morbidity, or bioaccumulation accounted for 20.0% together, which included BMAA f, Nodularin, DAB g, and anabaenopeptides. Toxins were unknown in 13.3% of cases. |
| Cyanobacterial species | Microcystis spp. were the most prevalent freshwater cyanobacterial species at 40.0%. Alarmingly, 28.9% of cases were unable to identify the cyanobacterial species responsible. Dolichospermum spp. contributed to the second largest number of cases at 22.2%. Oscillatoria spp. were present in 13.3% of cases, and Aphanizomenon spp. were present in 6.7%. Cylindrospermopsis spp., Arthrospira fusiformis, Nostoc spp. and species in the order Stigonematales each contributed to 4.4% of cases. The remaining species encompassed cyanobacterial species, dinoflagellates, and raphidophytes, some of which were marine algal species. These remaining species were all only present in one case each and included Plankothrix spp., Karenia brevis, Synechoccocus sp., Pleurochrysis pseudoroscoffensis, Chattonella marina, Gyrodinium uncatenum, Protoceratium reticulatum, Prorocentrum minimum, Phormidium terebriformis, Synechococcus bigranulatus, Spirulina subsalsa, and an unknown species of the order Chroococcales. |
| Taxonomic groups of affected species | The class Aves made up the majority of cases at 48.9% of all cases, followed by Mammalia with 33.3%. Insecta, Reptilia, and Amphibia were present in only 11.1%, 8.9%, and 6.6% of cases, respectively. The order Anseriformes accounted for the most cases, present in 17.8% of poisonings. Carnivora and Artiodactyla from class Mammalia were involved in a number of cases, both present in 13.3% of cases. The order Phoenicopteriformes from class Aves also contributed to a large total with 13.3% of cases. Many other orders from classes Mammalia and Aves were involved in cyanobacterial poisonings, each included in one to five cases. Testudines constituted four out of the five cases involving reptilians. Additionally, both chronic and acute cyanotoxin poisonings were detected within Chiroptera, contributing to a total of 4 (8.9%) cases. |
| Environmental contaminants | Pesticides and heavy metals were both present in 8.9% of all cases and always occurred in the presence of each other. Sewage was present in 4.4% of all cases, and fertiliser was present in 2.2% of all cases. Only one case existed where environmental contaminants were known to have contributed to the case. |
| Mitigation measures | Mitigation measures were implemented in only 9 (20.0% of all cases) cases, and no investigations had mitigation in place already. Of the 9 studies implementing mitigation, 4 were successful, 2 were unsuccessful, and the outcome of mitigation was unknown in 3. Mitigation measures were not implemented or mentioned in 80.0% of all cases. Treatment and management of water was the most common mitigation measure, mentioned in 8.9% of all cases. The movement and translocation of animals and habitat management each occurred in 4.4% of all cases. Surveillance as an outcome was only reported in one case. Education and veterinary treatment were not reported in any of these wildlife reports. |
| Method | Investigative Technique | Count (%) |
|---|---|---|
| Clinical diagnostic method | PME | 19(42.2) |
| Tissue sample (toxin identification) | 17(37.8) | |
| Histopathology | 10(22.2) | |
| Gut/stomach/faecal contents | 10(22.2) | |
| Clinical signs | 5(11.1) | |
| Blood sample | 2(4.4) | |
| Identification of cyanobacterial species | Microscopy | 19(42.2) |
| PCR | 3(6.7) | |
| Toxin presence and concentration | ELISA | 18(40.0) |
| HPLC (and multiple different detectors) | 17(37.8) | |
| Bioassay (cow, mouse, catfish hepatocyte, or brine shrimp) | 12(26.7) | |
| Mass spectrometry | 11(24.4) | |
| Protein phosphatase inhibition assay | 7(15.6) | |
| Cholinesterase inhibition assay | 1(2.2) | |
| HNMR a | 1(2.2) | |
| TLC b | 1(2.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ash, A.K.; Patterson, S. Reporting of Freshwater Cyanobacterial Poisoning in Terrestrial Wildlife: A Systematic Map. Animals 2022, 12, 2423. https://doi.org/10.3390/ani12182423
Ash AK, Patterson S. Reporting of Freshwater Cyanobacterial Poisoning in Terrestrial Wildlife: A Systematic Map. Animals. 2022; 12(18):2423. https://doi.org/10.3390/ani12182423
Chicago/Turabian StyleAsh, Alexandra K., and Stuart Patterson. 2022. "Reporting of Freshwater Cyanobacterial Poisoning in Terrestrial Wildlife: A Systematic Map" Animals 12, no. 18: 2423. https://doi.org/10.3390/ani12182423
APA StyleAsh, A. K., & Patterson, S. (2022). Reporting of Freshwater Cyanobacterial Poisoning in Terrestrial Wildlife: A Systematic Map. Animals, 12(18), 2423. https://doi.org/10.3390/ani12182423






