Effects of Three Feed Additives on the Culturable Microbiota Composition and Histology of the Anterior and Posterior Intestines of Zebrafish (Danio rerio)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Conditions
2.2. Feed Preparation
2.3. Sampling, Bacterial Isolation, and Identification
2.4. Semi-Quantitative Assessment of Bacterial Abundance
2.5. Histology
2.6. Statistical Analysis
3. Results
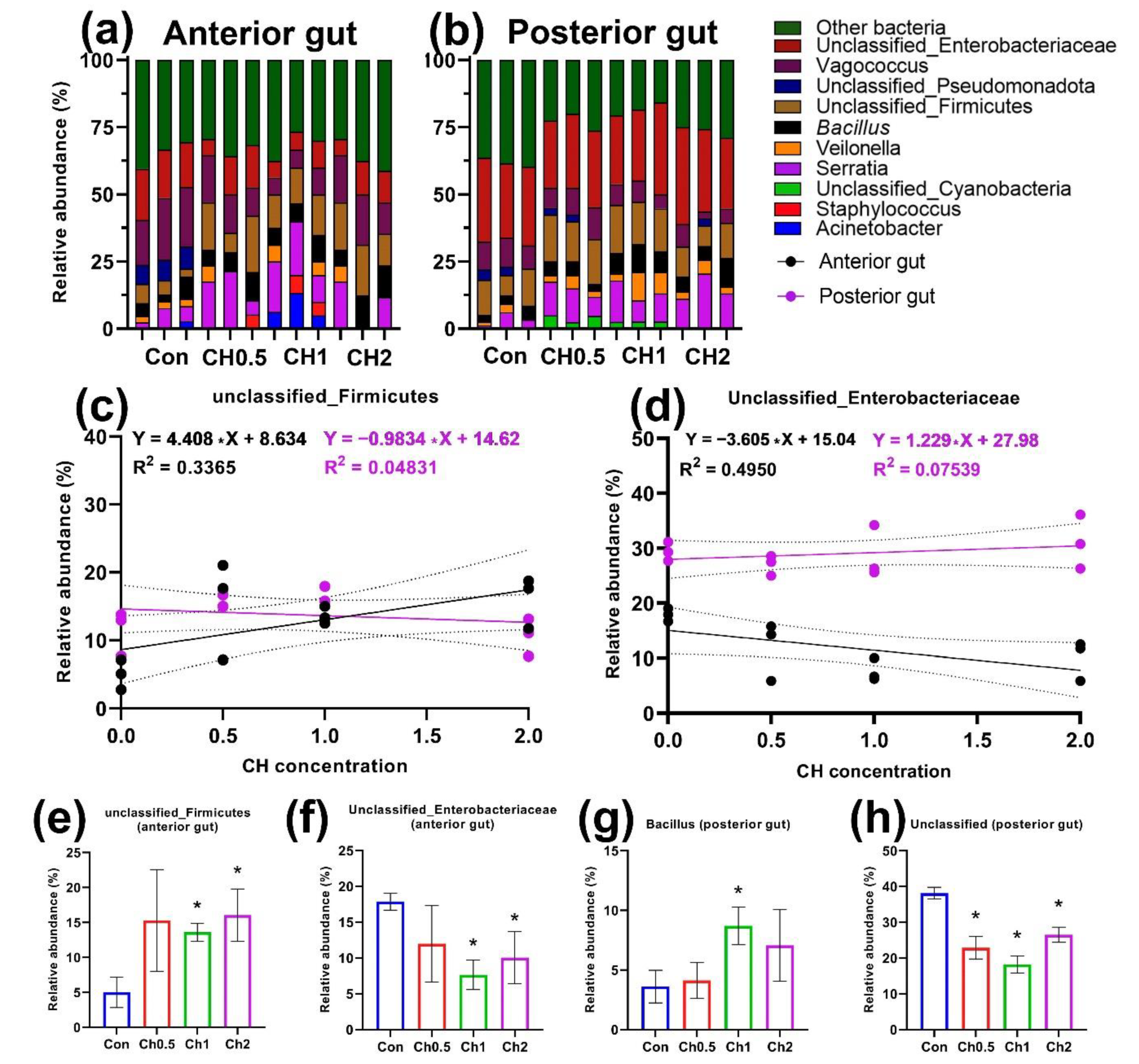
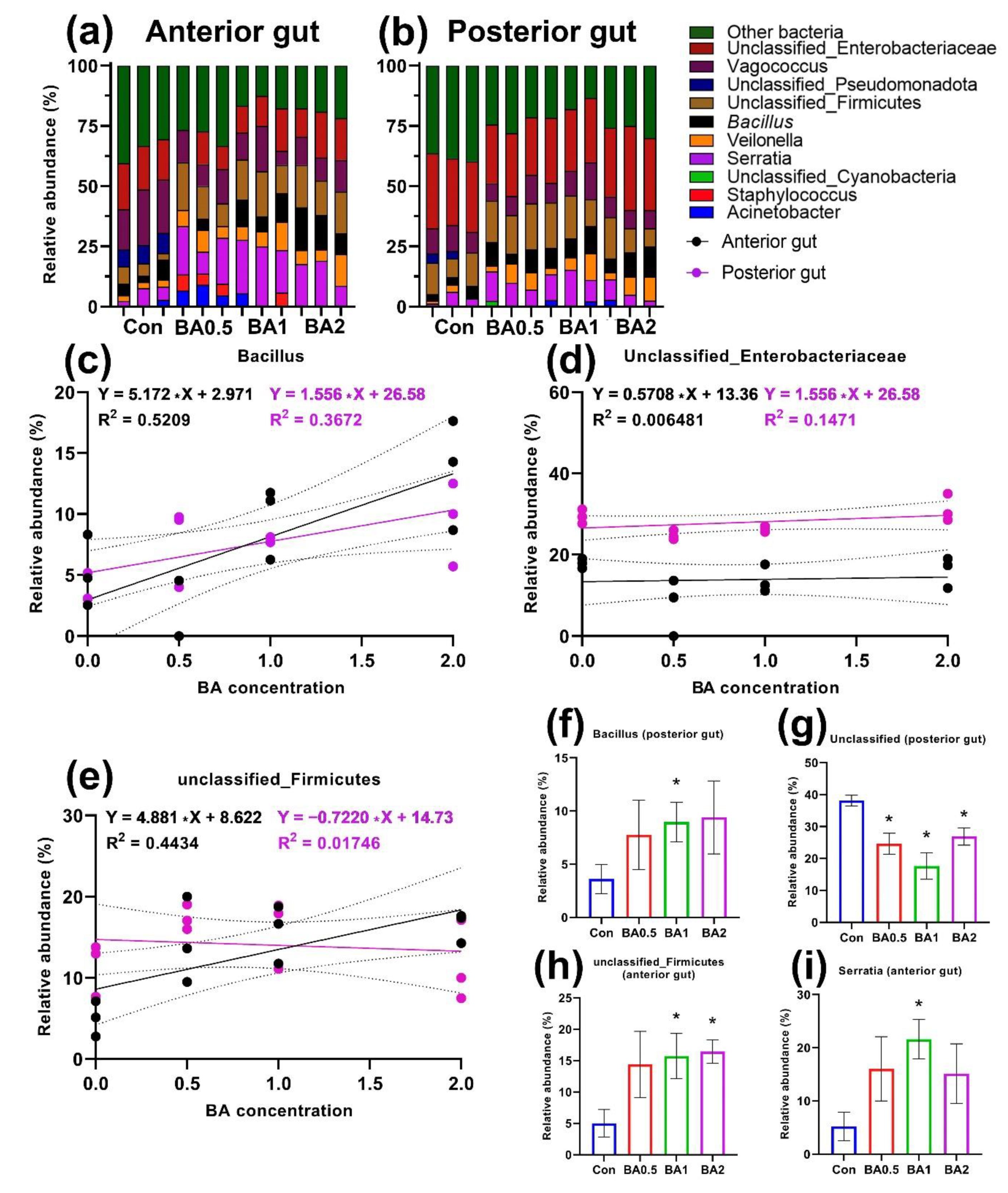
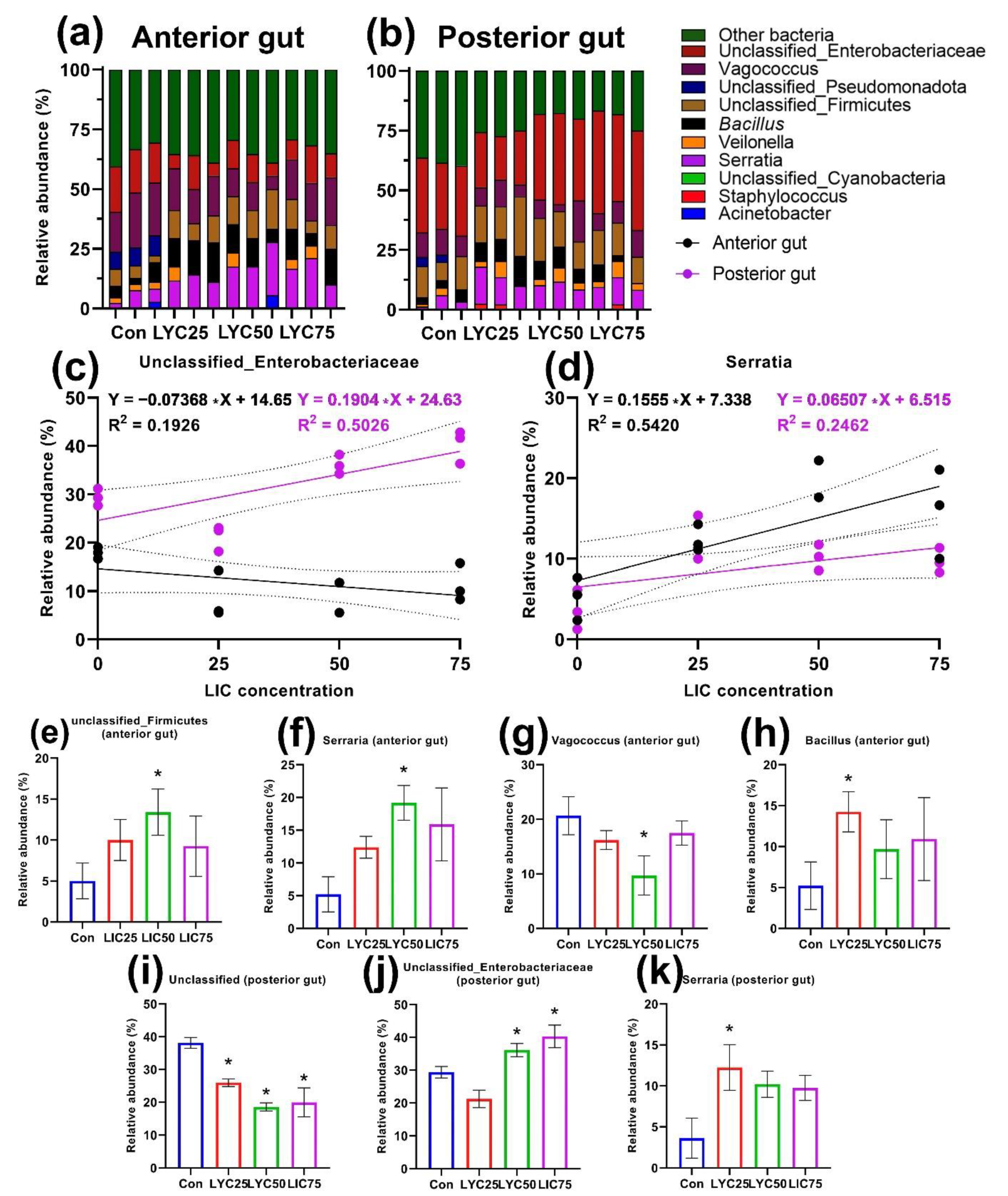
3.1. Cultured Intestinal Microbiota
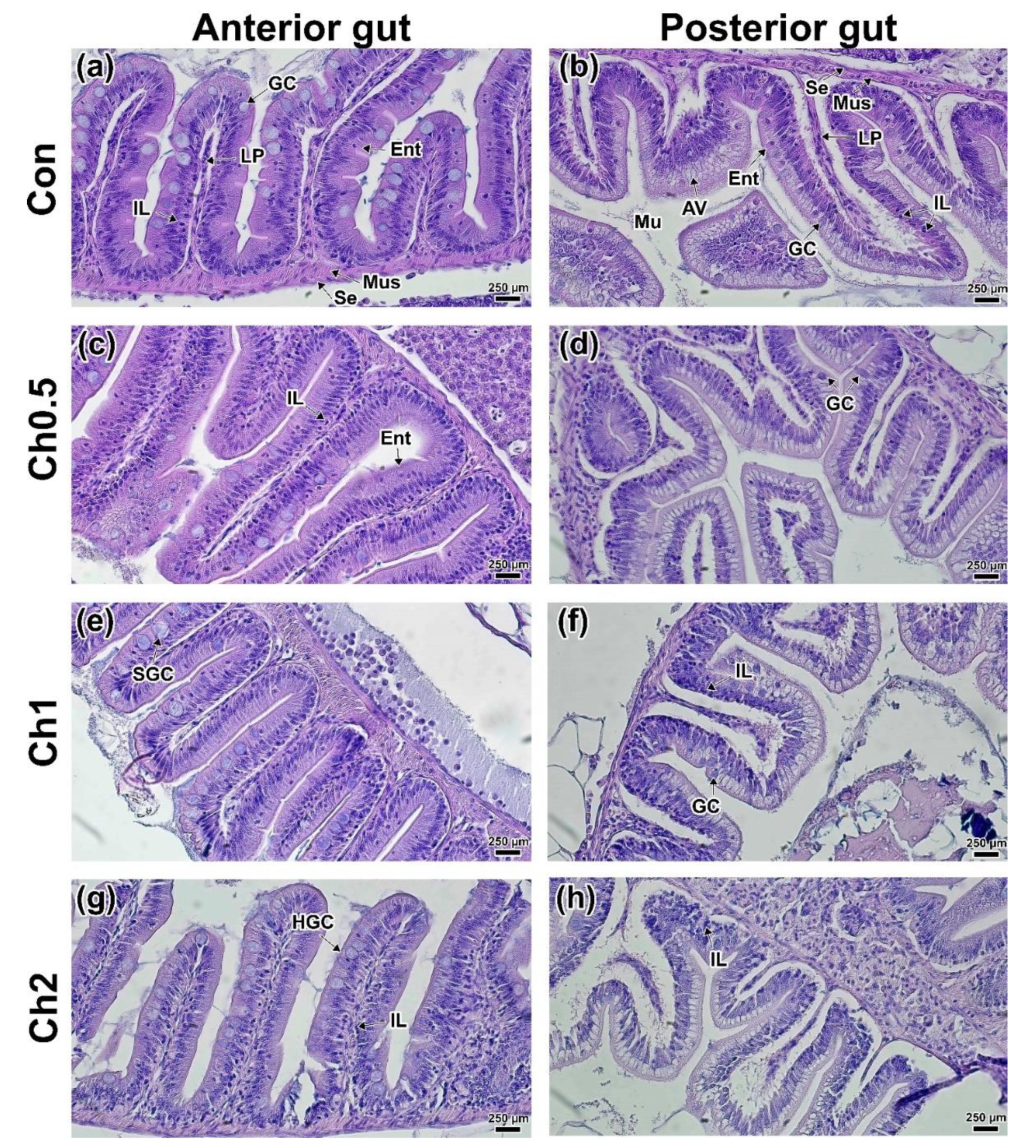
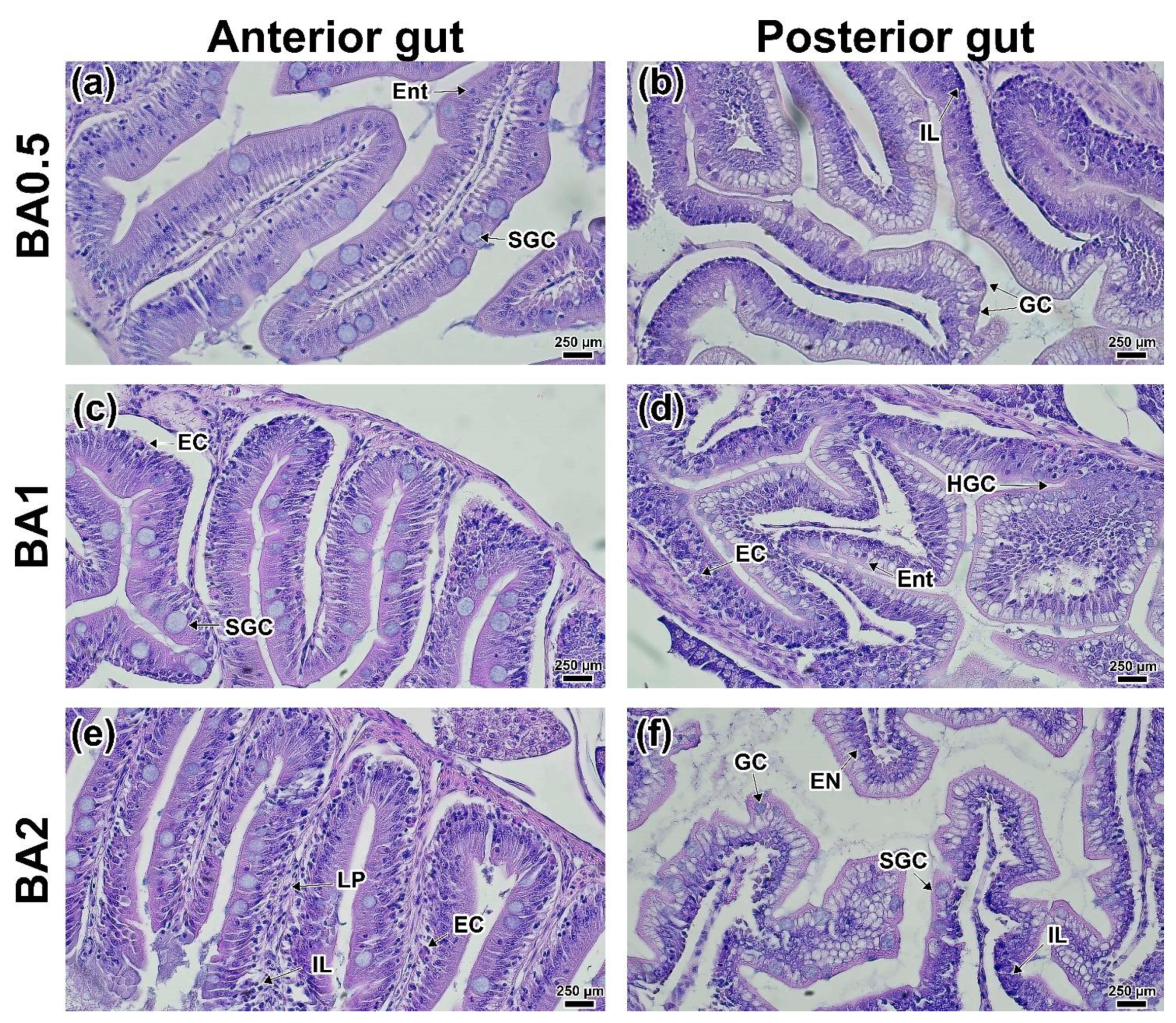
3.2. Intestinal Histology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bharathi, S.; Antony, C.; Rajagopalasamy, C. Functional Feed Additives Used in Fish Feeds. Int. J. Fish. Aquat. Stud. 2019, 7, 44–52. [Google Scholar]
- Ogunkalu, O. Effects of Feed Additives in Fish Feed for Improvement of Aquaculture. Eurasian J. Food Sci. Technol. 2019, 3, 49–57. [Google Scholar]
- Dimitroglou, A.; Merrifield, D.L.; Carnevali, O.; Picchietti, S.; Avella, M.; Daniels, C.; Güroy, D.; Davies, S.J. Microbial Manipulations to Improve Fish Health and Production—A Mediterranean Perspective. Fish Shellfish Immunol. 2011, 30, 1–16. [Google Scholar] [CrossRef]
- Bulfon, C.; Volpatti, D.; Galeotti, M. Current Research on the Use of Plant-Derived Products in Farmed Fish. Aquac. Res. 2013, 46, 513–551. [Google Scholar] [CrossRef]
- Villasante, A.; Patro, B.; Chew, B.; Becerra, M.; Wacyk, J.; Overturf, K.; Powell, M.S.; Hardy, R.W. Dietary Intake of Purple Corn Extract Reduces Fat Body Content and Improves Antioxidant Capacity and N-3 Polyunsaturated Fatty Acid Profile in Plasma of Rainbow Trout, Oncorhynchus mykiss. J. World Aquac. Soc. 2015, 46, 381–394. [Google Scholar] [CrossRef]
- Steiner, T. Phytogenics in Animal Nutrition: Natural Concepts to Optimize Gut Health and Performance; Nottingham University Press: Nottingham, UK, 2009. [Google Scholar]
- Lall, S.P.; Kaushik, S.J. Nutrition and Metabolism of Minerals in Fish. Animals 2021, 11, 2711. [Google Scholar] [CrossRef]
- Torno, C.; Staats, S.; Rimbach, G.; Schulz, C. Effects of Resveratrol and Genistein on Nutrient Digestibility and Intestinal Histopathology of Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2018, 491, 114–120. [Google Scholar] [CrossRef]
- Reda, R.M.; Selim, K.M. Evaluation of Bacillus amyloliquefaciens on the Growth Performance, Intestinal Morphology, Hematology and Body Composition of Nile tilapia, Oreochromis niloticus. Aquac. Int. 2014, 23, 203–217. [Google Scholar] [CrossRef]
- Guerreiro, I.; Couto, A.; Pérez-Jiménez, A.; Oliva-Teles, A.; Enes, P. Gut Morphology and Hepatic Oxidative Status of European Sea Bass (Dicentrarchus labrax) Juveniles Fed Plant Feedstuffs or Fishmeal-Based Diets Supplemented with Short-Chain Fructo-Oligosaccharides and Xylo-Oligosaccharides. Br. J. Nutr. 2015, 114, 1975–1984. [Google Scholar] [CrossRef]
- Brum, A.; Cardoso, L.; Chagas, E.C.; Chaves, F.C.M.; Mouriño, J.L.P.; Martins, M.L. Histological Changes in Nile tilapia Fed Essential Oils of Clove Basil and Ginger after Challenge with Streptococcus agalactiae. Aquaculture 2018, 490, 98–107. [Google Scholar] [CrossRef]
- Nguyen, C.D.H.; Amoroso, G.; Ventura, T.; Elizur, A. Assessing the Pyloric Caeca and Distal Gut Microbiota Correlation with Flesh Color in Atlantic Salmon (Salmo salar L., 1758). Microorganisms 2020, 8, 1244. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.R.; Ran, C.; Ringø, E.; Zhou, Z.G. Progress in Fish Gastrointestinal Microbiota Research. Rev. Aquac. 2017, 10, 626–640. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the Microbiota and the Immune System. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Zarkasi, K.Z.; Taylor, R.S.; Abell, G.C.J.; Tamplin, M.L.; Glencross, B.D.; Bowman, J.P. Atlantic Salmon (Salmo salar L.) Gastrointestinal Microbial Community Dynamics in Relation to Digesta Properties and Diet. Microb. Ecol. 2016, 71, 589–603. [Google Scholar] [CrossRef]
- Petra, A.I.; Panagiotidou, S.; Hatziagelaki, E.; Stewart, J.M.; Conti, P.; Theoharides, T.C. Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders with Suspected Immune Dysregulation. Clin. Ther. 2015, 37, 984–995. [Google Scholar] [CrossRef]
- Navarro-Barrón, E.; Hernández, C.; Llera-Herrera, R.; García-Gasca, A.; Gómez-Gil, B. Overfeeding a High-Fat Diet Promotes Sex-Specific Alterations on the Gut Microbiota of the Zebrafish (Danio rerio). Zebrafish 2019, 16, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-T.; Zou, S.-S.; Zhai, L.-J.; Wang, Y.; Zhang, F.-M.; An, L.-G.; Yang, G.-W. Pathogen Invasion Changes the Intestinal Microbiota Composition and Induces Innate Immune Responses in the Zebrafish Intestine. Fish Shellfish Immunol. 2017, 71, 35–42. [Google Scholar] [CrossRef]
- Castañeda-Monsalve, V.A.; Junca, H.; García-Bonilla, E.; Montoya-Campuzano, O.I.; Moreno-Herrera, C.X. Characterization of the Gastrointestinal Bacterial Microbiome of Farmed Juvenile and Adult White Cachama (Piaractus brachypomus). Aquaculture 2019, 512, 734325. [Google Scholar] [CrossRef]
- Sullam, K.E.; Essinger, S.D.; Lozupone, C.A.; O’connor, M.P.; Rosen, G.L.; Knight, R.; Kilham, S.S.; Russell, J.A. Environmental and Ecological Factors That Shape the Gut Bacterial Communities of Fish: A Meta-Analysis. Mol. Ecol. 2012, 21, 3363–3378. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, S.; Zeng, Z.; Fu, Z. Effects of Environmental Pollutants on Gut Microbiota. Environ. Pollut. 2017, 222, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cantas, L.; Sørby, J.R.T.; Aleström, P.; Sørum, H. Culturable Gut Microbiota Diversity in Zebrafish. Zebrafish 2012, 9, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Esteban, M.Á.; Cuesta, A.; Sun, Y.-Z. Prebiotics and Fish Immune Response: A Review of Current Knowledge and Future Perspectives. Rev. Fish. Sci. Aquac. 2015, 23, 315–328. [Google Scholar] [CrossRef]
- Martínez Cruz, P.; Ibáñez, A.L.; Monroy Hermosillo, O.A.; Ramírez Saad, H.C. Use of Probiotics in Aquaculture. ISRN Microbiol. 2012, 2012, 916845. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.; Neiger, R.D.; Brown, M.L. Gut Histology, Immunology and the Intestinal Microbiota of Rainbow Trout, Oncorhynchus mykiss. Aquac. Res. 2018, 49, 492–504. [Google Scholar] [CrossRef]
- González-Félix, M.L.; Gatlin, D.M.; Urquidez-Bejarano, P.; de la Reé-Rodríguez, C.; Duarte-Rodríguez, L.; Sánchez, F.; Casas-Reyes, A.; Yamamoto, F.Y.; Ochoa-Leyva, A.; Perez-Velazquez, M. Effects of Commercial Dietary Prebiotic and Probiotic Supplements on Growth, Innate Immune Responses, and Intestinal Microbiota and Histology of Totoaba macdonaldi. Aquaculture 2018, 491, 239–251. [Google Scholar] [CrossRef]
- Wang, Y.; Abdullah; Zhong, H.; Wang, J.; Feng, F. Dietary Glycerol Monolaurate Improved the Growth, Activity of Digestive Enzymes and Gut Microbiota in Zebrafish (Danio rerio). Aquac. Rep. 2021, 20, 100670. [Google Scholar] [CrossRef]
- Patula, S.; Wojno, M.; Pinnell, L.J.; Oliaro, F.; Cabay, C.; Molinari, G.S.; Kwasek, K. Nutritional Programming with Dietary Soybean Meal and Its Effect on Gut Microbiota in Zebrafish (Danio rerio). Zebrafish 2021, 18, 125–138. [Google Scholar] [CrossRef]
- Reveco, F.E.; Øverland, M.; Romarheim, O.H.; Mydland, L.T. Intestinal Bacterial Community Structure Differs between Healthy and Inflamed Intestines in Atlantic Salmon (Salmo salar L.). Aquaculture 2014, 420–421, 262–269. [Google Scholar] [CrossRef]
- Ingerslev, H.-C.; Strube, M.L.; von Gersdorff Jørgensen, L.; Dalsgaard, I.; Boye, M.; Madsen, L. Diet Type Dictates the Gut Microbiota and the Immune Response against Yersinia ruckeri in Rainbow Trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2014, 40, 624–633. [Google Scholar] [CrossRef]
- Ingerslev, H.-C.; von Gersdorff Jørgensen, L.; Lenz Strube, M.; Larsen, N.; Dalsgaard, I.; Boye, M.; Madsen, L. The Development of the Gut Microbiota in Rainbow Trout (Oncorhynchus mykiss) Is Affected by First Feeding and Diet Type. Aquaculture 2014, 424–425, 24–34. [Google Scholar] [CrossRef]
- Arballo, J.; Amengual, J.; Erdman, J.W. Lycopene: A Critical Review of Digestion, Absorption, Metabolism, and Excretion. Antioxidants 2021, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, L.; He, S.; Ren, H.-L.; Zhou, N.; Hu, Z.-M. Lycopene Alleviates Disc Degeneration under Oxidative Stress through the Nrf2 Signaling Pathway. Mol. Cell. Probes 2020, 51, 101559. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The Importance of Minerals in Human Nutrition: Bioavailability, Food Fortification, Processing Effects and Nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Walters, M.; Esfandi, R.; Tsopmo, A. Potential of Food Hydrolyzed Proteins and Peptides to Chelate Iron or Calcium and Enhance Their Absorption. Foods 2018, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Y.; Zhao, X.; Liu, C.; Wang, B.; Zhou, J. Mechanistic Basis and Preliminary Practice of Butyric Acid and Butyrate Sodium to Mitigate Gut Inflammatory Diseases: A Comprehensive Review. Nutr. Res. 2021, 95, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ohira, H.; Tsutsui, W.; Fujioka, Y. Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis? J. Atheroscler. Thromb. 2017, 24, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, M.; Wawrzyniak, D.; Rolle, K.; Chomczyński, P.; Oziewicz, S.; Jurga, S.; Barciszewski, J. Let Food Be Your Medicine: Nutraceutical properties of lycopene. Food Funct. 2019, 10, 3090–3102. [Google Scholar] [CrossRef]
- Petyaev, I.M. Lycopene Deficiency in Ageing and Cardiovascular Disease. Oxidative Med. Cell. Longev. 2016, 2016, 3218605. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Abdel-Tawwab, M.; Abdel-Latif, H.M.R. Lycopene Reduces the Impacts of Aquatic Environmental Pollutants and Physical Stressors in Fish. Rev. Aquac. 2020, 12, 2511–2526. [Google Scholar] [CrossRef]
- Seth, A.; Stemple, D.L.; Barroso, I. The Emerging Use of Zebrafish to Model Metabolic Disease. Dis. Models Mech. 2013, 6, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- López Nadal, A.; Ikeda-Ohtsubo, W.; Sipkema, D.; Peggs, D.; McGurk, C.; Forlenza, M.; Wiegertjes, G.F.; Brugman, S. Feed, Microbiota, and Gut Immunity: Using the Zebrafish Model to Understand Fish Health. Front. Immunol. 2020, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Nishimura, Y.; Zang, L.; Hirano, M.; Shimada, Y.; Wang, Z.; Umemoto, N.; Kuroyanagi, J.; Nishimura, N.; Tanaka, T. Diet-Induced Obesity in Zebrafish Shares Common Pathophysiological Pathways with Mammalian Obesity. BMC Physiol. 2010, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, P.E. Zebrafish as Animal Model for Aquaculture Nutrition Research. Front. Genet. 2014, 5, 313. [Google Scholar] [CrossRef]
- Roeselers, G.; Mittge, E.K.; Stephens, W.Z.; Parichy, D.M.; Cavanaugh, C.M.; Guillemin, K.; Rawls, J.F. Evidence for a Core Gut Microbiota in the Zebrafish. ISME J. 2011, 5, 1595–1608. [Google Scholar] [CrossRef]
- Oehlers, S.H.; Flores, M.V.; Chen, T.; Hall, C.J.; Crosier, K.E.; Crosier, P.S. Topographical Distribution of Antimicrobial Genes in the Zebrafish Intestine. Dev. Comp. Immunol. 2011, 35, 385–391. [Google Scholar] [CrossRef]
- Wallace, K.N.; Akhter, S.; Smith, E.M.; Lorent, K.; Pack, M. Intestinal Growth and Differentiation in Zebrafish. Mech. Dev. 2005, 122, 157–173. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Volynets, V. Nutritional Influences of Overfeeding on Experimental Outcomes in Laboratory Mice: Consequences for Gut Microbiota and Other Functional Studies. Int. J. Med. Microbiol. 2016, 306, 328–333. [Google Scholar] [CrossRef]
- Alexander, T.J.L. Methods of disease control. In Disease of Swine; Blackwell Science: Oxford, UK, 1992; pp. 808–836. [Google Scholar]
- Nikiforov-Nikishin, D.L.; Nikiforov-Nikishin, A.L.; Bugaev, O.G.; Kochetkov, N.I. Temperature Differentiation of Aquatic Microbiota of a Closed Water Supply System by the Example of Incubation of Microbiological Crops at 21 and 37 °C. IOP Conf. Ser. Earth Environ. Sci. 2021, 723, 042049. [Google Scholar] [CrossRef]
- Schreier, H.J.; Mirzoyan, N.; Saito, K. Microbial diversity of biological filters in recirculating aquaculture systems. Curr. Opin. Biotechnol. 2010, 21, 318–325. [Google Scholar] [CrossRef]
- Luca, C.; Ercolini, D. Molecular Techniques in the Microbial Ecology of Fermented Foods; Springer: New York, NY, USA, 2008. [Google Scholar]
- Cocolin, L.; Alessandria, V.; Dolci, P.; Gorra, R.; Rantsiou, K. Culture Independent Methods to Assess the Diversity and Dynamics of Microbiota during Food Fermentation. Int. J. Food Microbiol. 2013, 167, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Liu, X.; Huang, Z.; Li, Y.; Zhang, L.; Luo, Y. Effects of Chitosan Oligosaccharides on Microbiota Composition of Silver Carp (Hypophthalmichthys molitrix) Determined by Culture-Dependent and Independent Methods during Chilled Storage. Int. J. Food Microbiol. 2018, 268, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; AbÃcly, M.; Boyer, F.; Morville, P.; Pochart, P.; Suau, A. Low Species Diversity and High Interindividual Variability in Faeces of Preterm Infants as Revealed by Sequences of 16S RRNA Genes and PCR-Temporal Temperature Gradient Gel Electrophoresis Profiles. FEMS Microbiol. Ecol. 2006, 57, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Hiergeist, A.; Gläsner, J.; Reischl, U.; Gessner, A. Analyses of Intestinal Microbiota: Culture versus Sequencing. ILAR J. 2015, 56, 228–240. [Google Scholar] [CrossRef]
- Aleström, P.; D’Angelo, L.; Midtlyng, P.J.; Schorderet, D.F.; Schulte-Merker, S.; Sohm, F.; Warner, S. Zebrafish: Housing and Husbandry Recommendations. Lab. Anim. 2019, 54, 213–224. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Abdel-Tawwab, M.; Dawood, M.A.O.; Menanteau-Ledouble, S.; El-Matbouli, M. Benefits of Dietary Butyric Acid, Sodium Butyrate, and Their Protected Forms in Aquafeeds: A Review. Rev. Fish. Sci. Aquac. 2020, 28, 421–448. [Google Scholar] [CrossRef]
- Aalamifar, H.; Soltanian, S.; Vazirzadeh, A.; Akhlaghi, M.; Morshedi, V.; Gholamhosseini, A.; Torfi Mozanzadeh, M. Dietary Butyric Acid Improved Growth, Digestive Enzyme Activities and Humoral Immune Parameters in Barramundi (Lates calcarifer). Aquac. Nutr. 2019, 26, 156–164. [Google Scholar] [CrossRef]
- Gramm, S.; Sergey, B.; Nikita, K.; Viktor, K.; Alexei, N.-N.; Dmitry, N.-N. Histological Changes in the Liver, Intestines and Kidneys of Clarias gariepinus When Using Feed with Chelated Compounds. Int. J. Pharm. Res. 2020, 12, 2380–2391. [Google Scholar] [CrossRef]
- Nikiforov-Nikishin, D.; Kochetkov, N.; Klimov, V.; Bugaev, O. Effects of Chelated Complexes and Probiotics on Histological and Morphometric Parameters of the Gastrointestinal Tract of Juvenile Carp (Cyprinus carpio). N. Z. J. Zool. 2022, 1–12. [Google Scholar] [CrossRef]
- Mézes, M.; Erdélyi, M.; Balogh, K. Deposition of organic trace metal complexes as feed additives in farm animals. Eur. Chem. Bull. 2012, 1, 410. [Google Scholar]
- Langi, P.; Kiokias, S.; Varzakas, T.; Proestos, C. Carotenoids: From Plants to Food and Feed Industries. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; pp. 57–71. [Google Scholar] [CrossRef]
- Noakes, D.E.; Wallace, L.; Smith, G.R. Bacterial Flora of the Uterus of Cows after Calving on Two Hygienically Contrasting Farms. Vet. Rec. 1991, 128, 440–442. [Google Scholar] [CrossRef]
- Kim Suvarna, S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier: Oxford, UK, 2019. [Google Scholar]
- Menke, A.L.; Spitsbergen, J.M.; Wolterbeek, A.P.M.; Woutersen, R.A. Normal Anatomy and Histology of the Adult Zebrafish. Toxicol. Pathol. 2011, 39, 759–775. [Google Scholar] [CrossRef] [PubMed]
- Semova, I.; Carten, J.D.; Stombaugh, J.; Mackey, L.C.; Knight, R.; Farber, S.A.; Rawls, J.F. Microbiota Regulate Intestinal Absorption and Metabolism of Fatty Acids in the Zebrafish. Cell Host Microbe 2012, 12, 277–288. [Google Scholar] [CrossRef]
- Falcinelli, S.; Rodiles, A.; Hatef, A.; Picchietti, S.; Cossignani, L.; Merrifield, D.L.; Unniappan, S.; Carnevali, O. Influence of Probiotics Administration on Gut Microbiota Core. J. Clin. Gastroenterol. 2018, 52 (Suppl. S1), S50–S56. [Google Scholar] [CrossRef]
- Antony Jesu Prabhu, P.; Schrama, J.W.; Mariojouls, C.; Godin, S.; Fontagné-Dicharry, S.; Geurden, I.; Surget, A.; Bouyssiere, B.; Kaushik, S.J. Post-Prandial Changes in Plasma Mineral Levels in Rainbow Trout Fed a Complete Plant Ingredient Based Diet and the Effect of Supplemental Di-Calcium Phosphate. Aquaculture 2014, 430, 34–43. [Google Scholar] [CrossRef]
- Skrypnik, K.; Suliburska, J. Association between the Gut Microbiota and Mineral Metabolism. J. Sci. Food Agric. 2017, 98, 2449–2460. [Google Scholar] [CrossRef]
- Farzad, R.; Kuhn, D.D.; Smith, S.A.; O’Keefe, S.F.; Hines, I.S.; Bushman, T.J.; Galagarza, O.A.; Stevens, A.M. Effects of Selenium-Enriched Prebiotic on the Growth Performance, Innate Immune Response, Oxidative Enzyme Activity and Microbiome of Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2021, 531, 735980. [Google Scholar] [CrossRef]
- Yausheva, E.; Miroshnikov, S.; Sizova, E. Intestinal Microbiome of Broiler Chickens after Use of Nanoparticles and Metal Salts. Environ. Sci. Pollut. Res. 2018, 25, 18109–18120. [Google Scholar] [CrossRef]
- Gillingham, M.A.F.; Borghesi, F.; Montero, B.K.; Migani, F.; Béchet, A.; Rendón-Martos, M.; Amat, J.A.; Dinelli, E.; Sommer, S. Bioaccumulation of Trace Elements Affects Chick Body Condition and Gut Microbiome in Greater Flamingos. Sci. Total Environ. 2021, 761, 143250. [Google Scholar] [CrossRef]
- Stojković, D.; Kostić, M.; Smiljković, M.; Aleksić, M.; Vasiljević, P.; Nikolić, M.; Soković, M. Linking Antimicrobial Potential of Natural Products Derived from Aquatic Organisms and Microbes Involved in Alzheimer’s Disease—A Review. Curr. Med. Chem. 2020, 27, 4372–4391. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut Microbiome Alterations in Alzheimer’s Disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Yang, J.; Zhou, L.; Yang, Z.; Liang, J.; Liu, Y.; Ma, X.; Qian, Z.; Hong, P.; Kalueff, A.V.; et al. Marine Fungal Metabolite Butyrolactone I Prevents Cognitive Deficits by Relieving Inflammation and Intestinal Microbiota Imbalance on Aluminum Trichloride-Injured Zebrafish. J. Neuroinflamm. 2022, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Patsiou, D.; del Rio-Cubilledo, C.; Catarino, A.I.; Summers, S.; Mohd Fahmi, A.; Boyle, D.; Fernandes, T.F.; Henry, T.B. Exposure to Pb-Halide Perovskite Nanoparticles Can Deliver Bioavailable Pb but Does Not Alter Endogenous Gut Microbiota in Zebrafish. Sci. Total Environ. 2020, 715, 136941. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Limbu, S.M.; Shen, Z.; Zhai, W.; Qiao, F.; He, A.; Du, Z.; Zhang, M. Environmental Concentrations of Antibiotics Impair Zebrafish Gut Health. Environ. Pollut. 2018, 235, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Liu, C.; Li, C.-C.; Fu, M.; Takahashi, S.; Hu, K.-Q.; Aizawa, K.; Hiroyuki, S.; Wu, G.; Zhao, L.; et al. Dietary Tomato Powder Inhibits High-Fat Diet–Promoted Hepatocellular Carcinoma with Alteration of Gut Microbiota in Mice Lacking Carotenoid Cleavage Enzymes. Cancer Prev. Res. 2018, 11, 797–810. [Google Scholar] [CrossRef]
- Lin, F.; Wu, H.; Zeng, M.; Yu, G.; Dong, S.; Yang, H. Probiotic/Prebiotic Correction for Adverse Effects of Iron Fortification on Intestinal Resistance to Salmonella Infection in Weaning Mice. Food Funct. 2018, 9, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.K.; Islam, S.S.; Sithi, I.N.; Ghosh, A.K.; Banu, G.R.; Bir, J.; Huq, K.A. Effect of Probiotics on Immune Competence of Giant Freshwater Prawn Macrobrachium rosenbergii. Aquac. Res. 2018, 50, 644–657. [Google Scholar] [CrossRef]
- Venkat, H.K.; Sahu, N.P.; Jain, K.K. Effect of feeding Lactobacillus-based probiotics on the gut microbiota, growth and survival of postlarvae of Macrobrachium rosenbergii (de Man). Aquac. Res. 2004, 35, 501–507. [Google Scholar] [CrossRef]
- Amoah, K.; Huang, Q.C.; Tan, B.P.; Zhang, S.; Chi, S.Y.; Yang, Q.H.; Liu, H.Y.; Dong, X.H. Dietary supplementation of probiotic Bacillus coagulans ATCC 7050, improves the growth performance, intestinal morphology, microbiota, immune response, and disease confrontation of Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 87, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Wang, S.; Qiu, H.; Xie, R.; Zhang, H.; Chen, N.; Li, S. Modulation of Growth Performance, Antioxidant Capacity, Non-Specific Immunity and Disease Resistance in Largemouth Bass (Micropterus salmoides) upon Compound Probiotic Cultures Inclusion. Fish Shellfish Immunol. 2022, 127, 804–812. [Google Scholar] [CrossRef] [PubMed]
- López-Mondéjar, R.; Zühlke, D.; Becher, D.; Riedel, K.; Baldrian, P. Cellulose and Hemicellulose Decomposition by Forest Soil Bacteria Proceeds by the Action of Structurally Variable Enzymatic Systems. Sci. Rep. 2016, 6, 25279. [Google Scholar] [CrossRef]
- Abid, A.; Davies, S.J.; Waines, P.; Emery, M.; Castex, M.; Gioacchini, G.; Carnevali, O.; Bickerdike, R.; Romero, J.; Merrifield, D.L. Dietary synbiotic application modulates Atlantic salmon (Salmo salar) intestinal microbial communities and intestinal immunity. Fish Shellfish Immunol. 2013, 35, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Mackay, C.R. Diet, Gut Microbiota and Immune Responses. Nat. Immunol. 2010, 12, 5–9. [Google Scholar] [CrossRef]
- Vidyasagar, S.; Barmeyer, C.; Geibel, J.; Binder, H.J.; Rajendran, V.M. Role of Short-Chain Fatty Acids in Colonic HCO3 Secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G1217–G1226. [Google Scholar] [CrossRef]
- Annison, G.; Illman, R.J.; Topping, D.L. Acetylated, Propionylated or Butyrylated Starches Raise Large Bowel Short-Chain Fatty Acids Preferentially When Fed to Rats. J. Nutr. 2003, 133, 3523–3528. [Google Scholar] [CrossRef]
- Ward, F.W.; Coates, M.E. Gastrointestinal PH Measurement in Rats: Influence of the Microbial Flora, Diet and Fasting. Lab. Anim. 1987, 21, 216–222. [Google Scholar] [CrossRef]
- Hai, N.V. The use of probiotics in aquaculture. J. Appl. Microbiol. 2015, 119, 917–935. [Google Scholar] [CrossRef]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.H.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.L.; et al. The Mucus and Mucins of the Goblet Cells and Enterocytes Provide the First Defense Line of the Gastrointestinal Tract and Interact with the Immune System. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef]
- Reboul, E. Mechanisms of Carotenoid Intestinal Absorption: Where Do We Stand? Nutrients 2019, 11, 838. [Google Scholar] [CrossRef]
- Lyu, Y.; Wu, L.; Wang, F.; Shen, X.; Lin, D. Carotenoid Supplementation and Retinoic Acid in Immunoglobulin a Regulation of the Gut Microbiota Dysbiosis. Exp. Biol. Med. 2018, 243, 613–620. [Google Scholar] [CrossRef]
- Lan, C.-C.; Love, D.R. Molecular Characterisation of Bacterial Community Structure along the Intestinal Tract of Zebrafish (Danio rerio): A Pilot Study. ISRN Microbiol. 2012, 2012, 590385. [Google Scholar] [CrossRef][Green Version]
- Paul, H.A.; Bomhof, M.R.; Vogel, H.J.; Reimer, R.A. Diet-Induced Changes in Maternal Gut Microbiota and Metabolomic Profiles Influence Programming of Offspring Obesity Risk in Rats. Sci. Rep. 2016, 6, 20683. [Google Scholar] [CrossRef] [PubMed]
- Arias-Jayo, N.; Abecia, L.; Alonso-Sáez, L.; Ramirez-Garcia, A.; Rodriguez, A.; Pardo, M.A. High-Fat Diet Consumption Induces Microbiota Dysbiosis and Intestinal Inflammation in Zebrafish. Microb. Ecol. 2018, 76, 1089–1101. [Google Scholar] [CrossRef]
- Rossi, L.T.; Sharpen, A.R.; Zimmermann, J.A.; Olivero, C.R.; Zbrun, M.V.; Frizzo, L.S.; Signorini, M.L.; Bacchetta, C.; Cian, R.E.; Cazenave, J.; et al. Intestinal Microbiota Modulation in Juvenile Pacú (Piaractus mesopotamicus) by Supplementation with Pyropia Columbina and β-Carotene. Aquac. Int. 2020, 28, 1001–1016. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Mu, M.; Guo, M.; Yu, H.; Xing, M. Lycopene Alleviates Sulfamethoxazole-Induced Hepatotoxicity in Grass Carp (Ctenopharyngodon idellus) via Suppression of Oxidative Stress, Inflammation and Apoptosis. Food Funct. 2020, 11, 8547–8559. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E. Absorption of Vitamin a and Carotenoids by the Enterocyte: Focus on Transport Proteins. Nutrients 2013, 5, 3563–3581. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wu, J.; Li, J.; Bai, Y.; Luo, Y.; Ji, B.; Xia, B.; Liu, Z.; Tan, X.; Lv, J.; et al. Lycopene Alleviates DSS-Induced Colitis and Behavioral Disorders via Mediating Microbes-Gut–Brain Axis Balance. J. Agric. Food Chem. 2020, 68, 3963–3975. [Google Scholar] [CrossRef]
- Meng, Y.; Yang, Y.; Wang, J. Effects of lycopene supplement on the antioxidant capacity of Carassius auratus. J. Anhui Agric. Univ. 2011, 38, 59–64. [Google Scholar]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Danilenko, V.N.; Yunes, R.A.; Ilyasov, R.A.; Kovtun, A.S.; Yanenko, A.S.; Kozlovsky, Y.E.; Sidorenko, A.V.; Kolomiets, E.I. Research human and animal microbiome as a source of genetic and pharmacological resources for the development of innovative biotechnology in medicine, animal husbandry and agroindustrial complex. Adv. Mod. Biol. 2022, 142, 315–332. [Google Scholar]
- Danilenko, V.N.; Ilyasov, R.A.; Yunes, R.A.; Yanenko, A.S.; Kozlovsky, Y.E.; Sverchkova, N.V.; Kolomiets, E.I. Animal microbiome: Search for biologically active ingredients for creation of probiotics and pharmabiotics. Adv. Mod. Biol. 2022, 142, 333–348. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikiforov-Nikishin, A.; Smorodinskaya, S.; Kochetkov, N.; Nikiforov-Nikishin, D.; Danilenko, V.; Bugaev, O.; Vatlin, A.; Abrosimova, N.; Antipov, S.; Kudryavtsev, A.; et al. Effects of Three Feed Additives on the Culturable Microbiota Composition and Histology of the Anterior and Posterior Intestines of Zebrafish (Danio rerio). Animals 2022, 12, 2424. https://doi.org/10.3390/ani12182424
Nikiforov-Nikishin A, Smorodinskaya S, Kochetkov N, Nikiforov-Nikishin D, Danilenko V, Bugaev O, Vatlin A, Abrosimova N, Antipov S, Kudryavtsev A, et al. Effects of Three Feed Additives on the Culturable Microbiota Composition and Histology of the Anterior and Posterior Intestines of Zebrafish (Danio rerio). Animals. 2022; 12(18):2424. https://doi.org/10.3390/ani12182424
Chicago/Turabian StyleNikiforov-Nikishin, Alexei, Svetlana Smorodinskaya, Nikita Kochetkov, Dmitry Nikiforov-Nikishin, Valery Danilenko, Oleg Bugaev, Aleksey Vatlin, Nina Abrosimova, Sergei Antipov, Alexander Kudryavtsev, and et al. 2022. "Effects of Three Feed Additives on the Culturable Microbiota Composition and Histology of the Anterior and Posterior Intestines of Zebrafish (Danio rerio)" Animals 12, no. 18: 2424. https://doi.org/10.3390/ani12182424
APA StyleNikiforov-Nikishin, A., Smorodinskaya, S., Kochetkov, N., Nikiforov-Nikishin, D., Danilenko, V., Bugaev, O., Vatlin, A., Abrosimova, N., Antipov, S., Kudryavtsev, A., & Klimov, V. (2022). Effects of Three Feed Additives on the Culturable Microbiota Composition and Histology of the Anterior and Posterior Intestines of Zebrafish (Danio rerio). Animals, 12(18), 2424. https://doi.org/10.3390/ani12182424






